Abstract
Executive function is a neuropsychological construct that enables controlled cognitive processing, which has been hypothesized to enhance individuals’ resilience to stress. However, little empirical work has directly examined how executive function under different conditions mitigates the negative effects of stress exposure on health. To address this issue, we recruited 110 healthy young adults and assessed their recent life stress exposure, executive function in either a stressful or non-stressful context, and current health complaints. Based on existing research, we hypothesized that individuals exhibiting better executive function following a laboratory-based stressor (but not a control task) would demonstrate weaker associations between recent stress exposure and health because they perceived recent life stressors as being less severe. Consistent with this hypothesis, better executive function during acute stress, but not in the absence of stress, was associated with an attenuated link between participants’ recent life stress exposure and their current health complaints. Moreover, this attenuating effect was mediated by lesser perceptions of stressor severity. Based on these data, we conclude that better executive function under stress is associated with fewer health complaints and that these effects may occur by reducing individuals’ perceptions of stressor severity. The data thus suggest the possibility of reducing stress-related health problems by enhancing executive function.
Keywords: Cumulative life stress, executive function, stress perception, cognitive flexibility, resilience, STRAIN, mediated moderation, risk, health, disease
Introduction
Stress can negatively impact health (Kemeny & Schedlowski, 2007), and recent research has examined mechanisms that might mitigate these effects. One construct thought to influence how stress affects health is executive function, which enables controlled cognitive processing (Williams et al., 2009; Williams & Thayer, 2009). However, no research to date has directly tested whether executive function mitigates the stress-health link.
Executive function may mitigate the effects of stress on health by reducing perceptions of stress severity. Executive function facilitates cognitive reappraisal (Malooly et al., 2013), and cognitive reappraisal in turn reduces perceptions of stress severity (Lazarus & Folkman, 1984). Similarly, executive function facilitates emotion regulation and reduces experiences of negative affect (Schmeichel & Tang, 2015). As it is the subjective experience of stress that most strongly shapes the negative effects of stress on health (Slavich & Cole, 2013), individuals exhibiting better executive function during stressful circumstances may perceive such circumstances as less stressful and thus have fewer health problems. However, this hypothesis has not yet been tested.
Acute and chronic stress impair executive function (Alexander et al., 2007; Holmes & Wellman, 2009). However, there are substantial individual differences in this effect, with stress impairing executive function in some people more than others (Beilock & Carr, 2005; Pletzer et al., 2010; Schoofs et al., 2013; Shamosh & Gray, 2007). If executive function specifically during stressful circumstances is what most strongly shapes how people respond to stressors, then executive function assessed under stressful or “hot” conditions should be a stronger moderator of the stress-health link than executive function assessed under baseline or “cold” conditions.
Consistent with this possibility, executive function ability under “hot” and “cold” conditions is not always related (Raio et al., 2013). Additionally, some evidence supports the idea that executive function moderates the effects of stress on health only when executive function is assessed within a stressful context. Specifically, two recent studies found that executive function assessed at baseline was a poor predictor of depressive symptoms, whereas executive function assessed in a stressful context was associated with depressive symptoms in a cross-sectional study (Quinn & Joormann, 2015a) and predicted increases in depressive symptoms over time in a longitudinal study (Quinn & Joormann, 2015b). Although these studies did not examine whether executive function under stress moderates the stress-health link, they support the formulation that executive function under stress may be particularly important for health.
In the present study, we randomly assigned participants to either a socially stressful or non-stressful group task. We then assessed participants’ executive function following these tasks. Finally, we used an online, interview-based system to characterize participants’ recent stress exposure and perceived severity ratings for those exposures, and two well-validated questionnaires to assess participants’ health complaints. We hypothesized that executive function under acute stress would be associated with an attenuated relation between life stress exposure and health complaints. Additionally, we hypothesized that decreased perceptions of stress would mediate the association between better executive function under acute stress and an attenuated relation between life stress exposure and health complaints.
Method
Participants
We recruited 110 healthy young adults attending a large public university on the West coast. Because we were interested in factors influencing typical responses to stress, we excluded individuals with health conditions that could have confounded results. Specifically, we excluded people with a current medical illness, diabetes, history of stroke, neurological disorders, current or former diagnosis of posttraumatic stress disorder, hospitalization for a psychiatric disorder within the past year, current injury or illness within the past week, major sleep disturbances within the past six weeks, or consumption of more than eight caffeinated beverages a day, as well as people who were pregnant, were nursing, were on any form of medication (including any form of birth control and asthma medication), had taken any mood-altering medications within the past eight weeks, or had taken oral or injected corticosteroids within the past three months.
Of the 110 people recruited for this study, 55 participants (35 female) were randomized to the stress induction condition and 55 participants (37 female) were randomized to the non-stressful control condition. Participants ranged in age from 18 to 33 years-old (M = 19.91, SD = 2.0), and the sample was racially and ethnically diverse: 1.9% of people were American Indian or Alaskan Native, 58.9% were Asian, 1.9% were Native Hawaiian or Pacific Islander, 3.7% were Black or African American, 17.8% were White and 15.9% were Hispanic. Importantly, participants in the stress induction and control conditions did not differ with respect to sex, race, or age, ps > .23. All study procedures received Institutional Review Board approval.
Materials
Stress manipulation
This study employed a standard laboratory stressor called the Trier Social Stress Test for Groups (TSST-G; von Dawans et al., 2011) to induce stress in participants. This manipulation involves two conditions: a stress induction condition and a control condition.
Stress induction condition
In the stress induction condition, instructions appeared on a computer screen and informed each person that the next task would involve giving a three-minute speech describing why that person was the ideal candidate for a job of that person’s choice. The participant was also told that the speech would take place in front of a panel of evaluators trained in the evaluation of nonverbal behavior. To make the task more self-relevant (von Dawans et al., 2011), the instructions also told each person to use his or her actual qualifications in the speech, and that he or she must speak for the full 3 min. The instructions further informed each person that a camera would record his or her speech and that a panel of three professors from the psychology, sociology and communication departments would conduct a video analysis of their performance to identify nonverbal behaviors that distinguish a qualified job applicant from an unqualified one. The instructions then told each person to use the piece of scratch paper and the next 10 min to prepare for his or her speech. The final sentence of the instructions told participants that there would be “another task” following the speech. These instructions remained on the screen during the rest of this stressor anticipation phase.
After 10 min had elapsed, the experimenters opened each participant’s cubicle door, removed each participant’s chair and scratch paper from his or her cubicle, and instructed each participant to stand at the door of his or her cubicle. Experimenters then sat out of the participants’ view. The spatial layout of the testing environment ensured that participants could not see each other. Two evaluators then came into the testing room with a self-standing digital video camera and went to each participant’s cubicle one by one in an apparently random fashion, though the order was kept consistent across sessions. When the evaluators came to a participant’s cubicle, the evaluators informed that person to begin his or her speech. If a participant stopped speaking once before the full 3 min allotted to his or her speech had elapsed, then the evaluators prompted the participant to continue. If a participant stopped a second time before the 3 min had elapsed, then the evaluators stared in silence at the participant for 20 s or until the participant began talking again; if the full 20 s of silence elapsed, then the evaluators then asked participants scripted questions, such as, “Why can’t you continue talking?” Immediately after each participant finished the speech task, the evaluators went to another participant’s cubicle and instructed that person to begin his or her speech in the same fashion.
After all of the participants had finished their speeches, the evaluators again went to each of their cubicles one by one, in an apparently random fashion. Once at a participant’s cubicle, the evaluators instructed the participant to count aloud backwards from a four-digit number by 16s as quickly and accurately as possible. Each participant in a given experiment was given a different four-digit number, although the different four-digit numbers were kept constant across all study sessions. Each participant counted backwards by 16s for a total of 100 seconds. Thirty seconds and 70 seconds after the participant began, one of the evaluators instructed the participant to count faster using scripted statements. If the participant verbally paused, counted too slowly, or made an error, the evaluator instructed them to restart. After all participants finished the math task, the evaluators left the room, the experimenters returned the participants’ chairs to their cubicles, the participants returned to their computers, and the experimenters closed the participants’ cubicle doors.
Importantly, the stressfulness of this task did not differ depending upon the order in which participants completed the math task, due to the information given to participants. Participants were not informed about all the tasks that the stressor would entail. Instead, they were each told that they could be called upon by the evaluators at any time for a number of different reasons. In addition, the order of participants called on by the evaluators appeared randomized. As such, participants did not know that the stressor had finished when it had. Instead, they were left in a state of stressful anticipation until they were collectively told to return to their seats after the last person had finished the math task.
Control condition
The control condition of the TSST-G controls for cognitive load, as well as biological effects of standing and speaking, without inducing stress. Instructions presented on the computer informed each participant in the control condition that in 10 min he or she would quietly read an academic article aloud, which was currently face down on the person’s desk. The instructions further stated that all other people in the study would also read their articles quietly aloud at the same time. The instructions then informed each person that he or she should use the remaining time to read over the article face down on his or her desk in order to prepare to read the article aloud. The final sentence of the instructions told participants that there would be another task following the speech. These instructions remained on the screen during the rest of this control anticipation phase.
After the 10 minutes had elapsed, participants’ cubicle doors were opened and they were asked by one of the experimenters to stand and read their articles. The experimenter informed the participants that the experimenter would tell them when this task was done; then, the experimenter instructed them to begin. After the experimenter told them to begin, both experimenters sat out of participants’ view. The experimenters were instructed not to evaluate, correct, comment upon the performance of, or in any way engage with any of the participants in this condition. After 12 minutes of reading aloud (i.e. to equate the total time for the speech portion of the TSST-G between conditions), one experimenter told the participants that they would move on to the next task. The experimenter then instructed the participants to each count out loud, again in a quiet voice and while standing. The experimenter told the participants that each of them may choose to count by 3s, 5s, 10s or 20s, but to choose now and be consistent. Finally, the experimenter again told the participants that the experimenter would tell them when they would move on to the next task. After counting for 6 minutes and 40 seconds (i.e. to equate the total time for the math portion of the TSST-G between conditions), an experimenter told the participants to stop counting and return to their computers, after which time the experimenters closed the participants’ cubicle doors.
Manipulation check
We examined the success of the stress manipulation using self-report scales designed for assessing self-reported stressfulness of the laboratory task and affective responses to the laboratory task, as described below.
Self-reported stressfulness of the laboratory task
Participants in both the stress and non-stress TSST-G conditions used an unmarked scale ranging from 1 (Strongly Disagree) to 9 (Strongly Agree), with 5 (Neither Agree nor Disagree) as the midpoint, in order to indicate the extent to which they agreed with various statements that assessed the stressfulness of the stressor/control task. This questionnaire asked questions such as, “The speech and mathematics tasks were very stressful” and “The speech and mathematics tasks were very demanding.” Participants answered the questions in a randomized order. Reliability for this measure was excellent (α = .90).
Affective responses to the laboratory task
Because stress is known to induce negative affect (e.g. Schoofs et al., 2008), we used a self-report questionnaire assessing negative affect as an additional manipulation check. Prior to learning of the stressor task and immediately after this task, participants indicated on an unmarked 1–7 scale ranging from 1 (Not at All) to 7 (Very Much) to what extent they currently felt afraid, scared, nervous, negative, distressed, angry, ashamed, disgusted, frustrated, sad and down. To avoid demand characteristics that might have arisen had the questionnaire only assessed negative affect, participants also indicated the extent to which they currently felt several positive affective states, including happy, calm and proud. Participants responded to the emotions in a randomized order. Self-reports of the negative emotions were averaged at each time point to create indices of overall negative affect. Negative affect was assessed at baseline (α = .90) and after the stress manipulation (α = .94).
Executive function
To assess executive function, we employed the Berg Card Sorting Test (BCST), which is an open-source version of the Wisconsin Card Sorting Test (WCST) written in Psychology Experiment Building Language (PEBL), version 0.13 (Fox et al., 2013; Mueller & Piper, 2014; Piper et al., 2012). This executive function task was chosen because it is well-validated global executive function task, meaning that performance on it relies on all subcomponents of executive function (i.e. working memory, inhibition and cognitive flexibility; see Nyhus & Barceló, 2009). In healthy young adults, performance on this task is most closely related to performance on cognitive flexibility tasks (Miyake et al., 2000). However, neuroimaging and lesion work show that working memory and inhibition are also required for performance on this task in otherwise healthy people (Nyhus & Barceló, 2009). Of note, the automated BCST scoring in PEBL version 0.13 scores perseverative errors differently than the traditional scoring method of the WCST (Fox et al., 2013). As such, we rescored the BCST to match the standard scoring rules for scoring perseverative errors on the WCST.
Participants are required to sort a maximum of 128 cards into one of four decks based upon one of three rules (sort by color, number of figures, or shape of figure), the current of which is always unknown to them. Participants are given feedback after every response whether their answer was correct or not and are thus able to deduce the correct rule through trial-and-error. Once participants deduce the current rule and answer nine cards correctly in a row, the rule shifts. Participants are aware that the rule has shifted only because they are then presented with “Incorrect” feedback for what would have previously been a correct response; as such, they must realize that the rule has shifted and again deduce the correct answer. If a person continues to respond according to the previous set’s rule after the rule has shifted and the person has received “Incorrect” feedback, the response is labeled a perseverative error, as the person is perseverating on the previous rule. This error thus indicates cognitive inflexibility, as it reflects an inability to quickly transition to a new rule after an old rule had been established. Thus, the primary outcome of interest in this task is the number of perseverative errors a person makes during the task. As perseverative errors are errors, the outcomes of interest in this task indicate worse executive function. Higher scores thus represent worse performance.
Mental and physical health
We used the Kessler-6 item psychological distress inventory (K-6; Kessler et al., 2002) and Physical Health Questionnaire (PHQ; Schat et al., 2005) to assess mental health and physical health, respectively. The K-6 inquires about a variety of nonspecific poor mental health symptoms and the frequency that a person has experienced the symptoms over the past month, and higher scores on the measure indicate worse mental health. The PHQ inquires about a variety of nonspecific symptoms of poor physical health and the frequency of those symptoms over the past month, with higher scores indicating worse physical health. In prior work, the K-6 has shown good convergence with DSM-IV based measures of mental health symptoms (Kessler et al., 2002), and the PHQ has shown good convergence with general health and divergence with work stress (Schat et al., 2005). In the present study, the K-6 and PHQ both demonstrated good internal consistency (α = .87 and α = .82, respectively). To reduce the likelihood of committing a Type I error and because we had no a priori hypothesis differentiating the effects of stress on mental versus physical health, we standardized and averaged participants’ responses on these scales to create an index of overall health for analysis (α = .89). As questions on the K-6 and PHQ assess health complaints, higher scores on this index indicate worse health.
Recent life stress
We used the Transition to College module of the Stress and Adversity Inventory for Adults (Adult STRAIN-TTC) to assess participants’ exposure to recent life stress. The Adult STRAIN has good predictive validity, as evidenced by the fact that the main stress exposure summary scores from the instrument strongly predict mental and physical health complaints in young adults (Toussaint et al., 2016), metabolic health in adults (Kurtzman et al., 2012) and levels of depressive symptoms and fatigue in cancer patients (Bower et al., 2014; Dooley et al., 2017). We used the Transition to College (TTC) Stress Assessment Module because it specifically assesses recent life stressors that are very stressful and highly salient for participants in this young adult college population.
The STRAIN-TTC can either be self- or interviewer-administered, depending on the characteristics of the sample and needs of the study. Given the computer savvy nature of the present sample, participants self-administered the STRAIN-TTC in the lab after reading a brief introduction to the STRAIN and related instructions. Participants were then asked 14 core questions about different major life stressors that they could have experienced from when they first transitioned to college until the date of the interview. Example questions include “Did you end a relationship with a significant other when you moved to college?” and “Have you had any major fights or arguments with your roommate(s) or suitemates?” If a person endorses a particular stressor, the STRAIN system employs branching logic to obtain additional information about the specific frequency, timing, duration and perceived severity of each reported stressor. Most important for the present study is the fact that the STRAIN-TTC provides information about both stressor exposure (coded as 0 for absent and 1 for present for each possible stressor) and the perceived stressfulness of each reported exposure, with stressor severity scores ranging from 1 (Very Slightly or Not at All) to 5 (Extremely). The two main stress variables used in analyses were thus participants’ (a) stressor count, calculated as the sum of the stressor frequencies and (b) overall stressor severity, calculated as the sum of the perceived severities of reported stressors. Although these scores can be correlated, substantial individual differences exist in how people rate the severity of the stressors they have experienced, making each score independently informative. Additionally, obtaining perceived severity scores for experienced stressors has several important advantages over obtaining general perceived stress ratings that are unrelated to experienced stressors (e.g. the scores can be directly compared, etc.).
Procedure
Participants came to the lab at either 12 pm or 3 pm for 3-h, four-participant group sessions. Upon arrival, an experimenter immediately greeted each participant and brought the participant into a cubicle in order to prevent the participants from interacting with each other. Once in the cubicle, each participant provided informed consent and completed miscellaneous baseline measures, including measures assessing baseline affect. When finished with the measures, the participant’s computer reached a password-protected screen and instructed him or her to wait until the experimenter allowed them to continue. Participants waited until all other participants for the session arrived and completed the initial measures, upon which time the experimenter gave all participants the password to continue. Next, participants completed the stressor or control task, depending upon their time slot’s assigned condition. The stressor or control task lasted approximately 30 minutes, after which time participants immediately completed the post-stressor manipulation check. Five minutes after the offset of the stressor, participants completed the executive function task (i.e. BCST). Participants then completed filler measures for 90 minutes. One hundred minutes after the offset of the stressor, participants completed the recent life stress interview (i.e. the Adult STRAIN-TTC). This delay was intended to prevent the manipulation from influencing participants’ responses to stress interview. Following completion of the stress interview, participants completed the health questionnaires. Finally, the experimenter thanked and debriefed the participants.
Analytic strategy
Because we randomized sessions of participants to conditions (instead of individual participants), we used linear mixed models in analyses – nesting with participants within sessions to account for potential shared variability within sessions. All reported means and standard errors were least-squares means and their corresponding standard errors. Degrees of freedom were estimated using the Satterthwaite approximation, which relaxes the assumption of homogeneity of variance, but entails the reported degrees of freedom contain numbers that are not integers. In all primary analyses, the control condition was used as the reference, meaning that all lower-order effects aside from the effect of Condition that do not involve interactions with Condition are the parameter estimates obtained in the control condition. We conducted analyses of mediated moderation following the directions given by Muller et al. (2005). All continuous variables were standardized (which also mean-centers them) to enable interpretation of lower-order coefficients.
Results
Preliminary analyses
Effects of stress on negative affect and stress appraisals
To ensure that the stress condition did in fact induce stress to a greater degree than the control condition, we tested whether participants’ negative affect changed over time as a function of their experimental condition. The hypothesized two-way Condition × Time interaction was significant, F(1,110.0) = 16.10, p < .001, indicating that participants’ negative affect differentially changed over time as a function of their experimental condition (Figure 1(A)). In particular, whereas participants in the stress induction (M = 2.24, SE = 0.14) and control (M = 2.24, SE = 0.14) conditions did not differ in negative affect at baseline, t(62.3) = 0.03, p = .97, those in the stress induction condition reported significantly more negative affect post-stressor (M = 2.72, SE = 0.14) than did their counterparts in the control condition (M = 1.86, SE = 0.14), t(62.3) = 4.27, p < .001, d = 0.75.
Figure 1.
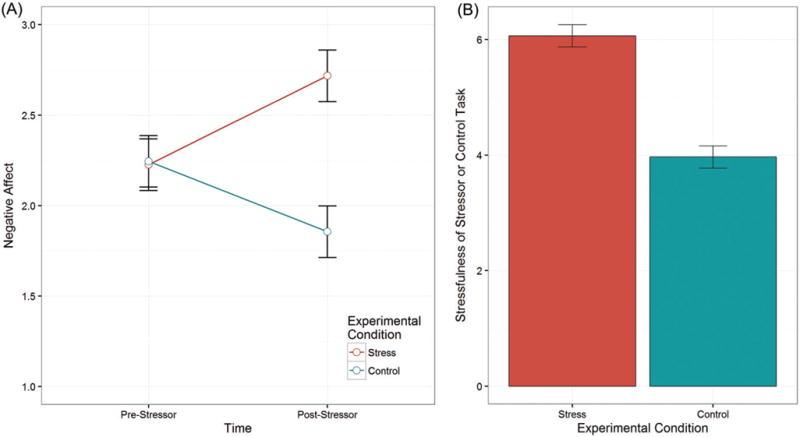
Manipulation check. Participants in the Trier Social Stress Test for Groups (TSST-G) stress induction condition did not differ from their counterparts in the TSST-G control condition with respect to pre-TSST-G negative affect (p = .94). However, (A) participants in the stress induction condition experienced significantly greater negative affect post-stressor than pre-stressor (p < .001), and they also experienced significantly greater negative affect after the stressor than did participants in the control condition (p < .001). Similarly, (B) after the laboratory-based social stressor, participants in the stress group reported significantly more stress when asked about the stressfulness of the preceding task than did participants in the control group (p < .001). Error bars represent ±1 SEM.
In addition, participants’ perceptions of stress during the laboratory task differed significantly between the two conditions. Specifically, participants in the stress induction condition found the stressor task to be significantly more stressful (M = 6.06, SE = 0.19) than did those in the control condition (M = 3.96, SE = 0.20), t(34.5) = 7.46, p<.001, d = 1.47 (Figure 1(B)). Therefore, the stressor manipulation successfully induced stress in participants in the stress induction condition to a significantly greater degree than it did for participants in the control condition.
Effects of acute stress exposure on executive function
Given prior research showing that stress impairs executive functions, we next examined whether participants in the acute stress induction condition committed more perseverative errors than participants in the control condition. Although participants in the stress condition descriptively committed more perseverative errors (M = 9.51, SE = 1.21) than those in the control condition (M = 9.15, SE = 1.21), this difference was not significant, t(42.5) = 0.22, p=.828.
Executive function, emotional reactivity to the stressor, and perceptions of the stressor
Because we assessed emotional reactions to and perceptions of the acute stress manipulation, we were able to examine whether participants’ executive function ability under stress moderated their psychological and affective responses to the laboratory stress task. As hypothesized, executive function scores under stress were inversely related to increases in negative affect in the stress condition, such that people with poorer executive function showed greater increases in negative affect, β = .203, t(110.0) = 1.70, p = .046 (one-tailed). In addition, although not significant, better executive function scores under stress were inversely related to perceptions of stressor severity, β = .137, t(87.4) = 1.39, p = .092 (one-tailed). In the control condition, executive function was not a predictor of changes in negative affect, β = −.153, t(110.0) = −1.20, p = .232, or perceived stressfulness of the control task, β = .112, t(80.0) = 1.00, p = .322. Thus, better executive function under stress was associated with lower emotional reactivity to acute stress.
Primary analyses
Better executive function following acute stress attenuates the link between recent stress exposure and health
To test our primary hypotheses, we first examined whether better executive function within a stressful context was associated with a weaker relation between recent life stress exposure and health complaints (Table 1). As expected, recent stress exposure significantly predicted participants’ health complaints, β = .41, t(110.0) = 2.97, p = .004, and this association was not greater in the stress induction condition than it was in the control condition, β = −.140, t(110.0) = −0.78, p = .436. Therefore, greater recent stress exposure was associated with poorer health for all participants, regardless of experimental condition.
Table 1.
The association between recent life stress exposure and health complaints is moderated by executive function in the acute stress induction (but not control) condition.
| Variable | β | p |
|---|---|---|
| Recent Life Stress Exposure | .41 | .004 |
| Perseverative Errors | −.03 | .81 |
| Condition | .23 | .19 |
| Recent Life Stress Exposure × Perseverative Errors | −.25 | .24 |
| Recent Stress Life Exposure × Condition | −.14 | .44 |
| Condition × Perseverative Errors | .36 | .07 |
| Recent Life Stress Exposure × Perseverative Errors × Condition | .61 | .02 |
Note: Significant effects are represented with a boldface p value.
So, recent life stress is associated with poorer health, but does executive function moderate this association? In the control condition, the answer is no (Table 1). The association between recent life stress exposure and health complaints did not change as a function of individuals’ executive function in the control condition, β = −.250, t(110.0) = −1.19, p = .239 (Figure 2). However, the moderating effect of executive function on the association between recent stress exposure and health complaints differed between the stress induction and control condition, β = .61, t(110.0) = 2.37, p = .019 (Figure 2). Although it could be thought that this effect was due to a restriction of range in the control group due to floor effects (i.e. no errors), there was no evidence of a floor effect in these data, as all participants completed at least one perseverative error. Moreover, the variability between the two experimental conditions was equivalent; Levene’s test was non-significant, F(1,108) = 0.08, p = .785, indicating that the variance of perseverative errors in the control group did not differ from the variance of perseverative errors in the stress induction group. Therefore, these effects were not due to floor effects or to a relative lack of variability within the control group.
Figure 2.
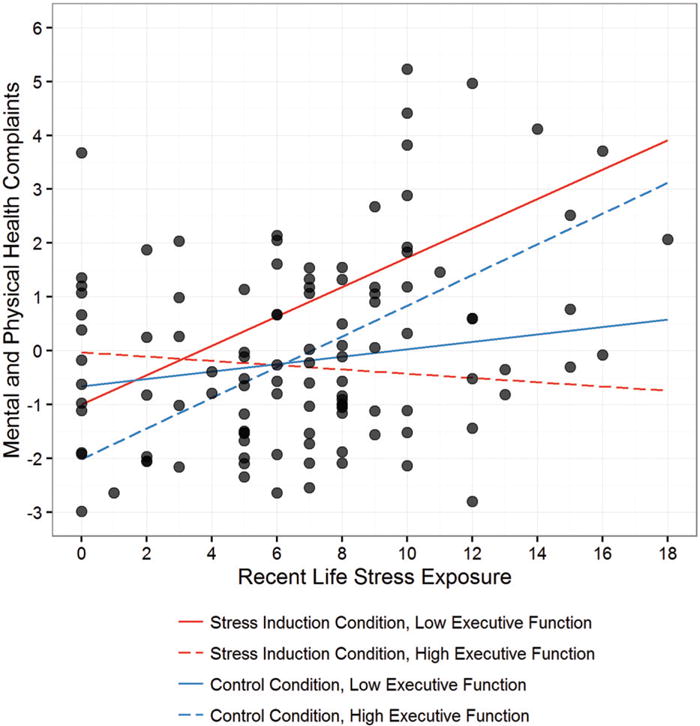
Associations between recent life stress exposure, executive function and health complaints for participants in the stress induction and control conditions. Better executive function in the stress induction condition moderated the effects of recent life stress exposure on health (p = .02), but these effects were not found for executive function assessed in the control condition (p = .24). Therefore, the strength of association between actual recent life stress exposure and mental and physical health depended on individuals’ executive function capabilities specifically under stress.
When we decomposed this interaction (Table 2), we found that poorer executive function in the stress induction condition was associated with a stronger association between recent life stress exposure and health complaints, β = .39, t(55.0) = 2.32, p = .024. To further decompose this interaction, we employed analyses of simple slopes, which revealed that, in the stress induction condition, recent stress exposure did not predict poorer health when executive function was high (1 SD below the mean error rate), β = −.10, t(51) −0.48, p = .64. Consistent with hypotheses, though, greater stress exposure was a moderately strong predictor of health complaints when executive function was at the mean, β = .29, t(51) = 2.26, p = .028, and a strong predictor of poor health when executive function was low (1 SD above the mean error rate), β = .68, t(51) = 3.01, p = .004. Together, these data indicate that better executive function when assessed in a stressful context – but not when assessed in a non-stressful context – was associated with an attenuated link between recent life stress exposure and health complaints in this sample.
Table 2.
For the acute stress induction condition, the association between recent life stress exposure and health complaints increased by half of one standard deviation for every one standard deviation increase in perseverative errors.
| Variable | β | p |
|---|---|---|
| Recent Life Stress Exposure | .24 | .05 |
| Perseverative Errors | .44 | .003 |
| Recent Life Stress Exposure × Perseverative Errors | .50 | .004 |
Note: Significant effects are represented with a boldface p value.
Mediation by perceptions of stressor severity
We next examined whether the link between executive function and health complaints was mediated by alterations in the perceived severity of the different life stressors that individuals experienced. Given that the STRAIN inquires about both the occurrence of stressors and individuals’ perceived severity of experienced stressors, this analysis enabled us to examine whether better executive function in a stressful context was associated with a moderated relation between recent life stress exposure and the perceived stressfulness of those same stressors. As hypothesized, the perceived severity of experienced stressors did not change as executive function decreased in the non-stressful control condition, β = −.14, t(106.2) = −1.37, p = .174 (Table 3). However, the moderating effect of executive function on the association between recent life stress exposure and perceptions of the severity of those stressors was significantly different between the two conditions, β = .32, t(110.0) = 2.46, p = .015 (Figure 3). Decomposing this interaction revealed that the strength of the association between recent stress exposure and perceived stressor severity increased as executive function decreased in the stress induction condition, β = .19, t(55.0) = 2.23, p = .030 (Table 4). In other words, as hypothesized, better executive function within a stressful context was associated with a weaker association between recent life stress exposure and perceived stress severity for the different life stressors that participants experienced.
Table 3.
The association between recent life stress exposure and recent life stress perceived severity is moderated by executive function ability in the acute stress induction (but not control) condition.
| Variable | β | p |
|---|---|---|
| Recent Life Stress Exposure | .94 | <.001 |
| Perseverative Errors | −.02 | .76 |
| Condition | −.14 | .10 |
| Recent Life Stress Exposure × Perseverative Errors | −.14 | .17 |
| Recent Life Stress Exposure × Condition | −.07 | .42 |
| Condition × Perseverative Errors | .20 | .04 |
| Recent Life Stress Exposure × Perseverative Errors × Condition | .32 | .02 |
Note: Significant effects are represented with a boldface p value. This test illustrates the first step in mediated moderation, as the proposed mediator (recent life stress severity) depends upon levels of the moderator.
Figure 3.
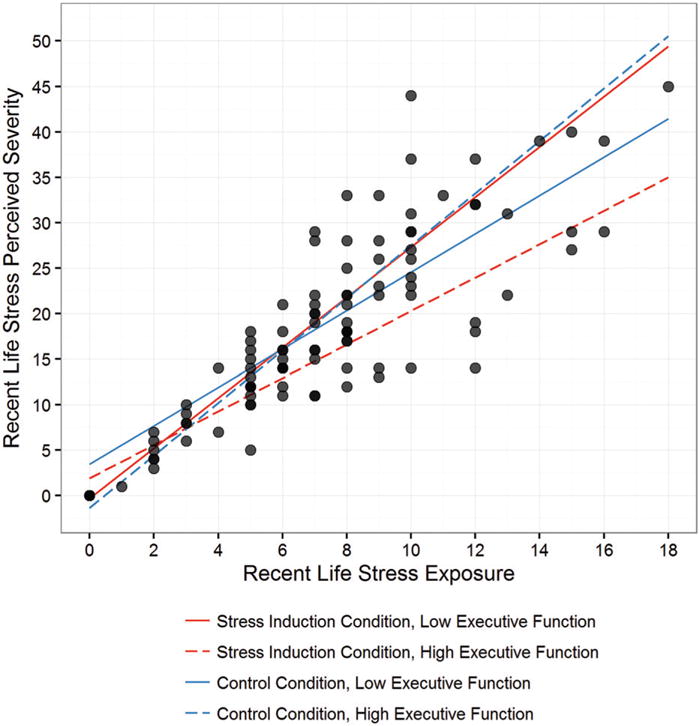
Better executive function under stress attenuates the relation between life stress exposure and severity. Better executive function in the stress induction condition was associated with an attenuated link between participants’ recent life stress exposure and the perceived severity of those exposures (p = .03). In contrast, in the control condition, there was no effect of executive function on the association between recent life stress exposure and recent life stress severity (p = .17). As such, better executive function as assessed under a stressful (but not control) context led to decreased perceptions of severity for the different stressors that participants experienced.
Table 4.
The moderating effect of executive function in the stress condition on the association between recent life stress exposure and health complaints is mediated by the perceived severity of recent life stressors.
| Variable | β | p |
|---|---|---|
| Recent Life Stress Exposure | −.12 | .66 |
| Recent Life Stress Perceived Severity | .57 | .04 |
| Perseverative Errors | −.02 | .89 |
| Condition | .31 | .07 |
| Recent Life Stress Exposure × Perseverative Errors | −.09 | .82 |
| Recent Life Stress Exposure × Condition | −.26 | .34 |
| Recent Life Stress Exposure × Perseverative Errors × Condition | .22 | .64 |
| Recent Life Stress Perceived Severity × Perseverative Errors | −.10 | .81 |
| Recent Life Stress Perceived Severity × Condition | .18 | .63 |
| Recent Life Stress Perceived Severity × Perseverative Errors × Condition | .19 | .68 |
Note: Significant effects are represented with a boldface p value. This test illustrates the final step in mediated moderation, as the mediator (Recent Life Perceived Stress Severity) is significant, whereas the former moderator (Recent Life Stress × Perseverative Errors × Condition) is no longer significant.
To fully demonstrate mediated moderation, the mediator must not only depend upon levels of the moderator (as demonstrated above), but in the complete model, the lower-order mediator coefficient must be significant and account for which the variance that the moderator formerly accounted (Muller et al., 2005). We thus tested this criterion in the present data (Table 5). As predicted, stressor severity was a significant predictor of health complaints, β = .57, t(110.0) = 2.12, p = .036, and this association was not significantly greater in the stress condition than in the control condition, β = .18, t(110.0) = 0.49, p = .626, indicating that, across both conditions, worse health was associated with greater perceived severity of recent stress exposure (Figure 4). Just as important for demonstrating mediation, the formerly significant condition by recent life stress by executive function interaction was no longer significant, β = .22, t(110.0) = 0.48, p = .64, indicating that stressor severity explained the variance in the relation of health complaints with better executive function under stress. In sum, consistent with hypotheses, individuals’ perceptions of the severity of their recent life stress exposure mediated the association between better executive function under acute stress and an attenuated relation between stress and health. Importantly, there was no main effect of condition on any of the recent life stress or health variables (see Table 5), indicating that self-reports of stress severity and health complaints were not influenced by the experimental manipulation.
Table 5.
Lack of differences in recent life stress and health complaints by experimental condition.
| Variable | Mstress | Mcontrol | t | p | d |
|---|---|---|---|---|---|
| Recent Life Stress Exposure | 6.8 | 6.8 | 0.00 | .999 | 0.00 |
| Recent Life Stress Severity | 16.2 | 17.8 | −0.75 | .456 | −0.14 |
| Health Complaints | 0.20 | −0.20 | 1.18 | .241 | 0.23 |
Figure 4.
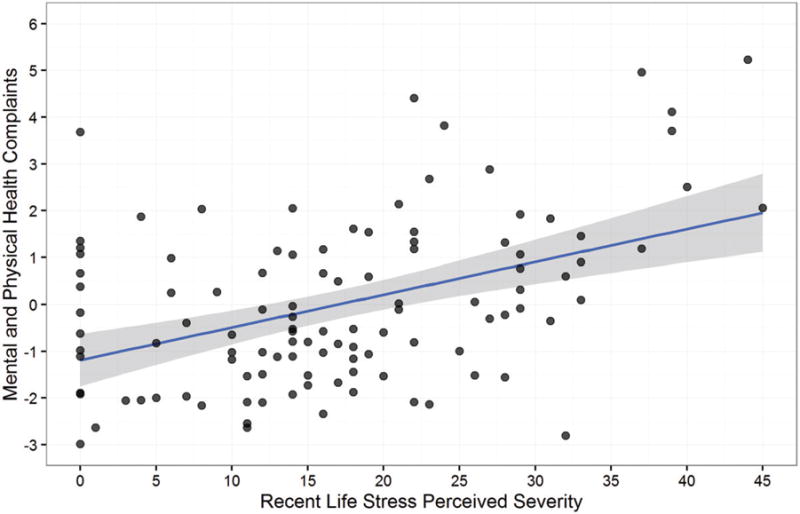
Effects of perceived stress severity on mental and physical health complaints. In both a bivariate association (p < .001) and in the complete model (p = .036), greater recent life stress severity predicted more mental and physical health complaints. Moreover, when accounting for the effects of stress severity, executive function under stress no longer moderated the effects of recent life stress exposure on health. These data suggest that the association of better executive function under stress with an attenuated link between stress and health was mediated by lesser perceptions of stress severity.
Additional, direct test of mediation
Although the random assignment of time slots (rather than participants) to experimental conditions requires the use of a multilevel model in order to control Type I error rates, the random effect of time slots was nearly zero in all analyses, all χ2s < 0.06, all ps>.99, indicating that all fixed effects reported here did not differ by time slot. Therefore, we also conducted a direct test of mediation using the mediate() function in the R package mediation in order to ensure that testing the mediation piecemeal did not incorrectly obscure or produce any results. Consistent with hypotheses and with the analyses described above, this direct test of mediation was also significant: β = .22, 95% CI of β [.03, .46], p = .013.
Discussion
The fact that stress exposure contributes to increased risk for a variety of disorders is well documented (Cohen et al., 2007; Slavich, 2016). Despite countless studies examining these associations, however, little is known about factors that moderate these effects and that could potentially be modified to reduce the deleterious impact that stress has on health. We addressed this important issue here by conducting a combined naturalistic, laboratory-based study in which we assessed participants’ recent life stress exposure and current health complaints, as well as their executive function capabilities under stressful and non-stressful conditions.
Consistent with hypotheses, we found that better executive function under acute laboratory stress conditions was associated with an attenuated link between recent life stress exposure and poor health. Additionally, this attenuated association between stress and health was mediated by lower perceived stressfulness of the actual life stressors that individuals experienced. These findings are consistent with prior research showing that executive function is a significant predictor of stress-related health outcomes only when executive function is assessed under stress (Quinn & Joormann, 2015a,b). To our knowledge, however, the present data are the first to provide empirical support for the formulation that better executive function under stress is associated with a weaker relation between actual life stress exposure and health. Specifically, we found that (a) individual differences in executive function relate to better stress-related health outcomes, but only when executive function is assessed under stressful conditions, and (b) executive function under stress relates to better health at least in part due to its association with lower perceptions of severity for the different life stressors that individuals have experienced.
Surprisingly, although participants in the laboratory stress condition showed some evidence of poorer executive function when compared to participants in the control condition, this difference was not significant. The lack of a group difference in executive function performance is consistent with a recent meta-analytic review on this topic, which found that stress effects on executive functions are weak to moderate and that these effects can be null due to random variation in performance (see Shields et al., 2016). However, it is important to note that this null effect is consistent with the idea that stress does not always impair executive functions and that it impairs executive functions in some people more than others. This is why we believe that assessing executive functions under stress is important for understanding stress-buffering effects of executive function. In support of this idea, a prior study found that post-stress, but not baseline, executive function performance predicted the development of depressive symptoms over time even though post-stress and baseline executive function performance did not differ (see Quinn & Joormann, 2015b), and this is similar to the pattern of effects observed in the present data.
The present findings highlight one cognitive process that may influence the effects of stress on health. These effects, however, occur through multiple pathways and involve multiple levels of analysis. As a result, there is a pressing need for more multi-level research on stress and health that examines how stress, stress-related cognitions, and other cognitive and biological processes combine to affect health (Slavich, 2015; Slavich & Cole, 2013; Slavich & Irwin, 2014). Although we did not assess biological processes in this study, for example, several may be relevant. For example, neural substrates of executive function also underlie the regulation of an immune system response to stress (Ohira et al., 2008, 2009), indicating that activity in these brain regions during stress may both produce better executive function and dampen inflammatory responses to stress, thereby reducing the effects of stress on negative health outcomes.
Several aspects of this study are notable. First, the integration of a gold-standard, laboratory-based stressor with the assessment of real-life stress exposure is a relatively uncommon practice, but important as it provides the information necessary to address questions about the moderating effects of cognitive processes under stress on association between actual stress exposure and health. Second, assessing recent life stress exposure using a sophisticated online system provided a highly efficient, low-cost method for collecting detailed information about both stress exposure and perceived severity of those exposures. Finally, executive function abilities were assessed using an ecologically valid, global executive function task, performance on which is predictive of real-world abilities and impairments (Kibby et al., 1998).
Some limitations should also be noted. First, although we experimentally manipulated participants’ experience of acute stress in the laboratory, the remainder of the data were cross-sectional, which prohibits causal claims regarding the associations observed herein. Additional research using experimental or longitudinal methods is thus needed to further test the working model described here. Second, we only assessed mental and physical health complaints using self-report measures. As such, future research should extend these findings using objective assessments of health. Third, the use of a relatively young, healthy sample provides a reasonable first test of these ideas, but additional research in more diverse populations is required to examine the generalizability of the effects. Future research could examine these associations in a sample not selected to be free of any health conditions that might affect acute stress reactivity in order to determine whether the effects observed here hold in other populations. Fourth, although there were no differences between groups in any recent life stress or health variable (Table 5), it is possible that the observed results may have been affected by the experimental design. Future research could address this concern by assessing recent life stress and health complaints on a different day than when the acute stress manipulation takes place. Fifth, we did not collect information on participants’ year in college, and it is possible that length of time in school could have influenced the salience of the stressors experienced. To address this point, we conducted additional analyses controlling for age (data not shown), but the results were identical to those reported above. Nonetheless, future research could improve upon the present design by collecting information on factors that might modify stressor salience or by standardizing the time between stressor occurrence and assessment. Sixth, the executive function task that we used is but one of many, and it is possible that other task (e.g. those not primarily utilizing cognitive flexibility) could yield different results. Finally, although the present data do not focus on biological aspects of the stress response, including such markers in future studies could be helpful for ensuring the validity of the stress manipulation and for examining biological factors, such as cortisol and pro-inflammatory cytokines, which might underlie associations between stress, executive function and health.
Notwithstanding these limitations, the present data are the first to demonstrate that better executive function within a stressful context is associated with an attenuated link between recent life stress exposure and current health and, moreover, that these effects are specific to executive function capability during a stressful (but not non-stressful) circumstance. In addition, the moderating effect of better executive function on the link between recent stress exposure and health appears to be mediated by perceptions of stressfulness for experienced stressors, illustrating one potential cognitive pathway through which executive function may lead to beneficial health effects. Additional research is needed to examine these possibilities and to explore other psychological, neural and physiologic processes that link stress and executive function with human health and well-being.
Acknowledgments
The authors thank members of the SNAP Lab for assistance with data collection and Alison Ledgerwood for helpful comments on a previous version of the manuscript.
Funding
This research was supported by a University of California, Davis Provost’s Fellowship to Grant S. Shields; a Hellman Foundation Fellowship to Wesley G. Moons; and National Institutes of Health grant K08 MH103443, NARSAD Young Investigator Grant 23958 from the Brain & Behavior Research Foundation, and a Society in Science—Branco Weiss Fellowship to George M. Slavich.
Footnotes
Disclosure statement
There are no conflicts of interest that could have inappropriately influenced the study or the reporting of the findings.
ORCID
Grant S. Shields http://orcid.org/0000-0002-0827-4669
George M. Slavich http://orcid.org/0000-0001-5710-3818
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–78. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH. When high-powered people fail: working memory and ‘choking under pressure’ in math. Psychol Sci. 2005;16:101–5. doi: 10.1111/j.0956-7976.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress risk factors for cancer-related fatigue. Clin Psychol Sci. 2014;2:108–15. doi: 10.1177/2167702613496243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Dooley LN, Slavich GM, Moreno PI, Bower JE. Strength through adversity: moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors. Stress Health. 2017 doi: 10.1002/smi.2739. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Mueller ST, Gray HM, Raber J, Piper BJ. Evaluation of a short-form of the Berg Card Sorting Test. PLoS One. 2013;8:e63885. doi: 10.1371/journal.pone.0063885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21:1009–18. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Schmitter-Edgecombe M, Long CJ. Ecological validity of neuropsychological tests: focus on the California Verbal Learning Test and the Wisconsin Card Sorting Test. Arch Clin Neuropsychol. 1998;13:523–34. [PubMed] [Google Scholar]
- Kurtzman L, O’Donovan A, Koslov K, Arenander J, Epel ES, Slavich GM. Sweating the big stuff: dispositional pessimism exacerbates the deleterious effects of life stress on metabolic health. Eur J Psychotraumatol. 2012;3:1. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer Publishing Company; 1984. [Google Scholar]
- Malooly AM, Genet JJ, Siemer M. Individual differences in reappraisal effectiveness: the role of affective flexibility. Emotion. 2013;13:302–13. doi: 10.1037/a0029980. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mueller ST, Piper BJ. The Psychology Experiment Building Language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–9. doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. J Pers Soc Psychol. 2005;89:852–63. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–51. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Ohira H, Fukuyama S, Kimura K, Nomura M, Isowa T, Ichikawa N, Matsunaga M, et al. Regulation of natural killer cell redistribution by prefrontal cortex during stochastic learning. NeuroImage. 2009;47:897–907. doi: 10.1016/j.neuroimage.2009.04.088. [DOI] [PubMed] [Google Scholar]
- Ohira H, Isowa T, Nomura M, Ichikawa N, Kimura K, Miyakoshi M, Iidaka T, et al. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. NeuroImage. 2008;39:500–14. doi: 10.1016/j.neuroimage.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Li V, Eiwaz MA, Kobel YV, Benice TS, Chu AM, Olsen RH, et al. Executive function on the psychology experiment building language tests. Behav Res Methods. 2012;44:110–23. doi: 10.3758/s13428-011-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Wood G, Moeller K, Nuerk HC, Kerschbaum HH. Predictors of performance in a real-life statistics examination depend on the individual cortisol profile. Biol Psychol. 2010;85:410–16. doi: 10.1016/j.biopsycho.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Quinn ME, Joormann J. Stress-induced changes in executive control are associated with depression symptoms: examining the role of rumination. Clin Psychol Sci. 2015a;3:628–36. [Google Scholar]
- Quinn ME, Joormann J. Control when it counts: change in executive control under stress predicts depression symptoms. Emotion. 2015b;15:522–30. doi: 10.1037/emo0000089. [DOI] [PubMed] [Google Scholar]
- Raio CM, Orederu T, Palazzolo L, Shurick A, Phelps EA. Cognitive emotion regulation fails the stress test. Proc Natl Acad Sci USA. 2013;110:15139–44. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat AC, Kelloway EK, Desmarais S. The Physical Health Questionnaire (PHQ): construct validation of a self-report scale of somatic symptoms. J Occup Health Psychol. 2005;10:363–81. doi: 10.1037/1076-8998.10.4.363. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Tang D. Individual differences in executive functioning and their relationship to emotional processes and responses. Curr Dir Psychol Sci. 2015;24:93–8. [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. Working memory is differentially affected by stress in men and women. Behav Brain Res. 2013;241:144–53. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–53. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. The relation between fluid intelligence and self-regulatory depletion. Cogn Emotion. 2007;21:1833–43. [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP. A meta-analytic review of the effects of stress on executive functions. Neurosci Biobehav Rev. 2016;68:651–88. doi: 10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci. 2013;1:331–48. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. Understanding inflammation, its regulation, and relevance for health: a top scientific and public priority. Brain Behav Immun. 2015;45:13–14. doi: 10.1016/j.bbi.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. Life stress and health: a review of conceptual issues and recent findings. Teach Psychol. 2016;43:346–55. doi: 10.1177/0098628316662768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, Slavich GM. Effects of lifetime stress exposure on mental and physical health in young adulthood: how stress degrades and forgiveness protects health. J Health Psychol. 2016;21:1004–14. doi: 10.1177/1359105314544132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, Heinrichs M. The Trier Social Stress Test for Groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2011;36:514–22. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37:126–40. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Williams PG, Thayer JF. Executive functioning and health: introduction to the special series. Ann Behav Med. 2009;37:101–5. doi: 10.1007/s12160-009-9091-x. [DOI] [PubMed] [Google Scholar]


