Abstract
Objective
To assess the association of markers for dietary protein intake, measures of dietary adherence and demographic variables with weight loss in the POUNDS Lost study over the first 6 months and again between 6 and 24 months using data from those who completed each period.
Methods
Secondary analysis of pooled data on completers assigned to one of four diets: 65%C/15%P/20%F (AP/LF); 55%C/25%P/20%F (HP/LF); 45%C/15%P/40%F (AP/HF); or 35%C/25%P40%F (HP/HF) in the POUNDS Lost study. Urinary nitrogen excretion, dietary adherence measured by 24 hour recall and attendance at sessions, age (above & below 50 years), sex, race/ethnicity, and activity by pedometry were analyzed.
Results
Increased spread between protein intake at baseline and protein at 6 or 24 months, assessed by urinary nitrogen excretion, was associated with greater weight loss from baseline to 2 years. At 6 and 24 months older age, male sex, BMI > 30 kg/m2 and adherence to the fat and protein diets was associated with more weight loss. None of these variables was associated with regain from 6 to 24 months. Weight regain for women in the highest carbohydrate (65%) group was significantly greater [−4.4kg [95% CI: −5.9, −3.0] than for women in the lowest carbohydrate group [−1.8kg (95% CI:−3.2, −0.4kg)] (p for interaction =0.012).
Conclusion
An increased spread in the difference between baseline and follow-up protein intake was associated with greater weight loss, consistent with the ‘protein spread theory’. Women eating the highest carbohydrate diet regained more weight from 6 to 24 months.
Keywords: Randomized clinical trial, high protein diet, high fat diet, high carbohydrate diet, low carbohydrate diet, gender, age, ethnicity
INTRODUCTION
Whether the macronutrient composition of diets might enhance weight loss has been studied for more than a century and has resulted in a large number of publications (1–12) as well as reviews and meta-analyses (13–27). A substantial body of evidence supports the idea that consumption of more dietary protein would be a successful strategy to treat obesity and prevent its relapse (18, 19, 23, 24, 26, 27, 28). Other contributors to successful weight loss are adherence to the dietary program (9, 12, 29, 30, 31, 32, 33) as well as earlier and more rapid loss (34, 35). Men lose more weight than women (32, 36, 37, 38, 39), and older adults lose 1–2 kg more than younger ones (32). In structured programs, White participants tend to lose 2–3 kg more than African-Americans (38).
The largest single study to date to examine this question of diet composition is The POUNDS Lost trial (12, 40–56). In this two year dietary intervention 811 individuals who were overweight or obese were randomly assigned in a 2X2 factorial design to one of four diets with target intakes of 15% (average protein =AP) or 25% protein (high protein = HP) and 20% fat (low fat = LF) or 40% fat (high fat = HF). This design provided assigned levels of carbohydrate, protein, and fat as % energy of: 65%C,15%P, 20%F (AP/LF); 55%C,25%P, 20%F (HP/LF); 45%C,15%P, 40%F (AP/HF); and 35%C, 25%P, 40%F (HP/HF). The main paper from this study used intent to treat analysis with imputations for missing data and reported that weight losses were similar for 4 diets. Weight losses were respectively 3.0 vs. 3.6 kg in the 15% and 25% protein diet at two years (P=0.2) (12). For those assigned to 20% fat or 40% fat diet, weight loss was 3.3 kg in both cases (P=0.94). The comparison of highest carbohydrate (AP/LF; 35% carbohydrate) and lowest carbohydrate (HP/HF; 65% carbohydrate) showed 2.9 vs. 3.4 kg loss at two years (P=0.4 without adjustment for multiple comparisons and using imputed data) (12). Thus, there were no macronutrient effects on weight loss or weight maintenance at two years in the intent to treat analysis (12). When analyzed by level of adherence to diet, the data showed that adhering to the diet and to a higher protein diet, led to more weight loss. In this secondary analysis we have re-examined this relationship in those who completed six months and in those who completed 24 months using urea excretion as a marker for protein intake. In contrast with macronutrient composition, the POUNDS Lost trial did show that adherence to the behavioral program, as reflected in attendance at group sessions produced more weight loss (12, 29, 30). In an editorial to this paper Astrup and Pedersen noted that “the POUNDS LOST trial does not allow us to draw conclusions about the efficacy of protein in inducing satiety and inhibiting caloric intake, but it does emphasize the difficulty in achieving adherence to diets when running long-term intervention studies” (25) because contrasts between groups were too small. This secondary analysis was designed to pool data from all 4 diets to examine the relation of protein intake, as reflected in urinary urea excretion and weight loss. We also examined the effect of sex on the response to carbohydrate.
The European Diogenes study of diets with higher and lower dietary levels of protein with and without variation in glycemic index demonstrated that a higher protein diet could be beneficial in helping participants maintain weight loss (28). Bosse and Dixon (27) proposed that providing a wider separation from habitual protein intake to the higher protein intake of a new diet is an important factor in determining the success of dietary protein intake in weight management. Their protein spread theory proposes that there must be a sufficiently wide spread in protein intake between groups expressed as % difference in g/kg/day during the protein intervention to see differences in body composition. This concept, known as the ‘protein spread hypothesis’ has been tested in this paper by using urinary urea excretion as a marker of protein intake in a pooled analysis. In this secondary analysis we have also examined the association of age, sex, ethnicity and diet with changes in body weight.
METHODS
Participants
The POUNDS Lost trial was conducted at two clinical research sites: The Harvard School of Public Health in Boston, MA and the Pennington Biomedical Research Center in Baton Rouge, LA (12). The study design (determined by statistical power analysis) called for recruiting 400 participants at each site, for a total of 800 volunteers. A total of 811 individuals were enrolled in the final sample. The protocol and consent process were approved by the Institutional Review Board at each site. All subjects gave written informed consent. The inclusion and exclusion criteria for the POUNDS Lost study have been described in detail previously (12). Briefly, participants were men and women aged 30 to 70 years who were overweight or obese (BMI ranged from 25 to 40 kg/m2), and who were otherwise healthy and able to participate in a two-year clinical trial. The recruitment, enrollment and retention of participants in the POUNDS Lost have been summarized in an earlier publication (12).
Study Design
Participants were randomly assigned to one of four macronutrient diets, stratified by gender (12). Randomization was done by a statistician using a computer-based program and occurred after verification that all baseline data had been collected. The first subject was randomized in October 2004 and final clinic visits were completed in December 2007. Participants in all four groups were instructed to follow calorie restricted diets that consisted of the following macronutrient compositions: 1) low fat (20% of energy), average protein (15% of energy)(LF/AP); 2) low fat (20%), high protein (25%)(LF/HP); 3) high fat (40%), average protein (15%)(HF/AP); and 4) high fat (40%), high protein (25%)(HF/HP). All four diets were low in saturated fat and energy intake was reduced by 750 kcal/day below the resting metabolic rate measured with a metabolic cart multiplied by an activity factor, but with a minimal caloric intake of 1200 kcal/day (41). Similar foods were used for all diets, but in different amounts. Adherence to the diet was based on the 24-hour recalls done in telephone interviews at 6 months in a 50% subset of participants at both sites. Reported data were compared to assigned goals and those within +5% were labeled as “adherent” to their diet.
A behavioral program of similar programmatic content and intensity was uniformly implemented across the four dietary interventions using personnel from each site who had been trained in these techniques (12). To encourage a similar level of physical activity nearly a quarter of the participants (N=241) received and wore pedometers that were attached at the belt line. Data for the number of steps was recorded at baseline and 6 months in this sub-sample from each of the 4 dietary groups.
Investigators and staff strived for trial-wide equipoise by teaching participants that each diet had an equal chance of success and by emphasizing the importance of macronutrient goals. The staff members responsible for outcome measurements were blinded to treatment assignment. The following variables were analyzed: age above and below 50 years, sex, race/ethnicity, body mass index above and below 30 kg/m2, adherence to the fat and protein diets as determined from the baseline to 6 and 24 month data using the dietary recall information. Change in urinary urea nitrogen excretion per gram of urinary creatinine excretion was calculated from baseline to 6 (N = 240) and 24 months (N= 96) in subjects for whom this information was available.
Statistical Analysis
The results are reported for completers at 6 months and 24 months. The age category was a split at age 50 years which is nearly the mean age of 51.1±9.01 years. We evaluated weight loss from baseline to 6 months and 24 months and from 6 months to 24 months by subgroups of completers. The analysis also compared two of the four diets, lowest (35%) and highest (65%) carbohydrate content during weight loss and weight regain (6 to 24 months). Simple main effects were tested using mixed models including age category, sex, race/ethnicity and BMI category. For the analysis of change in urea nitrogen/creatinine excretion, grams of urea nitrogen and creatinine in samples at baseline, 6 months and 24 months were measured and the percentage change in the urinary urea nitrogen/creatinine ratio was calculated by subtracting the month 6 or month 24 value from the baseline value and dividing by the 6 month or 24 month value. In each interval (baseline to 6 months, baseline to 2 years and 6 months to 2 years) quartiles were established as from the 6 month or 24 month value minus baseline divided by the month 6 or 24 value. Analyses were all conducted using SAS software, Version 9.3 of the SAS System for Windows or JMP-11 for windows. Baseline data are presented as the mean and standard deviation, and change from baseline as least square means and 95% confidence interval.
Results
Participants
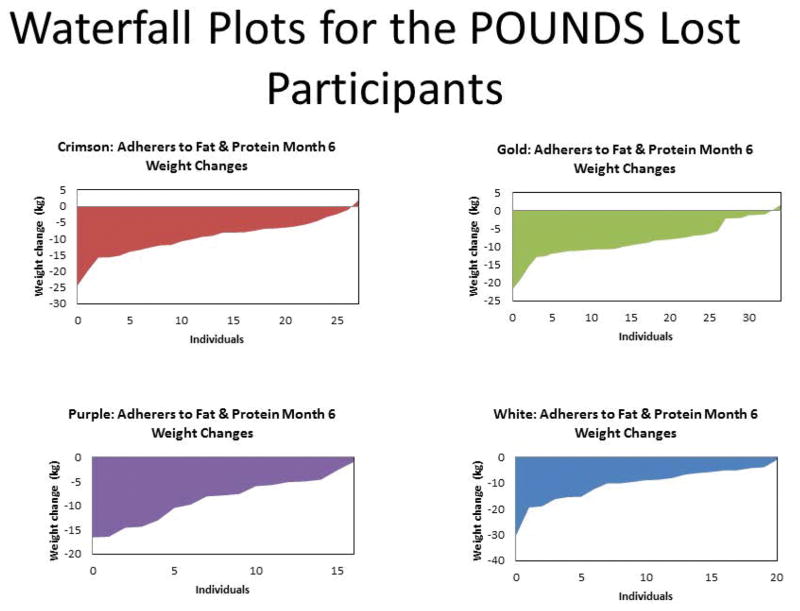
In the POUNDS Lost trial, a total of 811 individuals who were overweight or obese were randomized and 80% completed the two-year study. Retention ranged from 74% (high fat, average protein) to 84% (low fat, average protein, highest carbohydrate) for the four diets (Chi-square test, p=0.05 across diet groups)(12). In this secondary analysis of completers, we used only those individuals with complete data at baseline and 6 months (N=580) or at baseline and 24 months (N=392). Figure 1 shows the heterogeneity of weight loss from baseline to 6 months with individual participants in each diet group for whom data are available at both times ranked from the largest weight loss on the left to those who gained weight on the right. The great individual variability in response to each diet is evident with some participants losing more than 20 kg on each diet and others losing no weight or even gaining weight on each diet. This figure has the value of showing that a significant number of individuals on each diet actually gain weight. This differential response occurred in a group of participants who volunteered for a “weight loss study” and who received a standardized lifestyle intervention. One marker that the intervention was implemented similarly across groups is the amount of activity they performed as measured with pedometers. Individuals were asked to increase their activity by gradually increasing walking to 30 minutes per day. In the first 6 months 241 of the participants recorded their activity on pedometers. The average number of steps increased 1,151 ± 177 (P < 0.0001) with males (N= 116) walking significantly more steps than females (N=1,568 steps for men and 762 steps for women; P=0.023). There was no significant difference in steps walked between diets suggesting that activity increased similarly across the 4 diet groups (Data not shown).
Figure 1.
Weight change from baseline to 6 months for each individual participant in the 4 dietary assignment groups ranked from the largest loser on the left to the most weight gain on the right. Panel A (n=38 ) is the AP/LF group (15% protein, 20% fat, diet, 65% carbohydrate; Panel B (n=43 ) is the HP/LF group (25% protein, 20% fat, 55% carbohydrate); Panel C (n=28 ) is the HP/LF group (15% protein, 40% fat, 45% carbohydrate); and Panel D (n=30) is the HP/HF group (25% protein, 40% fat, 35% carbohydrate).
Table 1 shows the baseline weights and weight change to 6 months and 24 months for the pooled data. Older participants were lighter at baseline (91.6 kg; n=462) than younger ones (94.6 kg; n=349; P <0.01) and lost significantly more weight (8.11 kg vs 7.15 kg; P=0.023). Females were lighter (86.2±12.4 kg; n= 515) than males (104.5±13.5 kg; n= 296) at baseline (P < 0.001), and lost significantly more weight over 6 and 24 months. However, when expressed as percent weight loss, men and women were not different at 6 months [men −7.17% (95% CI −7.93, −6.40); women −6.87% (95% CI −7.41, −6.25)](P=0.45). Over the full 2 years, men lost marginally more body weight than women, on a percentage basis [Men; −4.72% (95% CI −6.75, −3.69; women; −3.60% (95%CI −4.38, −2.82)(P=0.070). There was no difference in baseline body weight between Whites (n=623) and African-Americans (n=175), and race/ethnicity did not influence weight loss over the first 6 months. The ‘Other category” consisted of Hispanics, Asian-Americans, Native Americans, Middle Easterners, Puerto Ricans and Native Hawaiian, who were not numerous enough to draw inferences. Individuals with a baseline BMI >30 kg/m2 lost more weight than those with a BMI <30 kg/m2 (P=0.0065). Adherence to the protein and fat diets was assessed by comparing those within 5% of their dietary goal on the 6-month dietary recall with those who didn’t achieve this goal. Those who adhered to the protein goal lost more weight in 6 months [−8.35kg; (95% CI −9.06, −7.65 kg; n=239)](P<0.0001) than those who did not [−4.80kg; (95% CI −5.75, −3.86 kg; n=106)]. To test the possibility that urinary protein might be a surrogate for adherence to the behavioral weight loss program we examined the relationship between attendance at group sessions and the change in urinary urea excretion from either baseline to 6 months or to 24 months. There was no correlation between the number of sessions attended and the change in urea nitrogen between baseline and 6 months (r=−0.03 p = 0.47) or 24 months (r=0.032, p = 0.50). Those who adhered to the low fat diet (n=136) lost slightly more weight (−8.13 kg) than those who did not (−6.70 kg) (P=0.021).
Table 1.
Baseline and Change from Baseline to 6 Months and 24 Months in the POUNDS Lost Study
Baseline weight and change in weight (%) from baseline to 6 and 24 months.
| Variable (Number at baseline) | Baseline Weight (kg) by Variable | Change in Weight from Baseline to 6 months (kg) mean & 95% CI | Change in Weight from Baseline to 24 months (kg) mean & 95% CI | P value for Change Baseline to 6 mos | P value for Change Baseline to 24 mos |
|---|---|---|---|---|---|
| Age | 0.023 | 0.0081 | |||
| < 50 yr (349) | 94.6 ± 15.8 | −7.15 (−8.97, −5.32) | −4.14 (−6.54, −1.73) | ||
| > 50 yr (462) | 91.6 ± 15.2 | −8.11 (−9,92, −6.31) | −5.70 (−8.07, −3.33) | ||
| Sex | 0.0005 | 0.0016 | |||
| M (296) | 104.5 ± 13.5 | −8.39 (−10.25, −6.54) | −5.87 (−8.31, −3.42) | ||
| F (515) | 86.2 ± 12.4 | −6.87 (−8.65, −5.09) | −3.98 (−6.31, −1.64) | ||
| Race | 0.19 | 0.64 | |||
| White (623) | 92.8 ± 15.5 | −6.11 (−6.64 −5.57) | −3.86 (−4.58, −3.13) | ||
| AA (175) | 93.3 ± 15.7 | −6.96 (−7.86, −6.05) | −3.52 (−4.78, −2.26) | ||
| Other (13) | 92.4 ± 15.3 | −8.38 (−12.5, −4.23) | −6.84 (−12.29, −1.30) | ||
| BMI | 0.0065 | 0.058 | |||
| < 30 (227) | 78.2 ± 9.7 | −6.99 (−8.89, −5.08) | −4.31 (−6.82, −1.80) | ||
| > 30 (584) | 98.6 ± 13.5 | −8.27 (−10.02, −6.53) | −5.53(−7.82, −3.24) | ||
| Adherence to Fat | 0.021 | 0.025 | |||
| Fat NO (209) | 94.96 ± 16.1 | −6.70 (−7.46, −5.93) | −4.21 (−5.51, −2.90) (N=108) | ||
| Fat YES (136) | 92.93 ± 16.0 | −8.13 (−9.08, −7.19) | −6.94 (−9.18, −4.70) (N=65) | ||
| Adherence to Protein | <0.0001 | 0.048 | |||
| Pro NO (106) | 91.84 ± 16.6 | −4.80 (−5.75, −3.86) | −3.93 (−5.66, −2.12) (N=78) | ||
| Pro YES (239) | 95.19 ± 15.8 | −8.35 (−9.06, −7.65) | −6.30 (−7.90. −4.71) (N=95) |
Baseline data is mean ± SD; Change data are mean and 95% confidence interval; Adherence to protein and fat was determined from the 6 and 24 month telephone dietary records as being within 5% of the dietary goal for either protein or fat.
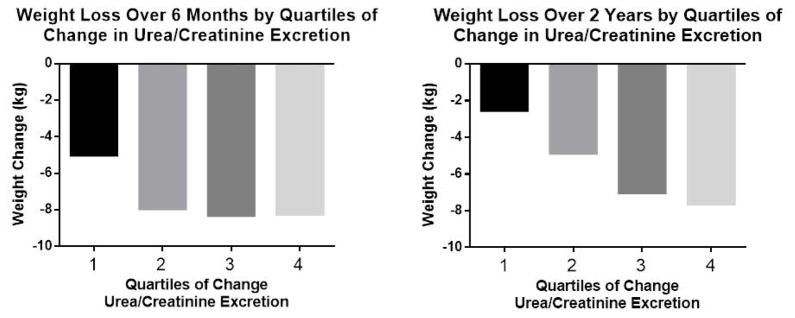
The nearly two-fold difference in weight loss for those who adhered to the protein diet led to a further analysis of change in protein intake using the change in urinary urea nitrogen excretion corrected for creatinine excretion as a surrogate for protein intake at 6 (n=581) and 24 months (n=393) (Figure 2). Panel A shows that individuals in the lowest quartile of change in urea nitrogen/creatinine (−58.7g/g to −14.2g/g) an indicator of reduced protein intake lost significantly less weight than those in the other 3 quartiles who had larger changes in protein intake from baseline to 6 months (−14.2g/g to 0.93g/g; 0.93g/g to 19.7g/g; 19.7g/g to 282.7g/g) (P<0.001 for quartile 2, 3, and 4 vs quartile 1; N=145 per quartile). Panel B shows quartiles of change in urea nitrogen/creatinine ratio to change in body weight from baseline to 2 years. (Quartile 1 =−62.9g/g to −10.9g/g; −10.9g/g to 2.4g/g; 2.4g/g to 23.0g/g; 23.0g/g to 203.8g/g) (N=68 per quartile). In this time interval all quartiles were significantly different from each other (P<0.05) except quartile 3 and 4. There was no relation between the number of sessions attended and the change in urea nitrogen between baseline and 6 months (r=−0.03 p = 0.47) or 24 months (r=0.032, p = 0.50).
Figure 2.
Change in body weight by quartiles of urea nitrogen/creatinine excretion. Panel A is the change in quartiles urea nitrogen/creatinine excretion from baseline to 6 months (quartile 1 is −58.7g/g to −14.2g/g; quartile 2 is −14.2g/g to 0.93g/g; quartile 3 is 0.93 g/g to 19.7 g/g; quartile 4 is 19.7g/g to 282.7g/g); Panel B is the change in quartiles of urea nitrogen/creatinine excretion from baseline to 24 months (quartile 1 is −62.9g/g to −10.9g/g; quartile 2 is −10.9g/g to 2.4g/g; quartile 3 is 2.4g/g to 23.0g/g; quartile 4 is 23.0g/g to 201.8g/g).
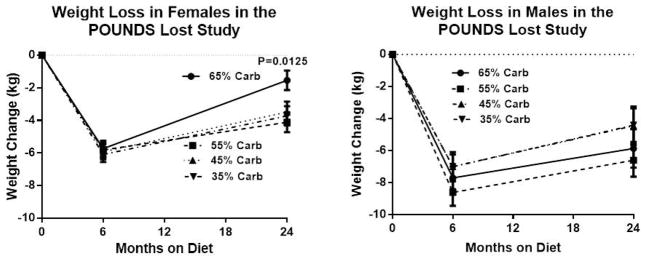
Table 2 shows the body weight at 6 months for each variable group, and the changes in body weight from 6 months to 24 months. During this time interval, average body weight increased significantly except in the small group of individuals classified as “other”. The significant relationships identified during the weight loss phase (Table 1) were no longer present. Thus, from 6 to 24 months, age, race, BMI and adherence to the protein or fat diet did not predict weight maintenance, but there was a significant age by sex interaction for the change in weight from 6 months to 24 months (P=0.0037) which was explored further. Figure 3 (left panel) explores this interaction by showing that the female completers eating the highest carbohydrate diet (AP/LF; 65% carbohydrate) were significantly less successful in maintaining their weight loss from 6 to 24 months (4.0±0.50 kg vs 2.3±0.50 kg P =0.022) and thus lost less from baseline to 24 months (1.5 kg vs 3.7 kg P=0.0125) than the women eating the lowest carbohydrate diet (HP/LF; 35% carbohydrate). This did not apply to men as seen in the right hand panel of figure 3.
Table 2.
Six Month Data and Change from 6 Months to 24 Months
Body weight at 6 months and change in weight (%) from 6 months to 24 months
| Variable (Number at 6 months) | 6 Month Weight (kg) | Change in Weight from 6 to 24 months (kg) (mean & 95% CI) | P value |
|---|---|---|---|
| Age | 0.52 | ||
| < 50 yr (239) | 88.6 ± 16.2 | 2.96 (1.21, 4.71) | |
| > 50 yr (368) | 85.0 ± 15.0 | 2.41 (0.68, 4.13) | |
| Sex | 0.15 | ||
| M (248) | 96.5 ± 13.8 | 2.66 (0.88, 4.44) | |
| F (369) | 80.2 ± 13.2 | 2.70 (1.01, 4.40) | |
| Race | 0.43 | ||
| White (464) | 86.4 ± 15.4 | 2.37 (1.82, 2.91) | |
| AA (133) | 86.5 ± 16.4 | 3.13 (2.18, 4.07) | |
| Other (11) | 86.5 ± 13.5 | 1.54 (−2.40, 5.48) | |
| BMI | 0.33 | ||
| < 30 (174) | 72.6 ± 10.3 | 2.63 (0.80, 4.46) | |
| > 30 (434) | 91.7 ± 13.9 | 2.73 (1.07, 4.39) | |
| Adherence to Fat | 0.23 | ||
| Fat NO (108) | 81.9 ± 10.6 | 1.98 (0.61, 3.25) | |
| Fat YES (65) | 86.7 ± 15.2 | 1.43 (−0.65, 3.38) | |
| Adherence to Protein | 0.25 | ||
| Protein NO (78) | 84.4 ± 15.3 | 1.84 (0.16, 3.52) | |
| Protein YES (95) | 83.0 ± 14.7 | 1.96 (0.43, 3.49) |
6 month data are mean ± SD; Change from 6 to 24 months are mean (95% Confidence Interval)
Figure 3.
Mean weight loss from baseline to 6 months and from 6 months to twenty-four months in females and male according to macronutrient assignment. For the 102 women on the lowest carbohydrate diets, significantly less weight loss was observed; weight change was −1.8% (95% CI −3.2%, −0.4%), significantly different from men [−5.5% (95% CI −7.2%, −3.7%)] (p=0.0016) and significantly less in women on the lowest carbohydrate diets [−4.4% (95% CI −5.8%, −3.0%)] (p=0.0116). Women lost significantly less weight (−3.2% [−4.3, −2.2]) on the low fat diets than men (−6.0% [−7.3, −4.6]) (p=0.0015), but the low fat macronutrient comparison combines the two highest carbohydrate diets (65% and 55%).
DISCUSSION
In the present study, we conducted a secondary analysis of the randomized POUNDS Lost study using pooled data to examine the extent to which protein and demographic variables influenced weight loss and weight maintenance or weight regain in men and women completing 6 or 24 months. Using urinary excretion as a marker of protein metabolism, the data shows that it was the “difference” or “spread” between baseline and actual protein intake was related to weight loss. Urine urea has been used as a surrogate for protein metabolism in numerous studies throughout the 20th and 21st centuries (57). Recognition of the microbiome as a contributor of human metabolism (58, 59, 60, 61) raises the interesting possibility that urinary urea may be influenced by microbial metabolism of urea which is a highly permeable molecule that surely must enter the gut. A higher plasma urea concentration reflecting higher intake of amino acids might provide the microbiome with more urea which would tend to blunt the effect we see. In one study of a single individual on 5 occasions over years Hibbert and Jackson showed the salvage of urea by the bowel (62). The authors concluded that measuring urea kinetics gives reproducible results and that the intra-individual variation in urea kinetics is much less than the inter-individual variation. A higher plasma urea concentration reflecting higher intake of amino acids might provide the microbiome with more urea which would tend to enhance breakdown. This might mean that the numbers used in our quartiles would underestimate the actual protein metabolism or protein spread, but the quartiles of weight loss would not be altered. With the data available from this study we are not able to test whether there was any such effect, but this is certainly an important question for future research.
The heterogeneity in weight loss among participants in each group was evident as was the overall similarity of weight loss across the 4 diet groups. This is consistent with what has been observed in other studies, including the Look AHEAD study (34, 35, 39), the Diabetes Prevention program (36, 37) and the Premiere study (38). The similarity of weight loss in the 4 diets led us to suggest that patients should select the diet which they prefer (12).
The similar variability of weight loss across the 4 diets assignments suggests that regardless of diet and possibly other treatment assignments, some people are able to lose weight and others are not. Such “individual” differences might reflect the numerous genetic differences which accounted for various responses in the POUNDS Lost study. Analysis of DNA samples from the participants in this study have predicted differential dietary responses to some of the metabolic variables (45–55). For example Xu et al (49) found that the C allele of PPM1K (protein phosphatase Mg/Mn 1K) was associated with less weight loss and a small decrease in both serum insulin and HOMA-IR in the group eating the high fat diet. In another report, we found that carriers of the risk allele for the FTO variant rs1558902 showed significant effects of dietary protein on 2-year changes in fat-free mass, whole body total percentage fat mass, total adipose tissue mass, visceral adipose tissue mass, and superficial adipose tissue mass (P < 0.05 for all interactions). There were also significant interactions observed at 6 months. Thus, a high-protein diet may be beneficial for weight loss and improvement in body composition and fat distribution in individuals with the risk allele of the FTO variant (rs1558902) (48).
There were differences in age, sex, race/ethnicity, and BMI that accounted for much of the variability in weight loss in this study. Older individuals lost more weight than younger ones, men lost more weight than women, and heavier individuals more than lighter ones, but African-Americans and Whites lost similar amounts of weight. This latter finding differs from observations in two large lifestyle intervention trials (37, 39), which show that African-Americans lose less weight, on average, when participating in lifestyle interventions, than do White Americans. The reason why our data differ is unclear. Interestingly, however, in a four year study of more than 2000 participants, initial one-year differences in weight loss by sex and ethnicity were also observed later at year four (36). Our study does confirm observations (35, 56) that older participants lose more weight when participating in lifestyle interventions than younger participants. However, after the weight loss phase age, sex and race/ethnicity were unrelated to the degree of weight regain from 6 to 24 months.
There have been many studies testing the efficacy of high protein diets compared to conventional lower fat diets for weight loss using both generic diets such as the diet in this paper and those reviewed by Bosse & Dixon(27) and diets named after individual investigators (2–12), such as the Ornish Diet, the Zone Diet or the Atkins Diet (See Refs 8 & 9). The present study uses urinary urea corrected to creatinine excretion to explore the changes in protein intake from baseline to 6 and 24 months. Adherence to the protein assignment was a significant predictor of successful weight loss which was consistent with the protein intake data in the main paper (12). When we pooled data on urea excretion as an index of protein intake from all 4 diets we found that changes of protein intake affected weight loss. Over the 2 years of the study there was a graded and statistically significant increase in weight loss across quartiles of increasing differences in protein intake (urea excretion). From baseline to 6 months, the quartile which had the least spread of protein intake lost significantly less weight than the other 3 quartiles, indicating that a difference of 25% in protein intake was sufficient to see a significantly greater weight loss. We conclude that increasing the spread of protein intake may enhance weight loss independent of initial diet. This is consistent with the “protein spread hypothesis’ of Bosse and Dixon (27). They proposed that providing a deviation of 38% on average from habitual protein intake is a sufficient to see an effect of a higher protein intake on weight loss. To test the possibility that urinary protein might be a surrogate for the adherence to the behavioral weight loss program we examined the relationship between attendance at group sessions and the change in urinary urea excretion from either baseline to 6 months or to 24 months. The correlation coefficients were essentially zero indicating that the change in urea excretion was not a surrogate for behavioral adherence. Thus, our data would suggest that decreases in protein from initial levels blunt weight loss whereas higher deviations in protein intake would reduce body weight.
In this secondary analysis using only completers there was also an effect of sex on the response to carbohydrate. Women on the highest carbohydrate (AP/LF; 65% carbohydrate) versus the lowest carbohydrate diet (HP/HF; 35% carbohydrate) lost similar amounts of weight in the first 6 months, but were less successful in maintaining the weight loss from 6 to 24 months than women eating the lowest carbohydrate (highest fat) diet. In contrast, men did not show this difference from 6 to 24 months. Our finding that women assigned to highest carbohydrate (lowest fat) diet were less successful in maintaining weight loss might fit the observations of Shai et al. (7) who found that a high carbohydrate (low fat diet) was associated with a shift in trajectory of weight loss during the maintenance phase after 9–12 months. In that study, as in ours high protein diets had similar efficacy in women and men. In another study where gender effects are evident, Gardner et al. (8) randomized premenopausal women to one of four diets: Atkins (low carbohydrate), Zone (even macronutrient distribution), Ornish (low fat) and the balanced calorie restricted diet with the LEARN manual. The LEARN manual by Dr. Brownell provides detailed steps for introducing a behavioral modification into a weight loss program. In this study, the group eating low carbohydrate diet (Atkins) lost more weight at one year compared to the other groups. Another study that only lasted three months and only included women, also reported superiority for the low carbohydrate diet (10). Our finding that women, but not men, eating the highest carbohydrate had a significantly greater increase in weight from 6 to 24 months, i.e., poorer weight maintenance suggests that this issue needs further evaluation in studies designed to answer this question specifically.
This present study has both strengths and limitations. One strength is the large group of individuals who were randomized to the treatment groups. Another is the 80% follow-up that was achieved at 2 years. One limitation is that the study was not designed and powered to address the questions of gender differences in response specifically. Second, the present study reports findings from a completers analysis which limited the data at 6 months and even more at 24 months. Third, our study encouraged participants to come for follow-up measurements at two years, but adherence with the dietary recommendations by this stage of the study was variable.
In summary, our findings show that individuals with a higher spread of protein intake from their baseline values, regardless of diet assignment have greater weight loss over 2 years which is consistent with the ‘protein spread hypothesis’ of Bosse & Dixon (26). Men lost more weight in the first 6 months than women and this was not affected by diet assignment. We also show that women lose less weight than men and that older individuals lose more weight than younger ones. However, women assigned to the highest carbohydrate (lowest fat) diet had more difficulty maintaining their weight loss after 6 months
Acknowledgments
Supported, in part, by a cooperative agreement award HL073286 from National Heart, Lung, and Blood Institute, National Institutes of Health; NIH General Clinical Research Center grant RR-02635 and NIH Nutrition Obesity Research Center grant P30DK072476.
Author contributions:
Study Design: Overall design GB FS, DR, DW, JR, CC
Data Collection: GB, FS, DR, DW.
Data Analysis: GB, WJ, CJ
Data Interpretation: GB, DR
Literature Search: GB, DR
Generation of Figures: GB
Writing of Manuscript: Drafted by GB and DR and review and edited by all authors
Footnotes
Registry: (ClinicalTrials.gov number, NCT00072995)
Conflicts of Interest: None of the authors reported relationships with industry or potential conflicts of interest in relationship to the work described in this manuscript.
Conflict of Interest. None of the authors had a conflict of interest related to his paper.
References
- 1.Banting W. Letter on corpulence, addressed to the public. 3. London: Harrison; 1864. [DOI] [PubMed] [Google Scholar]
- 2.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 3.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;324:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 4.Stern L, Iqbal N, Seshadri P, et al. The effects of a low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–57. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal N, Vetter ML, Moore RH, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity. 2010;18:1733–8. doi: 10.1038/oby.2009.460. [DOI] [PubMed] [Google Scholar]
- 7.Shai I, Schwarzfuchs D, Henkin Y, et al. the Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 8.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 9.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Brehm BJ, Seeley RJ, Daniels SR, D’Alesso DA. A Randomized Trial Comparing a Very Low Carbohydrate Diet and a Calorie-Restricted Low Fat Diet on Body Weight and Cardiovascular Risk Factors in Healthy Women. JCEM. 2003;88(4):1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 11.Soenen S, Bonomi AG, Lemmens SG, Scholte J, Thijssen MA, van Berkum F, Westerterp-Plantenga MS. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol Behav. 2012 Oct 10;107(3):374–80. doi: 10.1016/j.physbeh.2012.08.004. Epub 2012 Aug 19. [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Bray GA, Carey FJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray GA, Siri-Tarino PW. The Role of Macronutrient Content in the Diet for Weight Management. Endocrinol Metab Clin N Am. 2016;45:581–604. doi: 10.1016/j.ecl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MD, Ryan DH, Apovian CM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. Epub 2013 Nov 12. [DOI] [PubMed] [Google Scholar]
- 15.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014 Sep 3;312(9):923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 16.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 17.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013 Aug;23(8):699–706. doi: 10.1016/j.numecd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012 Dec;96(6):1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 19.Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013 Apr 15;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011 Sep;124(9):841–51. doi: 10.1016/j.amjmed.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Avenell A, Broom J, Brown TJ, et al. Systematic review of long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8(21):i–ix. 1–458. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 22.Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 2012 Dec 6;345:e7666. doi: 10.1136/bmj.e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 24.Astrup A, Pedersen SD. Is a protein calorie better for weight control? Am J Clin Nutr. 2012;95:535–536. doi: 10.3945/ajcn.111.031625. [DOI] [PubMed] [Google Scholar]
- 25.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet vs. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178–87. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 26.Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101( Suppl):1320S–1329S. doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 27.Bosse JD, Dixon BM. Dietary protein in weight management: a review proposing protein spread and change theories. Nutr Metab (Lond) 2012 Sep 12;9(1):81. doi: 10.1186/1743-7075-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen TM, Dalskov SM, van Baak M, et al. Diet, Obesity, and Genes (Diogenes) Project. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010 Nov 25;363(22):2102–13. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A to Z weight loss study. Intern J Obes. 2008;32:985–991. doi: 10.1038/ijo.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson DA, Anton SD, Han H, et al. Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J Behav Med. 2010 Aug;33(4):305–14. doi: 10.1007/s10865-010-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson DA, Anton SD, Han H, et al. Adherence is a multi-dimensional construct in the POUNDS LOST trial. J Behav Med. 2010 Feb;33(1):35–46. doi: 10.1007/s10865-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17:713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadden TA, Neiberg RH, Wing RR, et al. and the Look AHEAD Research Group. Four-Year Weight Losses in the Look AHEAD Study: Factors Associated with Long-Term Success. Obesity. 2011 Oct;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espeland MA, Bray GA, Neiberg R, et al. Describing patterns of weight change using principal components analysis: results from the Action for Health in Diabetes (LOOK AHEAD) research group. Ann Epidemiol. 2009;19:701–710. doi: 10.1016/j.annepidem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neiberg RH, Wing RR, Bray GA, et al. the Look AHEAD Research Group. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD study. Obesity. 2012;20:2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the diabetes prevention program (DPP) Diabetes Care. 2013;36(1):34–40. doi: 10.2337/dc12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerome GM, Myers VH, Young DR, et al. Psychological predictors of weight loss by race and sex. Clin Obes. 2015 Oct 21; doi: 10.1111/cob.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of African American, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity. 2008 Jun;16(6):1413–20. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 39.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, et al. Reduction in weight and cardiovascular disease (CVD) risk factors in individuals with Type 2 Diabetes: one year results of Look AHEAD Trial. Diab Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray GA, Smith SR, de Jonge L, et al. Effect of diet composition on energy expenditure during weight loss: The POUNDS LOST study. Int J Obes (Lond) 2012 Mar;36(3):448–55. doi: 10.1038/ijo.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jonge L, Bray GA, Smith SR, et al. Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity (Silver Spring) 2012 Dec;20(12):2384–9. doi: 10.1038/oby.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr. 2012 Mar;95(3):614–25. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anton SD, Gallager J, Carey VJ, et al. Diet Type and Changes in Food Cravings following Weight Loss: Findings from the POUNDS Lost Trial. Eat Weight Disorders. 2012 Jun;17(2):e101–8. doi: 10.1007/BF03325333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anton SD, LeBlanc E, Allen HR, Karapetian AC, Sacks FM, Bray GA, Williamson DA. Use of a Computer Tracking System to Monitor and Provide Feedback on Dietary Goals for Calorie Restricted Diets: The POUNDS LOST Study. J Diab Sci Technology. 2012;6(5):1216–25. doi: 10.1177/193229681200600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Qi Q, Liang J, Bray GA, Champagne CM, Hu FB, Sacks FM, Qi L. Neuropeptide Y promoter polymorphism modifies effects of a weight-loss diet on 2-year changes of blood pressure: the preventing overweight using novel dietary strategies trial. Hypertension. 2012 Nov;60(5):1169–75. doi: 10.1161/HYPERTENSIONAHA.112.197855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. Weight-loss diets modify glucose-dependent insulinotropic polypeptide receptor rs2287019 genotype effects on changes in body weight, fasting glucose, and insulin resistance: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr. 2012 Feb;95(2):506–13. doi: 10.3945/ajcn.111.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Qi Q, Zhang C, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012 Nov;61(11):3005–11. doi: 10.2337/db11-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. APOA5 genotype modulate 2-year changes in lipid profile in response to weight-loss diet intervention: the Pounds Lost Trial. Am J Clin Nutr. 2012 Oct;96(4):917–22. doi: 10.3945/ajcn.112.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M, Qi Q, Liang J, Bray GA, Frank Hu F, Sacks F, Qi L. Genetic Determinant for Amino Acid Metabolites and Changes in Body Weight and Insulin Resistance in Response to Weight-loss Diets: the POUNDS LOST Trial. Circulation. 2013;127:1283–89. doi: 10.1161/CIRCULATIONAHA.112.000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirzaei K, Xu M, Qi Q, Bray GA, Frank Sacks F, Qi L. Glucose and circadian related genetic variants affect response of energy expenditure to weight-loss diets: the POUNDS LOST Trial. Am J Clin Nutr. 2014 Feb;99(2):392–399. doi: 10.3945/ajcn.113.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang T, Huang J, Qi Q, Li Y, Bray GA, Rood J, Sacks FM, Qi L. PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the POUNDS LOST trial. Diab Care. 2015 Mar;38(3):439–44. doi: 10.2337/dc14-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi Q, Durst R, Schwarzfuchs D, et al. CETP genotype and changes in lipid levels in response to weight-loss diet intervention: gene-diet interaction analysis in the POUNDS Lost and DIRECT randomized trials. J Lipid Res. 2015 Mar;56(3):713–21. doi: 10.1194/jlr.P055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y, Huang T, Zhang X, Rood J, Bray GA, Sacks FM, Qi L. Dietary fat modified the effects of FTO genotype o changes in insulin sensitivity. J Nutr. 2015 May;145(5):977–82. doi: 10.3945/jn.115.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M, Ng SS, Bray GA, Ryan DH, Sacks FM, Ning G, Qi L. Dietary fat intake modifies the effect of a common variant in the LIPC gene on changes in serum lipids during a long-term intervention: The POUNDS Lost trial. J Nutr. 2015 Jun;145(6):1289–94. doi: 10.3945/jn.115.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin X, Qi Q, Zheng Y, Huang T, Lathrop M, Diana Zelenika D, Bray GA, Sacks FM, Lianf L, Qi L. Neuropeptide Y genotype, central obesity and abdominal fat distribution: the POUNDS Lost trial. Am J Clin Nutr. 2015 Aug;102(2):514–9. doi: 10.3945/ajcn.115.107276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoskin MA, Bray GA, Hattaway K, et al. for the Diabetes Prevention Research Group. Prevention of Diabetes Through the Lifestyle Intervention: Lessons Learned from the Diabetes Prevention Program and Outcomes Study and its Translation to Practice. Curr Nutr Rep. 2014 Dec 1;3(4):364–378. doi: 10.1007/s13668-014-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Report of a Joint FAO/WHO/UNU Expert Consultation. Energy and protein requirements. World Health Organization Technical Report Series 724. World Health Organization; Geneva: 1985. [PubMed] [Google Scholar]
- 58.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Doré J, Zucker JD, Clément K, Ehrlich SD ANR MicroObes consortium. Dietary intervention impact on gut microbial gene richness. Nature. 2013 Aug 29;500(7464):585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 59.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O MetaHIT consortium. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013 Aug 29;500(7464):541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 60.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K MICRO-Obes Consortium. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016 Mar;65(3):426–36. doi: 10.1136/gutjnl-2014-308778. Epub 2015 Jun 22. [DOI] [PubMed] [Google Scholar]
- 61.Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015 Jan;38(1):159–65. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 62.Hibbert JM, Jackson AA. Variation in measures of urea kinetics over four years in a single adult. Eur J Clin Nutr. 1991 Jul;45(7):347–51. [PubMed] [Google Scholar]
- 62.Akins RC. Dr. Atkins’ Diet Revolution: The High Calorie Way to Stay Thin Forever. New York: D Mackay Co; 1972. [Google Scholar]
- 63.Sears B, Lawren B. Enter The Zone: A Dietary Roadmap. New York: 1995. [Google Scholar]