Abstract
We present a robust strategy to covalently link proteins and DNA using HUH-endonuclease domains as fusion partners (HUH-tags). We show that HUH-tags react robustly with specific sequences of unmodified single-stranded DNA, and we have identified five tags that react orthogonally with distinct DNA sequences. We demonstrate the versatility of HUH-tags as fusion partners in Cas9-mediated gene editing and the construction of doubly DNA-tethered proteins for single-molecule studies. Finally we demonstrate application to cellular imaging in live and fixed cells.
Graphical abstract
INTRODUCTION
Combining the prolific functional properties of proteins with the highly programmable nature of DNA has been exploited in diverse applications such as precise DNA-guided control of protein localization and function,1–5 single-molecule manipulation of proteins tethered to DNA structures,6,7 cellular imaging (e.g., DNA-PAINT)8 and barcoding,9 and “smart” delivery of therapeutic proteins.10 Though improvements in cost and efficiency of constructing DNA nanostructures are proceeding at a breakneck pace, the full potential of protein–DNA chimeras has not been realized due to difficulties in specifically attaching proteins to DNA. Current strategies to link DNA to proteins using aptamers11 or zinc-finger-binding motifs12 offer specificity but are non-covalent. Conversely, covalent linkage of proteins to DNA using thiol and amine chemistry lacks specificity.13 Popular SNAP14 (New England Biolabs) and Halo15 (Promega) fusion tags provide both covalent and specific linkages between proteins and DNA, but require incorporation of expensive modified target bases into oligos and additional purification steps. Moreover, current technology is limited to two or three orthogonal attachment sites. We explored the potential of using HUH-endonuclease domains as new fusion tags for covalent protein/DNA conjugation. HUH-endonucleases, so-named because they contain a conserved pair of metal-coordinating histidines (H) separated by a hydrophobic residue (U), participate in cellular processes involving a transition from double-stranded to single-stranded DNA, such as rolling-circle replication in viruses and bacterial plasmid conjugation.16 The endonuclease first nicks single-stranded DNA at a specific sequence at the origin of replication (ori) followed by formation of a covalent phosphotyrosine intermediate, whereby the 5′ end of the DNA strand becomes linked to a specific tyrosine in the HUH-protein. While the phosphotyrosine linkage is an intermediate in vivo, purified HUH-proteins are able to form stable covalent bonds in vitro with synthetic oligonucleotides bearing their ori sequence.16 HUH-tags offer several advantages over current methods, including specific covalent coupling without the requirement for unnatural DNA bases in conjugation sequence and the possibility of massive orthogonality, given that the rapid evolution of viruses and mobile plasmids has yielded many examples of these proteins in nature with distinct target sequences. Here we have adapted members of the diverse HUH-endonuclease protein family as fusion-tags and demonstrate their uses in vitro and in cultured cells. We establish that HUH-tags do not disrupt the function when fused to several examples of nuclear, cytoplasmic, and cell-surface target proteins, that the chemistry occurs robustly in vitro and in mammalian cells, and that multiple proteins can be labeled simultaneously in vitro and in cells.
RESULTS AND DISCUSSION
We identified a panel of HUH-endonuclease domains for initial studies from literature and sequence similarity databases (Table 1), focusing on proteins with known structures. We selected endonucleases that represent the different biological functions that the HUH-endonucleases exhibit in nature. Despite similar catalytic mechanisms, different classes of HUH-proteins exhibit potentially useful differences when used as a fusion tag. For example, the HUH-endonucleases that participate in rolling circle replication in viruses are generally quite small (10–12 kDa) but have less sequence specificity than the relaxases, which are much larger (25–30 kDa). Moreover, the tyrosine at the site of covalent attachment is near the C-terminus in the viral classes while it is near the N-terminus in the relaxase class, which could have important implications for the design of molecules for single-molecule measurements or FRET biosensors. It should be noted that during preparation of this manuscript, a subset of HUH-endonucleases from the relaxase family were used to assemble proteins on DNA.17 We will refer to the HUH-tags by their shortened names depicted in Table 1.
Table 1.
HUH-Tags Used in This Study
| HUH-tag | full name | PDB ID | MW (kDa) | pI | ori sequence |
|---|---|---|---|---|---|
| PCV218 | porcine circovirus 2 | 2HW0 | 13.4 | 9.5 | aagtattaccagaaa |
| DCV | duck circovirus | 13.4 | 5.4 | ||
| FBNYV31 | fava bean necrosis yellow virus | 2HWT | 11.3 | 8.6 | |
| RepB32 | replication protein RepB Streptococcus agalactiae | 3DKY | 15.2 | 9.4 | tgcttccgtactacgacccccca |
| RepBm | RepB Fructobacillus tropaeoli | 14.7 | 5.5 | ||
| TraI19 | conjugation protein TraI Escherichia coli | 1P4D | 36.4 | 5.6 | tttgcgtggggtgtggtgcttt |
| mMobA33 | mobilization protein A Escherichia coli | 2NS6 | 20.9 | 6.3 | ccagtttctcgaagagaaaccggtaagtgcaccctccc |
| NES27 | nicking enzyme Staphylococcus aureus | 4HT4 | 25.9 | 6.7 | acgcgaacggaacgttcgcataagtgcgcccttacgggatttaac |
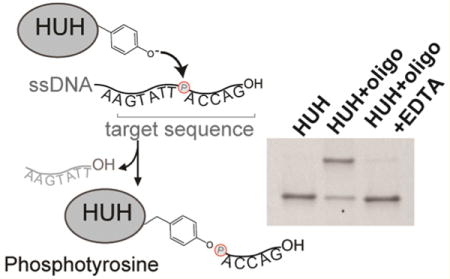
We first evaluated the biochemical requirements for formation of covalent adducts between the HUH-tags and single-stranded DNA (Figure 1). We expressed HUH-endonucleases in E. coli in fusion with an N-terminal His6-SUMO domain, and purified them using affinity chromatography and size exclusion chromatography. Reacting recombinant SUMO–DCV with a single-stranded oligo bearing its target sequence in the presence of Mn2+ results in formation of a characteristic covalent adduct,18 which runs slower on SDS-PAGE (Figure 1A). Treating the protein first with EDTA results in no covalent adduct, as previously shown.18 We next compared the rate of covalent adduct formation with the well-known SNAP-tag.14 Notably, linking SNAP-tagged proteins to DNA involves additional steps compared to the HUH-tags; we first chemically linked the benzylguanine SNAP substrate to a DNA-oligo to result in a substrate that would produce a shift on SDS-PAGE analogous to the HUH-tag. We then reacted recombinant SNAP-tag and SUMO–DCV in their respective optimal buffers with 4-fold excess of their respective target oligos and analyzed the reaction by SDS-PAGE (Figure 1B). Both reactions robustly form covalent adducts, with the DCV reaction achieving maximal yield in under 5 min, compared to 10 min for the SNAP-tag. To further explore the activity of the enzyme, we took advantage of the HUH-tag’s nuclease activity and monitored the cleavage reaction using an oligo containing a donor-fluorophore and quencher flanking the HUH nicking site (Figure 1C and Figure S1). Nicking results in dequenching of the fluorophore, allowing activity to be monitored by fluorescence. Reacting such an oligo with varying concentrations of SUMO-FBNYV shows efficient cleavage even at 1:1 HUH:oligo and achieving maximal cleavage rates at ∼4x HUH-protein (Figure 1C). We used this assay to explore the optimal conditions for HUH-endonuclease activity, where we found an optimal pH range of 7–8 for both FBNYV and mMobA (Figure S1) and determined that additives such as reducing agents, calcium, and BSA do not appreciably affect the enzymatic activity (Figure S1). In nature, the protein–DNA conjugate is reversible. We tested whether we could reverse this reaction in vitro by adding an excess of the cleavage product of the endonuclease reaction with a free 3′-OH group that is not covalently conjugated (Figure S2). At stoichiometries close to 1:1 with respect to the HUH-protein, no reversibility is observed. However, high molar excesses of 3′-OH oligo do indeed reverse the covalent complexes of both DCV and mMobA, which could be useful for some labeling applications.
Figure 1.
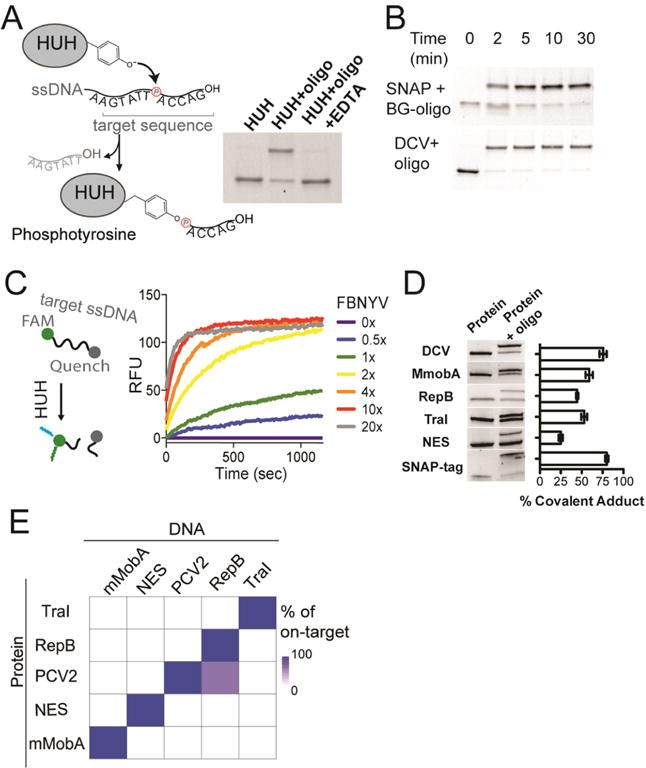
Covalent protein–DNA adduct formation using HUH-tags. (A) Cartoon of reaction chemistry showing catalytic tyrosine in HUH-endonuclease nicking ssDNA and forming a covalent phosphotyrosine adduct. SDS-PAGE showing formation of covalent adduct between DCV and target ori oligonucleotide in the presence of Mn2+ and EDTA (B) Timecourse of covalent adduct formation of the HUH-tag DCV and the SNAP-tag with 4-fold excess of target DNA using SDS-PAGE. (C) Dequenching assays monitor nicking of an HUH-target oligo flanked by donor and quencher dyes, which leads to appearance of fluorescence. Here, varying concentrations of FBNYV HUH-tag (62.5–2500 nM) were added to 125 nM quenched PCV-target oligo, and FAM fluorescence monitored as a function of time using a fluorescence plate reader (D) Comparison of covalent adducts formed by reaction of five HUH-tags with 10-fold excess of respective target oligos for 15 min at 37 °C on SDS-PAGE. Yield of covalent adduct was calculated for three replicates using ImageJ gel-band quantitation functions. Error bars report standard error. (E) Heat map of DNA target sequence preferences of HUH-tags. Indicated HUH-proteins were incubated individually with a 10-fold excess of preferred oligonucleotide target sequences for each HUH-protein; the reaction products were analyzed by SDS-PAGE and quantified.
A significant advantage of using HUH-tag fusion partners is that there are several classes of HUH-endonucleases with divergent structures, DNA recognition motifs and functions which allow them to recognize distinct sequences of ssDNA,16 leading to the potential for highly multiplexed labeling of multiple species in a single reaction. We tested five SUMO-HUH fusions for their ability to form covalent adducts by SDS-PAGE (Figure 1D), though it should be noted that TraI required the removal of the SUMO tag prior to reaction with target DNA due to the involvement of its N-terminal methionine in DNA-binding.19 All reactions resulted in covalent adduct formation, with yields ranging from 25 to 80%. The most efficient HUH-tag, PCV2 (and its homologues DCV and FBNYV) has activity similar to the heavily engineered SNAP protein. We next tested the sequence specificity of these tags. We reacted each HUH-protein with a 10-fold excess of each target DNA, and quantitated the formation of covalent adducts (Figure S3), as displayed in the heat map (Figure 1E). The HUH-tags generally displayed stringent sequence specificity, with the exception of slight cross-reactivity between PCV2 and RepB. We originally observed cross-reactivity between NES and the mMobA ori sequence. However, we were able to both improve the formation of covalent adduct and abrogate cross-reactivity with NES by altering single nucleotides in the mMobA target sequence (Figure S4).
We next aimed to fuse HUH-tags to recombinant proteins of interest where it could be potentially beneficial to covalently attach ssDNA (Figure 2). One application that could benefit from an efficient strategy to covalent tether DNA to recombinant proteins is single-molecule force spectroscopy (Figure 2A). DNA handles are routinely added to proteins using cysteine chemistry to isolate them from surfaces and beads as well as to provide a mechanical signature. However, cysteine chemistry requires mutation of internal cysteines in a protein, does not permit control over orientation and is not permissive for disulfide containing proteins. We envisioned sandwiching a protein of interest between two HUH-tags for use in this application. As a proof-of-concept for this strategy, we prepared a recombinant protein comprised of tandem HUH-tags linked by a protein sequence containing an MMP2 proteolytic cleavage site. We then added two oligos with distinct target sequences containing Cy3 or Cy5 dyes. Fluorescent imaging shows robust dual labeling of the protein, and treating the protein with MMP2 protease results in splitting of the dual-tagged protein into two single tagged proteins. This molecule can now be attached to two double-stranded DNA containing complementary overhangs24 to the oligos conjugated to the protein or in a similar way to complementary oligos presented by a DNA nanoswitch.6 We also tested the assembly of proteins on DNA nanostructures using HUH-tags (Figure S6). We observed a shift of the tetrahedron DNA in the presence of PCV2 and RepB and observed a similar shift of mMobA on a larger six-helix bundle nanostructure (Figure S6), consistent with recent work.17
Figure 2.
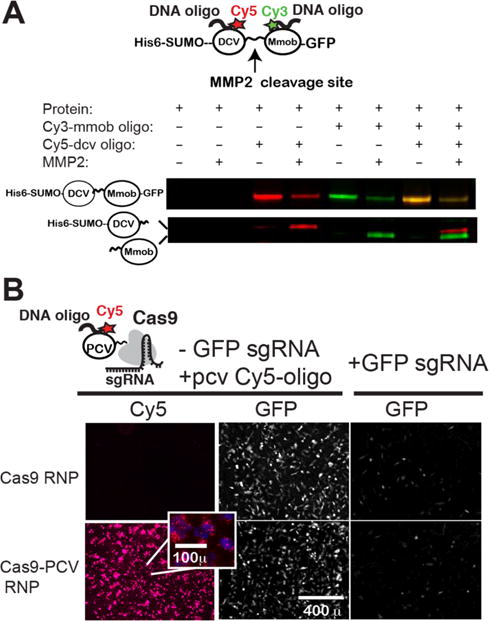
Uses of HUH-tagged recombinant proteins: covalent tethering of ssDNA to Cas9. (A) A recombinant tandem HUH-tagged protein containing DCV and mMobA domains linked by a protein sequence containing an MMP2 cleavage site was expressed and purified from E. coli. Two oligos bearing the appropriate target sequence and Cy3 or Cy5 dyes were added under normal HUH reaction conditions. Half of each reaction was subjected to mock or MM2 enzyme. Reaction products were run on SDS-PAGE and imaged on a Typhoon gel imager. (B) Testing function of Cas9 fusion to HUH-tag. Cy5-conjugated oligo bearing the PCV target sequence was reacted with recombinant Cas9 or PCV-Cas9 and delivered to HT1080 cells stably expressing inducible GFP with and without an sgRNA targeted to the GFP locus. GFP expression was induced 12 h after Cas9 treatment, and GFP and Cy5 fluorescence measured by fluorescence microscopy 4–8 h later.
The next example we thought of is Cas9, which is routinely delivered to cells as a recombinant protein via transfection or electroporation as a ribonucleoprotein (RNP) with in vitro transcribed guide RNA.20 We reasoned that we could use the HUH-tag to specifically label the RNP with organic fluorophores for quantitation purposes or for potential multiplexed covalent labeling of genomic loci21,22 with an organic fluorophore or other modification that can be encoded on an oligo. We first tested that the recombinant PCV-Cas9 (Figure S5) was functional by measuring its ability to cleave and disrupt GFP fluorescence in a stably expressing GFP cell line (Figure 2B). The number of GFP expressing cells was reduced by ∼80% for both Cas9 and PCV-Cas9 (Figure S5c). In addition to exhibiting similar levels of GFP knockdown, the PCV fusion tag allowed us to also codeliver a Cy5-labeled oligo into the cells. Interestingly, while the Cy5-oligo tethered to Cas9 was most efficiently delivered into cells when delivered with cationic lipid (Figure2B), an appreciable amount of the RNP-oligo complex also entered the cells even in the absence of cationic lipid, suggesting that the additional positive charges from the PCV enhance its ability to cross the cell membrane (Figure S5b,d,e).23
We next tested the activity of HUH-tags in mammalian cells (Figure 3) for potential use in cellular imaging applications such as barcoding25 and DNA-PAINT.8 We expected that characteristic high isoelectric points/embedded nuclear localization signals would make their use in mammalian cells difficult. We were able to identify a subset of HUH-tags to be compatible with proper localization in mammalian cells— mMobA, TraI, and RepBm, a homologue of RepB with lower isoelectric point. We initially transfected U2OS cells with HUH-tags cloned in pcDNA3 for constitutive expression. Standard preparation of cell lysates followed by incubation with a 100 nM solution of 3′-TAMRA target oligonucleotides and Mn2+ showed labeling of mMobA and TraI by fluorescent SDS-PAGE (Figure S7). To assess the use of HUH-tags for labeling in fixed cells and effects on cellular localization, we fused TraI and mMobA to the N-terminus of human β-actin and expressed the constructs in U2OS cells. Labeling the fixed TraI/mMobA-β-actin cells with 3′-Alexa647 target oligos showed expected labeling of both actin filaments and cytoplasmic actin; counterstaining with phalloidin-Alexa488 showed that the fusion protein was efficiently incorporated into actin filaments (Figure 3a, Figure S8). Control cells, transfected with EGFP-β-actin and mock labeled with either fluorescent target, showed no fluorescence in the far-red region, indicating that nonspecific sticking of the DNA is not responsible for labeling (Figure S8).26
Figure 3.
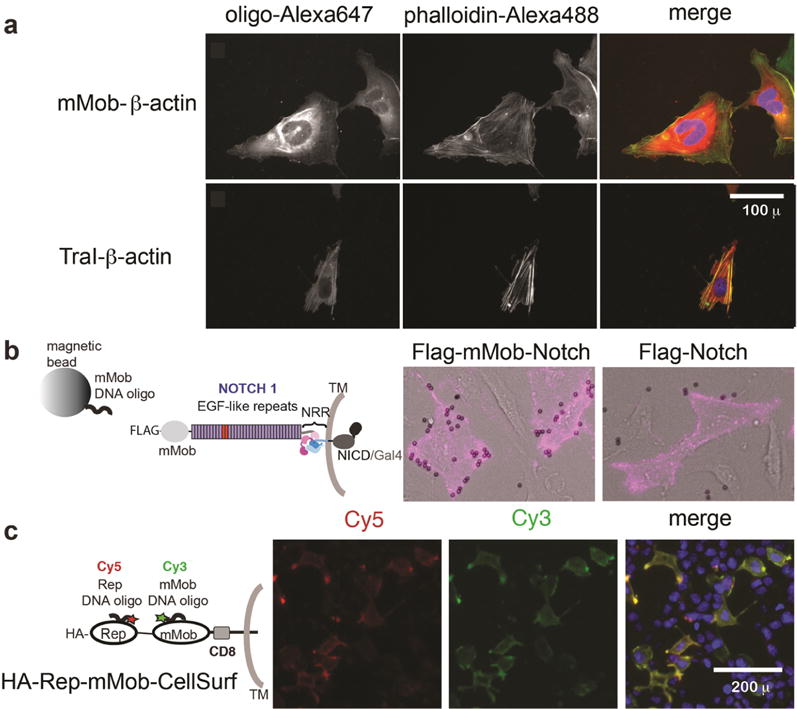
Uses of HUH-tags in live and fixed cellular imaging. All images were collected on an EVOS-FL-AUTO widefield fluorescence microscope using standard Plan Fluorite objectives (a) Intracellular imaging. U2OS cells were transfected with vectors expressing HUH-actin fusion proteins, fixed after 24 h, and stained using Alexa647-labeled target oligonucleotides (red in merge). Cellular actin filaments were stained with Alexa488-phalloidin (green in merge) and DNA stained with DAPI. (b) Attaching magnetic beads to Cell-surface Notch receptors. Full-length N-term Flag-tagged Notch receptors with or without an HUH-tag were transfected into U2OS cells. After 24 h, cells were treated with an APC-conjugated antibody recognizing the Flag epitope tag and magnetic beads coated with oligos harboring the mMobA target sequence. Excess magnetic beads were washed from cells and live cells imaged in brightfield and Cy5 channels. (c) Orthogonal labeling of tandem HUH-tags on the cell surface. A transmembrane construct encoding tandem HUH-tags was transiently transfected in HEK293T cells. After 24 h, the cells were labeled in full media with Cy3- and Cy5-labeled oligonucleotides harboring the target sequences of two HUH-tags. Cells were washed once in PBS and imaged in clear-bottom 96-well plates. After washing they were stained with Hoesht and imaged live.
We next tested HUH-tags in live-cell labeling. We first fused mMobA and RepBm to the N-terminus of cell-surface Notch receptors also containing a FLAG-tag.30 Both fusions exhibited good cell-surface trafficking in U2OS cells and normal capability to signal in comparison to a SNAP-fused Notch receptor, as shown by labeling the FLAG-epitope tag with an APC conjugated antibody and in luciferase signaling assays30 (Figure S9). Treating the cells expressing HUH-fusion tags with magnetic beads coated with oligos bearing the target sequence (Figure 3b) or Cy3 containing target oligos (Figure S9) only labeled the HUH-tag containing receptors. To test the orthogonality of labeling HUH-tags in the cellular context, we expressed a transmembrane receptor encoded with tandem HUH-tags in the extracellular domain. We added 3′Cy3-oligos and 3′Cy5-oligos bearing mMobA and RepBm respective target sequences (Figure 3c, Figure S10), which results in fast (Figure S11) and robust “one-pot” live-cell labeling. Cells expressing receptors containing only one HUH-tag are only labeled by the oligo containing the correct target sequence (Figure S10) and only cells that also are immunostained with an anti-HA antibody are labeled in the case of HA-tag containing receptors, showing that nonspecific sticking of DNA is not responsible for labeling. Optimal labeling occurred using 200–250 nM fluorescent target oligo in standard serum-containing media, supplemented with Mn2+ and salmon sperm DNA, for 15–20 min at 37 °C. Regarding live-cell fluorescent labeling of intracellular targets, oligonucleotides do not freely travel into cells. However, we performed proof-of-concept live-cell labeling of cells transfected with TraI-β-actin by delivering its fluorophore-conjugated target oligo using cationic lipid (Figure S12). Multiplexed intracellular labeling could be further optimized by exploring alternative methods to deliver oligos into cells.
CONCLUSIONS
We have shown that HUH-endonucleases are ideal fusion tags due to their efficient formation of covalent bonds, requirement for a specific sequence of DNA rather than expensive modified bases, and potential for multiplexed “one-pot” labeling. Moreover, some applications may benefit from the ability to reverse the covalent bond under certain conditions. We have demonstrated examples of both N-terminal and C-terminal fusions that do not disrupt normal protein function of cytoplasmic, nuclear, and cell-surface proteins. The reaction is compatible with a variety of in vitro conditions, standard cell-culture media, cellular lysates, and cell fixation. HUH-tags greatly expand the protein-labeling toolkit for in vitro applications such as DNA nanotechnology, where one could potentially immobilize up to five HUH-tagged proteins expressed in the same cell lysate directly onto a DNA origami structure, without intermediate purification steps. DNA-based cellular imaging applications such as proximity-ligation assays27 or DNA-PAINT would also benefit from the simplicity and specificity that HUH-tags offer. Tandem HUH-tags will be useful for single-molecule studies or FRET-biosensors using organic fluorophores. Moreover, we have shown that optimization of the target sequence for one HUH-tag enhanced both yield of covalent complex and specificity, and existing studies provide precedents for mutating amino acids in the HUH-endonucleases to alter DNA sequence specificity.28,29 This suggests that novel protein tags could be created by rational engineering of existing protein/DNA sequences, directed evolution strategies, and further exploration of untested HUH-endonucleases.
Supplementary Material
Acknowledgments
This study was supported by an NIH NIGMS R35 grant to W.R.G. (1R35GM119483), funds to W.R.G. from the Pew Biomedical Scholar Program (29634), and UMN startup funds. We would like to thank Peter Gordon for the kind gift of inducible GFP cell lines. We also would like to thank Eric Hendrickson and Brian Ruis for helpful discussions about the Cas9 experiments. Typhoon FLA9500 images were collected at the University of Minnesota Imaging Center.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b02572.
Full experimental methods, Figures S1–S12, and protein and oligonucleotide sequences (PDF)
ORCID
Wendy R. Gordon: 0000-0001-7696-5560
Notes
The authors declare no competing financial interest.
References
- 1.Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Science. 2012;338(6107):662. doi: 10.1126/science.1226734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariadi RF, Cale M, Sivaramakrishnan S. Proc Natl Acad Sci USA. 2014;111(11):4091. doi: 10.1073/pnas.1315923111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw A, Lundin V, Petrova E, Fördős F, Benson E, Al-Amin A, Herland A, Blokzijl A, Högberg B, Teixeira AI. Nat Methods. 2014;11(8):841. doi: 10.1038/nmeth.3025. [DOI] [PubMed] [Google Scholar]
- 4.Engelen W, Janssen BMG, Merkx M. Chem Commun (Cambridge, U K) 2016;52(18):3598. doi: 10.1039/c5cc09853j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu J, Yang YR, Johnson-Buck A, Liu M, Liu Y, Walter NG, Woodbury NW, Yan H. Nat Nanotechnol. 2014;9:531. doi: 10.1038/nnano.2014.100. [DOI] [PubMed] [Google Scholar]
- 6.Halvorsen K, Schaak D, Wong WP. Nanotechnology. 2011;22(49):494005. doi: 10.1088/0957-4484/22/49/494005. [DOI] [PubMed] [Google Scholar]
- 7.Cecconi C, Shank EA, Bustamante C, Marqusee S. Science. 2005;309:2057. doi: 10.1126/science.1116702. [DOI] [PubMed] [Google Scholar]
- 8.Jungmann R, Avendaño MS, Woehrstein JB, Dai M, Shih WM, Yin P. Nat Methods. 2014;11(3):313. doi: 10.1038/nmeth.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, Aach J, Lee J-H, Levner D, Nip L, Church GM. Nat Methods. 2013;10(5):403. doi: 10.1038/nmeth.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas SM, Bachelet I, Church GM. Science. 2012;335(6070):831. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Lin C, Li H, Yan H. Angew Chem Int Ed. 2005;44(28):4333. doi: 10.1002/anie.200501089. [DOI] [PubMed] [Google Scholar]
- 12.Nakata E, Liew FF, Uwatoko C, Kiyonaka S, Mori Y, Katsuda Y, Endo M, Sugiyama H, Morii T. Angew Chem Int Ed. 2012;51(10):2421. doi: 10.1002/anie.201108199. [DOI] [PubMed] [Google Scholar]
- 13.Stephanopoulos N, Francis MB. Nat Chem Biol. 2011;7(12):876. doi: 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- 14.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. Nat Biotechnol. 2003;21:86. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 15.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. ACS Chem Biol. 2008;3(6):373. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 16.Chandler M, de la Cruz F, Dyda F, Hickman AB, Moncalian G, Ton-Hoang B. Nat Rev Microbiol. 2013;11(8):525. doi: 10.1038/nrmicro3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagredo S, Pirzer T, Aghebat Rafat A, Goetzfried MA, Moncalian G, Simmel FC, de la Cruz F. Angew Chem Int Ed. 2016;55(13):4348. doi: 10.1002/anie.201510313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega-Rocha S, Byeon I-JL, Gronenborn B, Gronenborn AM, Campos-Olivas R. J Mol Biol. 2007;367(2):473. doi: 10.1016/j.jmb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Larkin C, Schildbach JF. Structure. 2003;11(11):1369. doi: 10.1016/j.str.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen Z-Y, Liu DR. Nat Biotechnol. 2015;33(1):73. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma H, Tu L-C, Naseri A, Huisman M, Zhang S, Grunwald D, Pederson T. Nat Biotechnol. 2016;34(5):528. doi: 10.1038/nbt.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Proc Natl Acad Sci USA. 2015;112(10):3002. doi: 10.1073/pnas.1420024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Staahl BT, Alla RK, Doudna JA. eLife. 2014;3:e04766. doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nastasijevic B, Becker NA, Wurster SE, Maher LJ. Biochem Biophys Res Commun. 2008;366(2):420. doi: 10.1016/j.bbrc.2007.11.169. [DOI] [PubMed] [Google Scholar]
- 25.Mali P, Aach J, Lee J-H, Levner D, Nip L, Church GM. Nat Methods. 2013;10(5):403. doi: 10.1038/nmeth.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius K-J, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson L-G, Landegren U. Nat Methods. 2006;3(12):995. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 27.Edwards JS, Betts L, Frazier ML, Pollet RM, Kwong SM, Walton WG, Ballentine WK, Huang JJ, Habibi S, Del Campo M, Meier JL, Dervan PB, Firth N, Redinbo MR. Proc Natl Acad Sci USA. 2013;110(8):2804. doi: 10.1073/pnas.1219701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harley MJ, Schildbach JF. Proc Natl Acad Sci USA. 2003;100(20):11243. doi: 10.1073/pnas.2035001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders C, Niewoehner O, Jinek M. Methods Enzymol. 2015;558:515. doi: 10.1016/bs.mie.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon WR, Zimmerman B, He L, Miles LJ, Huang J, Tiyanont K, McArthur DG, Aster JC, Perrimon N, Loparo JJ, Blacklow SC. Dev Cell. 2015;33(6):729. doi: 10.1016/j.devcel.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vega-Rocha S, Gronenborn B, Gronenborn AM, Campos-Olivas R. Biochemistry. 2007;46(21):6201. doi: 10.1021/bi700159q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boer DR, Ruíz-Masó JA, López-Blanco JR, Blanco AG, Vives-Llàcer M, Chacón P, Usón I, Gomis-Rüth FX, Espinosa M, Llorca O, del Solar G, Coll M. EMBO J. 2009;28(11):1666. doi: 10.1038/emboj.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monzingo AF, Ozburn A, Xia S, Meyer RJ, Robertus JD. J Mol Biol. 2007;366(1):165. doi: 10.1016/j.jmb.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


