There are several significant limitations to progress in studying cardiac disorders, including the lack of relevant tissue samples, inability to study human cardiomyocytes longitudinally, and lack of a patient-specific drug testing platform1. Traditionally, researchers have relied on cell-based assays or animal models to understand disease progression and develop therapeutic interventions2. However, these models have well-known limitations in reproducing human pathophysiology. Additionally, such models fail to recapitulate the considerable genetic variation that exists within disease populations, which may play a role in dictating the extent of disease severity and spectrum of patient responses to medical therapy. Clinicians typically rely on the patient’s history, clinical examination, and test results to formulate a clinical diagnosis and choose the presumed appropriate pharmacotherapy. However, clinical diagnoses often fail to consider diversity in underlying etiologies that could lead to similar clinical presentations. Conventionally, patients with similar clinical presentations will still receive the same medications on the basis of their symptoms, ignoring patient-specific factors that may affect response to therapy3. Therefore, there is a compelling need for better models to gain insights into patient-specific disease mechanism and clinical pharmacotherapy4.
The emerging human induced pluripotent stem cell (iPSC) technology has considerable advantages over classical models by overcoming limitations associated with other models of human disease. Because iPSCs are surrogates of human cardiomyocytes, they can be derived from healthy versus diseased patients to provide a robust alternative to animals for researchers to model human diseases5–7. Additionally, iPSCs are patient-specific, allowing them to more faithfully recapitulate the genotype encoded by original donor; this enables researchers to understand disease mechanisms at an individual patient level, potentially allowing screening of individual drugs for efficacy and toxicity. For these reasons, a precise prediction of each patient’s unique responses to different drugs is now within reach under the iPSC-based model as it becomes an increasingly valuable drug screening tool to guide clinical pharmacotherapy1, 4, 8, 9 (Figure 1).
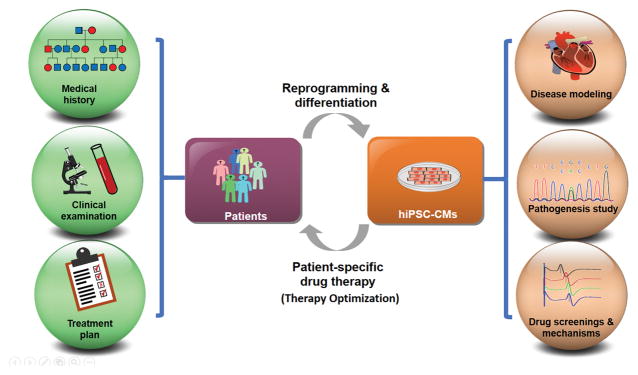
Figure 1.
Patient-specific pharmacotherapy using iPSC-CMs. Conventionally, clinicians rely on patients’ medical histories, clinical examinations, and test results to choose the presumed appropriate treatment plans. This is an imperfect approach as patients may experience potentially serious side effects because important relevant genetic information specific to individual patients is not considered beforehand. By contrast, an iPSC-based model is expected to enable patient-specific disease modeling, pathogenesis study, and drug screenings of some candidate drugs on patient-specific iPSC-differentiated cells. This makes possible mechanism studies and more accurate predictions of individual patients’ responses to different drugs. A working iPSC model could greatly optimize treatment plans with the patient-specific pharmacotherapy
In this issue of the Journal, Maizels et al.10 established a patient- and disease-specific iPSC-cardiomyocyte (CM) model that recapitulates an autosomal-recessive type of catecholaminergic polymorphic ventricular tachycardia type 2 (CPVT2) with the D307H-CASQ2 mutation in vitro. By simultaneously recording Ca2+ transients and optical action-potentials, they observed a complex interaction between Ca2+ handling abnormalities and membrane potential changes. This study therefore provided mechanistic insights into CPVT Type 2 (CPVT2) disease pathogenesis and treatment. The CPVT2 phenotype is aggravated by adrenergic stimulation, in which the ectopic Ca2+ releases and membrane depolarizations could trigger each other, and its abnormal Ca2+-handling is associated with delayed afterdepolarizations (DADs), early afterdepolarizations (EADs), and triggered arrhythmias. Maizels et al. showed that CPVT2 iPSC-CMs could model the cellular CPVT phenotype, which had significant Ca2+ handling abnormalities, diastolic Ca2+ leakage, and arrhythmic activity.
The findings by Maizels et al.10 are consistent with prior findings of published iPSC-CM disease models of CPVT1 and CPVT2. Those studies found a propensity for diastolic Ca2+ leak, increased incidence of EADs and DADs, and favorable therapeutic responses to beta blockers and flecainide. Furthermore, Maizels et al. modeled store overload–induced Ca2+ release (SOICR) events in the CPVT2 iPSC-CMs, thereby revealing new mechanistic insights into the pathogenesis of CPVT2 by demonstrating a decreased threshold for SOICR in the affected iPSC-CMs. These results further elucidated the mechanistic nature of CPVT2 arrhythmogenicity, supporting the potential physiological roles of CASQ2 in luminal Ca2+ sensing and in ryanodine receptor (RyR2) stabilization.
Unlike prior CPVT iPSC modeling studies, Maizels, et al. also screened multiple pharmacological compounds (propranolol, labetalol, JTV519, carvedilol, flecainide and riluzole) using patient-specific CPVT2 iPSC-CMs in clinically relevant drug concentrations, generating novel insights into the anti-arrhythmic mechanisms of the drugs tested on CPVT. These choices were not haphazard. Beta-blockers are the established first choice therapy for CPVT11 and flecainide is receiving growing attention12. The interest in labetalol and carvedilol reflects the fact that these two beta-blockers also have some alpha-adrenergic receptors blocking effect; this is the consequence of the significant efficacy demonstrated by left cardiac sympathetic denervation13,14. They found that carvedilol exerts a favorable effect by stabilizing RyR2 and increasing threshold for SOICR. These results also show that the salutary effect of beta blocker therapy is not a class wide effect and that carvedilol was superior to propranolol and labetalol in suppressing arrhythmia or SOICR at the cellular level. Moreover, flecainide appears to exert its therapeutic effect by suppressing the incidence of triggered activity rather than stabilizing the RyR2. These findings resolved past controversies regarding the mechanisms of action for some agents with potential therapeutic efficacy.
To evaluate the potential of this CPVT2 iPSC-based model in prospectively predicting clinical drug effects in CPVT2, Maizels et al.10 then compared the in vitro drug testing findings in iPSC-CMs with the drug responses of the same patient. The authors found that flecainide significantly reduced isoproterenol and phenylephrine-induced arrhythmia in iPSC-CMs, which was congruent with patient exercise test results showing that treatment with flecainide ameliorated exercise-induced ventricular tachycardia. Interestingly, labetalol did not reduce the incidence of arrhythmia at the single cell level or clinically. Propranolol treatment resulted in partial reduction of arrhythmic burden in iPSC-CMs and improvement in (but not resolution of) exercise-induced ventricular tachycardia clinically. The partial success of the therapies used reflects the well-known difficulties in managing genetic disorders when the patients are homozygous for the same mutation, as this increases clinical severity. This is the case for the two channelopathies in which sympathetic activation is the main trigger for life-threatening arrhythmias: CPVT2 and the Jervell-Lange-Nielsen syndrome15. Although this is a single-case proof of principle, these are encouraging results attesting to the power of iPSC-CMs in predicting patient-specific drug responses in CPVT patients.
Although iPSC-CMs have great potential as a platform for disease modeling and drug screenings, they currently have several limitations16. One limitation is that iPSC-CMs do not reach the full adult native phenotype of cardiomyocytes. Another limitation is the lengthy time required to reprogram somatic cells to iPSCs and to subsequently differentiate them to functional cell types (about 3 months). In addition, due to low incidence of rare disease syndromes, current models are obtained usually from a small number of patients. Therefore, the results of the studies may not necessarily be generalizable to larger populations of patients with inherited disorders such as CPVT.
In summary, Maizels et al.10 were able to demonstrate that iPSCs can recapitulate the disease phenotype of CPVT2. Their study provides important insights into disease and drug therapy mechanisms. Future improvements in iPSC-CM maturation17, optimization of protocols for faster yield of iPSC-CMs18, and establishing iPSC biobanks with larger population of affected patients19 will enable more precise iPSC modeling of diseases20. Bench-to-bedside correlations utilizing iPSC-CMs will become increasingly important in future studies of cardiovascular disease to fully leverage the broad utility of the human cellular model, thereby bringing precision medicine closer to reality1,4,8,21.
Acknowledgments
Sources of Funding: We thank funding support from National Institutes of Health (NIH) R01 HL128170, R01 HL133272, R01 HL123968, R01 HL126527, and Burroughs Wellcome Foundation 1015009.
Footnotes
Disclosures: None
References
- 1.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz PJ. Do animal models have clinical value? Am J Cardiol. 1998;81:14D–20D. doi: 10.1016/s0002-9149(98)00148-9. [DOI] [PubMed] [Google Scholar]
- 3.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Matsa E, Ahrens JH, Wu JC. Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol Rev. 2016;96:1093–1126. doi: 10.1152/physrev.00036.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallam K, Li Y, Sager PT, Houser SR, Wu JC. Finding the rhythm of sudden cardiac death: new opportunities using induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2015;116:1989–2004. doi: 10.1161/CIRCRESAHA.116.304494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallam K, Kodo K, Wu JC. Modeling inherited cardiac disorders. Circ J. 2014;78:784–794. doi: 10.1253/circj.cj-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, Zhang Y, Vermglinchan V, Lan F, Gu M, Gong T, Zhuge Y, He C, Ebert AD, Sanchez-Freire V, Churko J, Hu S, Sharma A, Lam CK, Scheinman MM, Bers DM, Wu JC. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of brugada syndrome. J Am Coll Cardiol. 2016;68:2086–2096. doi: 10.1016/j.jacc.2016.07.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016;13:333–349. doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maizels L, Huber I, Arbel G, Tijsen AJ, Gepstein A, Khoury A, Gepstein L. Patient-Specific Drug Screening Utilizing a Human Induced Pluripotent Stem Cell Model of CPVT-2. Circ Arrhythm Electrophysiol. 2017;10:e004725. doi: 10.1161/CIRCEP.116.004725. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 12.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haissaguerre M, Knollmann BC, Wilde AA. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 14.De Ferrari GM, Dusi V, Spazzolini C, Bos JM, Abrams DJ, Berul CI, Crotti L, Davis AM, Eldar M, Kharlap M, Khoury A, Krahn AD, Leenhardt A, Moir CR, Odero A, Olde Nordkamp L, Paul T, Roses INF, Shkolnikova M, Till J, Wilde AA, Ackerman MJ, Schwartz PJ. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–2193. doi: 10.1161/CIRCULATIONAHA.115.015731. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, Shkolnikova M, Berul CI, Bitner-Glindzicz M, Toivonen L, Horie M, Schulze-Bahr E, Denjoy I. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 16.Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx V. Stem cells: disease models that show and tell. Nat Methods. 2015;12:111–114. doi: 10.1038/nmeth.3263. [DOI] [PubMed] [Google Scholar]
- 20.Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12:669–677. doi: 10.1016/j.stem.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]