Abstract
Prenatal alcohol exposure often results in fetal alcohol syndrome and fetal alcohol spectrum disorders. Mechanisms of fetal brain damage by alcohol remain unclear. We used baboons (Papio spp.) to study alcohol-driven changes in the fetal cerebral artery endocannabinoid system. Pregnant baboons were subjected to binge alcohol exposure via gastric infusion three times during a period equivalent to the second trimester of human pregnancy. A control group was infused with orange-flavored drink that was isocaloric to the alcohol-containing solution. Cesarean sections were performed at a time equivalent to the end of the second trimester of human pregnancy. Fetal cerebral arteries were harvested and subjected to in vitro pressurization followed by pharmacological profiling. During each alcohol-infusion episode, maternal blood alcohol concentrations (BAC) reached 80 mg/dL, that is, equivalent to the BAC considered legal intoxication in humans. Circulating anandamide (AEA) and 2-arachidonoylglycerol (2-AG) remained unchanged. Ultrasound studies on pregnant mothers revealed that fetal alcohol exposure decreased peak systolic blood velocity in middle cerebral arteries when compared to pre-alcohol levels. Moreover, ethanol-induced dilation was observed in fetal cerebral arteries pressurized in vitro. This dilation was abolished by the mixture of AM251 and AM630, which block cannabinoid receptors 1 and 2, respectively. In the presence of AM251, the cannabinoid receptor agonist AEA evoked a higher, concentration-dependent dilation of cerebral arteries from alcohol-exposed fetuses. The difference in AEA-induced cerebral artery dilation vanished in the presence of AM630. CB1 and CB2 receptor mRNA and protein levels were similar in cerebral arteries from alcohol-exposed and control-exposed fetuses. In summary, alcohol exposure dilates fetal cerebral arteries via endocannabinoid receptors and results in an increased function of CB2.
Keywords: fetal cerebral artery, baboon pregnancy, maternal alcohol consumption, fetal alcohol exposure, binge drinking during pregnancy
Introduction
Alcohol consumption during pregnancy is a serious public health problem and a leading preventable cause of birth defects and developmental disabilities (Basavarajappa, 2015). The adverse effects of alcohol on the developing fetus result in a spectrum of structural abnormalities, behavioral defects, and neurocognitive disabilities termed fetal alcohol spectrum disorders (FASD). The most severe cases are diagnosed as fetal alcohol syndrome (FAS). FASD is estimated to affect 2–5% of children in the U.S. and Western European countries (www.cdc.gov; May & Gossage, 2001; 2011; May et al., 2009). In some regions of the world, however, FAS/FASD affects up to 29% of live births (Olivier, Curfs, & Viljoen, 2016).
It is widely documented that prenatal alcohol (ethanol) exposure causes abnormalities in several organs and systems of the body, such as the heart, kidney, liver, gastrointestinal tract, and the endocrine and immune systems (Caputo, Wood, & Jabbour, 2016; Gauthier, 2015). However, the brain constitutes the most severely affected organ, exhibiting both structural and functional abnormalities in response to prenatal alcohol exposure (Caputo et al., 2016; Mattson, Schoenfeld, & Riley, 2001). Unfortunately, the mechanisms of cerebral damage due to prenatal alcohol exposure are poorly understood and thus, treatment is limited (Murawski, Moore, Thomas, & Riley, 2015).
The developing brain is critically dependent on a sufficient blood supply, which ensures delivery of oxygen and other nutrients. Remarkably, the prevailing neurological symptoms in FAS and FASD, such as memory deficit, low processing speed, lack of cognitive flexibility, and many others are similar to those observed in individuals with progressive neurodegenerative disorders, in which cerebrovascular dysfunction is being increasingly recognized as one of the major pathophysiological mechanisms (Gorelick et al., 2011; Mattson & Riley, 1998; Olson, Feldman, Streissguth, Sampson, & Bookstein, 1998; Zlokovic, 2011).
Endocannabinoid (eCB) signaling is critical for regulating arterial contractility in response to physiological and pathological stimuli (Engeli et al., 2012; Lípez-Miranda, Herradón, & Martín, 2008). The endocannabinoid system (eCBS) consists of eCB-producing enzymes, eCB ligands, eCB receptors (mostly CB1 and CB2 types), and the eCB degradation machinery (Lu & Mackie, 2016). Endocannabinoid production, functional activity of CB1 and CB2 receptors, and eCB breakdown within the vascular smooth muscle have all been reported (Benyó, Ruisanchez, Leszl-Ishiguro, Sándor, & Pacher, 2016; Czikora et al., 2012; Rajesh et al., 2008). It remains unknown whether alcohol in general, and prenatal alcohol exposure in particular, affects the components of the vascular eCB system and its regulation of cerebrovascular contractility. Thus, we hypothesized that fetal alcohol exposure in utero targeted key components of the eCB system within the fetal cerebral artery.
In the current work, we use a model of binge alcohol administration in pregnant baboons via gastric infusion of alcohol-containing fluid during mid-pregnancy. Non-human primate models of alcohol exposure are widely used in alcohol-related studies (Baker, Farro, Gonzales, Helms, & Grant, 2014; Clarren & Bowden, 1982; Weerts, Goodwin, Kaminski, & Hienz, 2006). A substantial number of studies use several forms of alcohol self-administration (Grant & Bennett, 2003; Grant et al., 2008). The use of anesthesia to monitor fetal cerebral artery function by an ultrasound exam during an alcohol exposure episode, however, is required and is utilized in our current work, following previously accepted models of ethanol administration (Kochunov et al., 2010). Furthermore, the model we chose offers several advantages. First, the effect of alcohol during mid-pregnancy can be estimated without exposing the ovary or fetus to alcohol at earlier intervals, as would be necessary during development of alcohol preference in a self-administration protocol. Lack of alcohol exposure prior to conception is of particular advantage, as transgenerational effects of binge drinking have been reported in a primate model of alcohol self-administration (VandeVoort, Grimsrud, Midic, Mtango, & Latham, 2015). Second, the only data available on alcohol’s effects on fetal cerebral artery function in non-human primates is reported in an identical protocol (Kochunov et al., 2010). Thus, our findings can be easily compared with previous observations.
To our knowledge, the possibility that the fetal cerebral artery endocannabinoid system is targeted by maternal alcohol drinking has not been previously investigated. While largely exploratory in nature, our current work reveals that alcohol-induced dilation of fetal cerebral arteries is mediated by endocannabinoid receptors, with CB2 receptors representing the novel targets of fetal alcohol exposure in cerebral arteries.
Materials and Methods
Study approval
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution.
Animal subjects
A total of seven alcohol-naïve Papio hamadryas anubis dams (ages 7–15 years) were enrolled into the study. Dams were received from the University of Oklahoma Primate Research Center before reaching 80 days of gestation. Then, dams were given 10 days to acclimate to the new environment at UTHSC. Baboons were singly housed in standard baboon cages, with visual and auditory access to each other. A maximum of four baboons (cages) were housed per room, on a 12-h light/dark cycle (lights on at 6:00 AM) without access to natural light. Baboons were fed twice a day, with each feeding consisting of the High Protein Monkey Diet (~15 biscuits per meal, 21 kcal/biscuit) to sustain the baboons’ weight gain as expected throughout the pregnancy. Each feeding was also supplemented by two pieces of fresh fruits or vegetables and two tablespoons of peanut butter. Drinking water was available ad libitum. The facilities were maintained in accordance with the USDA and AAALAC standards.
Alcohol infusion procedure
Dams were randomly assigned to the experimental groups. Dams were subjected to either alcohol or control infusion procedures at 90, 100, and 110 days of gestation as confirmed by an ultrasound exam. Prior to each procedure, the animals were fasted for 12 h. On the day of the experiment, the animal sedation was induced by a single shot of ketamine hydrochloride (Ketaset®, 10 mg/kg of body weight). Anesthesia was maintained with isoflurane (1.5–2.0%) throughout the infusion, followed by maternal blood sampling and Doppler evaluation of fetal cerebral artery function. A rectal suppository of indomethacin (25 mg) was used to prevent labor during the alcohol/control infusion. An intravenous (IV) catheter was placed into the saphenous vein for blood collection and another catheter was placed into the cephalic vein for IV sodium chloride infusion. Animal monitoring of vital signs and depth of anesthesia consisted of electrocardiography, pulse oximetry, capnography, non-invasive blood pressure, and temperature measurements. A gastric catheter was introduced into the stomach and the infusion was administered over 10 min. For the experimental group, the infusion contained 1.8 g/kg ethanol (ultrapure, 200 proof; American Bioanalytical) diluted in reverse osmosis-purified drinking water. The control group of animals received an isocaloric solution containing orange-flavored Tang® powder (Kraft Foods). In both cases, the total volume of fluid infused was equal to 200 mL. Experimental blood collection occurred at 10 time points (see below) over 180 min, and Doppler evaluation of fetal middle cerebral artery (MCA) function was performed immediately before and at 120 min post-infusion. Before awakening from anesthesia, both groups of animals received a single intramuscular injection of carprofen (Rimadyl, 4.4 mg/kg of body weight), aimed to alleviate symptoms of hangover following alcohol drinking. At 120 days of gestation, Cesarean sections (C-sections) were performed using standard methodology that included maternal anesthesia. During C-section, fetuses were euthanized by exsanguination. In the control group, two fetuses were female and one was male; in the alcohol-exposed group, all four fetuses were male.
Blood alcohol concentration (BAC) measurements
Blood was collected immediately prior to the gastric infusion of either control or alcohol infusion, and at 10, 30, 60, 75, 90, 105, 120, 160, and 180 min following the infusion episode. Blood samples were collected through an IV catheter inserted into the saphenous vein. Blood alcohol levels were detected using the Nicotinamide Adenine Dinucleotide-Alcohol Dehydrogenase Reagent kit following the manufacturer’s instructions (Sigma-Aldrich). Samples and reagent mixture were pipetted into 96-well plates, each sample being prepared in duplicates. Absorbance was measured at 340 nm using a Synergy microplate reader (BioTek).
Doppler ultrasonography of fetal MCA
Doppler ultrasonography was performed on anesthetized pregnant baboons immediately prior to and 120 min following gastric infusion of control or alcohol-containing infusion fluid at 90, 100, and 110 days of gestation. At 120 days of gestation, Doppler ultrasonography was performed immediately prior to C-section. Ultrasounds were performed using SonoAce R3 sonographer (Samsung) and GE Voluson E (GE). These sonographers had negligible inter-instrument measurement variability. The MCA was studied just distal to the circle of Willis and the angle of insonation was maintained near 0 degrees. Peak systolic velocity (PSV) and resistive index (RI) were calculated. Each measurement was taken three times, and the average was presented as a single data point.
Liquid chromatography-tandem mass spectrometry
Sample preparation
Fifty μL of methanol was added to a 50-μL aliquot of plasma sample. The mixture was vortexed for 30 sec, and then centrifuged for 15 min at 4 °C (circa 13,000 × g). Seventy-five μL of supernatant was transferred to another vial containing 50 μL of methanol. The mixture was again vortexed for 30 sec, and centrifuged for 15 min at 4 °C (circa 13,000 × g). One hundred μL of supernatant was carefully transferred into a 96-well plate (max. 400 μL per well). Ten μL of IS solution (20 ng/mL AEA-d4 and 200 ng/mL 2-AG-d8 in methanol) was added, and an aliquot of 10 μL was injected into the LC-MS/MS system. To build the concentration calibration curve, analyte standards ranging from 0.02 to 50 ng/mL for AEA, and from 0.2 to 500 ng/mL for 2-AG were prepared in methanol solution. Similarly, 100 μL of each standard solution were transferred to a 96-well plate, and 10 μL of IS solution (20 ng/mL AEA-d4 and 200 ng/mL 2-AG-d8 in methanol) was added followed by a 10-μL aliquot injection into the LC-MS/MS system.
LC-MS/MS Measurements
LC-MS/MS measurements were carried out using a Triple Quad 5500 tandem mass spectrometer from AB Sciex LLC (Framingham, MA), equipped with a Turbo V™ ion source for electrospray ionization (ESI) with the TurboIonSpray probe, a Shimadzu (Columbia, MD) Nexera XR LC system with CBM20Alite Controller, LC20ADXR pumps, SIL20ACXR AutoSampler, and CTO20AC column oven. Chromatographic separation of analytes was completed using a Discovery HS C18 column of 50 × 4.6 mm (length × internal diameter, and 3-μm particle size (Sigma-Aldrich, St. Louis, MO), maintained at 40 °C. The mobile phases (A: water, and B: methanol, each containing 0.1% formic acid and 2 mM ammonium acetate) were eluted at a flow rate of 0.35 mL/min. The gradient started at 50% of mobile phase B for 0.5 min, then rose to 95% from 0.5 to 1.0 min, and from 1.0 to 6.0 min rose to 100%. Subsequently, the eluent composition was changed to initial condition, 50% of mobile phase B. The total run time was 7 min with an extra 1.0-min equilibration time before each injection. A switching valve directed the mobile phase to the MS system between 3.5–6.5 min. The electrospray ion source was operated in a positive ionization mode. The typical ion source parameters were: capillary 5.5 kV, entrance potential (EP) 10 V, channel electron multiplier (CEM) 2300 V, source temperature 600 °C. Curtain Gas, GS1, and GS2 were set at 20, 60, and 50 psi, respectively. Quantification was performed in the multiple reaction-monitoring mode (MRM). Analytical data were processed using the software program Analyst (Version 1.6.2).
Pressurized fetal cerebral artery diameter monitoring
Artery diameter monitoring was performed following a general methodology described in our previous work (Bukiya, Vaithianathan, Kuntamallappanavar, Asuncion-Chin, & Dopico, 2011). An arterial segment from the fetal MCA tree was dissected out of the brain and cannulated at each end in a temperature-controlled, custom-made perfusion chamber using a Dynamax RP-1 peristaltic pump (Rainin Instruments, Inc., Oakland, CA). The chamber was continuously perfused at a rate of 3.75 mL/min with physiologic saline solution (PSS) (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose, and 24 NaHCO3. The PSS was equilibrated at pH 7.4 with a 21/5/74% mix of O2/CO2/N2 and maintained at 35–37 °C. Arteries were observed with a CCD camera (Sanyo VCB-3512T; Sanyo Electric Co., Moriguchi, Japan) attached to an inverted microscope (Nikon Eclipse TS100; Nikon). The artery external wall diameter was measured using the automatic edge-detection function of IonWizard software (IonOptics, Milton, MA) and was digitized at 1 Hz using a personal computer.
Steady-state changes in intravascular pressure were achieved by elevating an attached reservoir filled with PSS and were monitored using a pressure transducer (Living Systems Instruments, Burlington, VT). Arteries were first incubated at an intravascular pressure of 10 mmHg for 10 min. The intravascular pressure then was increased to 30 mmHg and held steady throughout the experiment to develop and maintain the arterial myogenic tone. Drugs were dissolved to make stock solutions, diluted in PSS to their final concentration, and applied to the artery via chamber perfusion. The effect of a drug application was evaluated at the time it reached a maximal, steady level.
Western immunoblotting
The technique was done as described previously (Zabirnyk, Liu, Khalil, Sharma, & Phang, 2010). Prior to loading, total protein level in each sample was quantified using BCA protein assay kit (Thermo Fisher Scientific, Waltham MA); samples were diluted to yield equal amounts of total protein in each sample. The anti-cannabinoid receptor 1 (ab3559; Abcam, Cambridge, UK) and anti-cannabinoid receptor 2 (ab3560; Abcam, Cambridge, UK) antibodies were used according to the manufacturer recommendations. Specificity of the anti-CB receptor antibodies was validated using non-transfected HEK cells versus adult mouse whole-brain lysate. Corresponding HRP-labeled secondary antibodies were used at the concentration of 1:10,000. Membranes were developed using SuperSignal West Pico chemiluminescent substrate kit (Thermo Scientific, Rockford, IL). Each sample has been probed in duplicate. Results were analyzed using ImageJ software (https://imagej.nih.gov/).
Quantitative PCR
The technique was applied as described previously (Zabirnyk et al., 2010). Total RNA was isolated using RNeasy Kit (Qiagen, Valencia, CA) and complementary DNA was synthesized using SuperScriptTM III First-Strand Synthesis SuperMix (Invitrogen, Carlsbad, CA). Expression of the gene of interest was measured by TaqMan quantitative PCR using Roche LC480 cycler (Roche Diagnostics Corporation, Indianapolis, IN). TaqMan probes labeled with a specific fluorophore and primers for genes of interest were both designed using the www.universalprobelibrary.com website against the cDNA sequences of Papio anubis and bought from IDT (IDT, Newark, NJ). The cDNA was pre-amplified using TaqMan PreAmp Master Mix Kit (Applied Biosystems, Foster City, CA). Kappa probe fast qPCR Kit Master mix (2x) Universal (Kappa Biosystems, Boston, MA) was used to run the Real-Time PCR. Each primer pair was validated for an efficiency of around 100%. The primer pairs and probes were as follows:
CB1: F ctggtgctgtgtgtcatcct, R aggctgccaatgaagtgatag, probe 75
CB2: F ctctgtgggtagcctgctg, R tttgtaggagggtgggtagc, probe 13
Beta-actin: F attggcaatgagcggttc, R tcaggacaccgtaggtgc, probe 11.
The samples were run in duplicate for both target and reference (beta-actin) genes. The results were analyzed using Light Cycler 480 software (Roche Diagnostics Corporation, Indianapolis, IN).
Chemicals
AEA, Tocrisolve, AM251, and AM630 were purchased from Tocris Bioscience (Bristol, United Kingdom). Ethanol (ultrapure, 200 proof) was purchased from American Bioanalytical (Natick, MA). To minimize the contact time between ethanol and humid air that could result in ethanol contamination with water, ethanol was aliquoted into smaller vials upon arrival. Each aliquot was used quickly, and vials were closed immediately after a dosage of ethanol was retrieved. All storage and handling of ethanol was performed per manufacturer’s recommendations. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistics
Final plotting, fitting, and statistical analysis of the data were conducted using Origin 8.5.1 (OriginLab) and InStat 3.0 (GraphPad). Due to the nature of our study (e.g., the use of non-human primates), sample sizes in our work are small (does not exceed 4). Statistical analysis was conducted on groups of data points using either paired or unpaired t test, as t test is sensitive for data sets with limited sample sizes (de Winter, 2013). p < 0.05 was considered statistically significant. Data are expressed as the mean ± S.E.M., and n/N = number of measurements/number of fetuses. Due to the small sample sizes, in some instances, our study is underpowered to detect subtle differences between the groups.
Results
Alcohol gastric infusion in a baboon model of mid-pregnancy
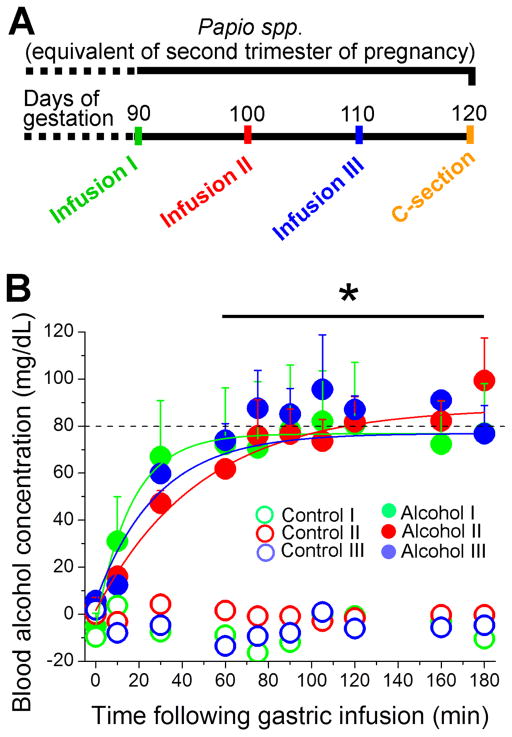
Four baboons underwent alcohol gastric administration (1.8 g/kg) at 90, 100, and 110 days of gestation (Fig. 1A), following the general layout of a previously published protocol (Kochunov et al., 2010) with some modifications, as detailed in Material and Methods. Considering that the total length of pregnancy in baboons is 180 days (that is, about two-thirds of the length of pregnancy in humans), the selected timing of drinking procedures corresponds to the second trimester of pregnancy in humans (Stevens, 1997). Upon alcohol infusion, maternal blood alcohol concentration reached approximately 80 mg/dL within 90 min (Fig. 1B). This pattern of drinking meets the definition of binge alcohol consumption, i.e., a pattern of drinking that results in a BAC of 80 mg/dL (https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking). In our experimental model, blood alcohol concentration remained at 80 mg/dL for up to 180 min following the drinking episode (Fig. 1B). For comparison, the control group of animals (3 baboons) received orange-flavored infusion fluid that was isocaloric to the alcohol-containing solution. Alcohol concentration in the blood of animals receiving control infusion was undetectable (Fig. 1B). Mass spectroscopy data from maternal blood serum failed to detect a significant difference in circulating anandamide (AEA) and 2-arachydonoylglycerol (2-AG) between control- and alcohol-infused groups.
Fig. 1. Alcohol gastric infusion in a baboon model of pregnancy.
A. Schematic representation of experimental design. Each pregnant baboon underwent three infusion episodes within the human equivalent of the 2nd trimester of pregnancy. Fetal cerebral arteries were harvested following Cesarean section at 120 days of baboon gestation. B. Alcohol level in maternal blood as a function of time following gastric infusion of alcohol-containing fluid (N = 4). Animals receiving control fluid did not have detectable amounts of alcohol in the blood (for visual clarity, data points from only one out of three baboons in the control-infused group are shown). Data at each time point in alcohol- versus control-receiving groups were compared using independent unpaired t tests. *Significantly different from blood alcohol level in control-receiving group, applicable to each alcohol-/control-receiving episode, p < 0.05.
Effect of fetal alcohol exposure on middle cerebral artery (MCA) function in vivo
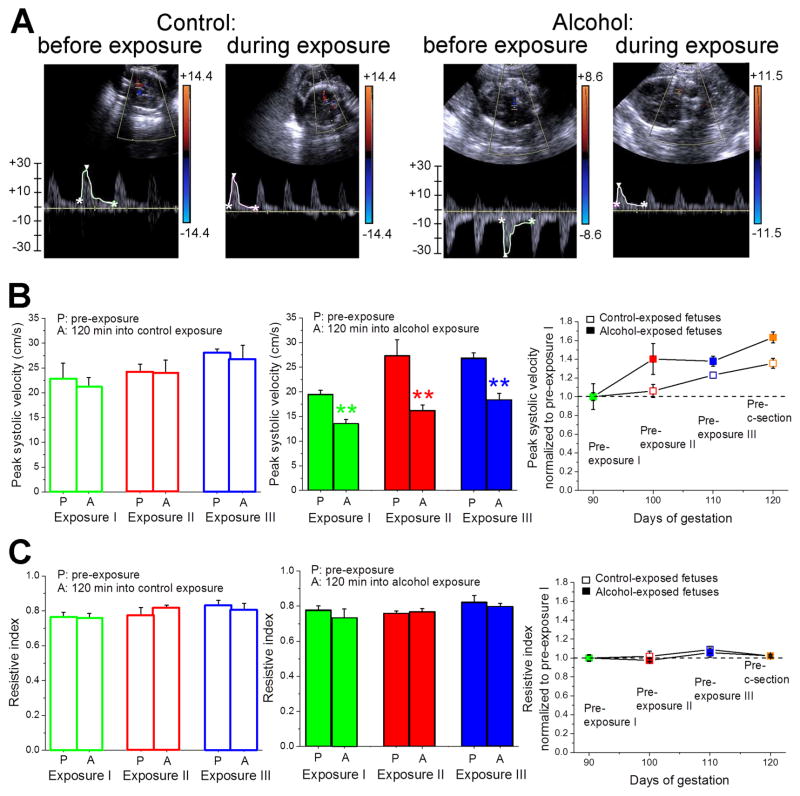
Peak systolic velocity (PSV) of fetal MCA at 120 min following each alcohol exposure episode was significantly diminished when compared to pre-exposure levels as revealed by Doppler sonography (p < 0.01) (Fig. 2A & B). As part of normal fetal development, PSV slightly increased during the course of the second trimester (Fig. 2B). Although there seemed to be a trend toward an increased fetal cerebral artery PSV in the alcohol-exposed group when compared to controls, this increase in PSV did not reach statistical significance (p > 0.05) (Fig. 2B, right panel). Finally, alcohol exposure did not affect the resistive index (RI) of fetal MCA (Fig. 2C). Furthermore, RI remained unchanged at 120 days of gestation. Overall, the reduced PSV of fetal MCA at 120 min following each alcohol exposure episode is consistent with the possibility that alcohol administration to the mothers during the second trimester of pregnancy causes dilation of fetal MCA.
Fig. 2. Doppler sonography evaluation of middle cerebral artery (MCA) function following maternal gastric infusion of either control- or alcohol-containing fluid.
A. Snapshots showing fetal MCA blood flow velocity before and 120 min following maternal gastric infusion of control (two left panels) vs. alcohol-containing fluid (two right panels). B. Averaged data showing a statistically significant drop in fetal cerebral artery peak systolic velocity following maternal gastric infusion of alcohol-containing but not control fluid. Maternal alcohol infusion does not affect gestational age-dependent increase in peak systolic velocity (panel on the right). In B and C, data at each exposure were compared using independent paired t tests. **Significantly different from pre-exposure level in corresponding exposure episode, p < 0.01. C. Averaged data showing lack of statistically significant difference in resistive index of fetal cerebral arteries from control- vs. alcohol-infused mothers. Control-infused: N = 3; alcohol-infused: N = 4.
Effect of fetal alcohol exposure on MCA function in vitro
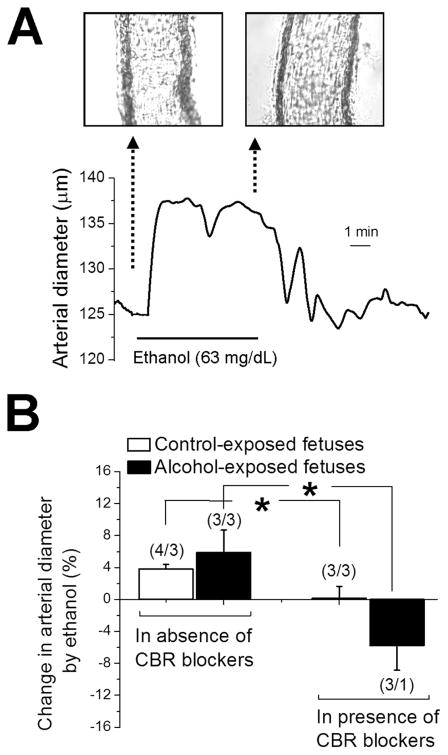
To test the hypothesis that ethanol exposure dilates fetal cerebral arteries, and to eventually begin the exploration of the cellular mechanisms contributing to this alcohol action, we switched to an in vitro model. At 120 ± 5 days of gestation, fetuses were harvested via C-section. Fetal middle cerebral arteries were dissected out and pressurized at 30 mmHg. At this time point during pregnancy, fetal cerebral arteries were consistently able to develop myogenic tone in response to 30 mmHg intravascular pressure. However, higher pressures sometimes resulted in arteries that failed to develop tone or respond to depolarization (see below). We did not find significant differences between fetal arteries from control- vs. alcohol-exposed fetuses for the following parameters: time to tone development, degree of myogenic tone, and arterial constriction in response to high (60 mM) KCl solution, an indicator of depolarization-induced constriction. However, ethanol (63 mg/dL; i.e., average concentration detected in amniotic fluid following maternal drinking) in vitro exposure for 10–15 min caused increases in diameter over pre-ethanol values in fetal arteries from both control- and alcohol-exposed fetuses: an average of 3.7 and 5.9%, respectively (Fig. 3A & B). Because diameter is linked to fluid flow by a power of 4 (Poiseuille’s law), ethanol-induced dilation of fetal cerebral arteries would result in ~16 and ~26% increase in blood flow for control- and alcohol-exposed fetuses, respectively. Thus, this ethanol-induced dilation of cerebral arteries in vitro is consistent with the decreased PSV observed in vivo and strongly suggests that the former is largely determined by vasodilation. More important, the fact that ethanol dilates isolated fetal cerebral artery segments under continuous perfusion indicates that this ethanol action does not require systemic metabolism (e.g., in liver) of alcohol, vasoactive factors from the systemic circulation, or intact autonomic regulation of arterial function. Rather, the molecular effectors of ethanol action are located within the fetal cerebral artery itself.
Fig. 3. Ethanol-induced dilation of in vitro pressurized fetal MCA.
A. Trace showing fetal cerebral artery dilation in response to 63-mg/dL ethanol. B. Bar graph displays averaged data: ethanol-induced dilation of fetal cerebral artery vanishes in presence of CB1 and CB2 receptor blockers AM251 and AM630, respectively. Data from control- and alcohol-exposed fetuses were compared using independent unpaired t tests. *Significantly different from data in absence of CBR blockers, p < 0.05. (n/N) = number of artery segments/number of fetuses.
The role of cannabinoid receptors in ethanol-induced dilation of pressurized fetal cerebral arteries
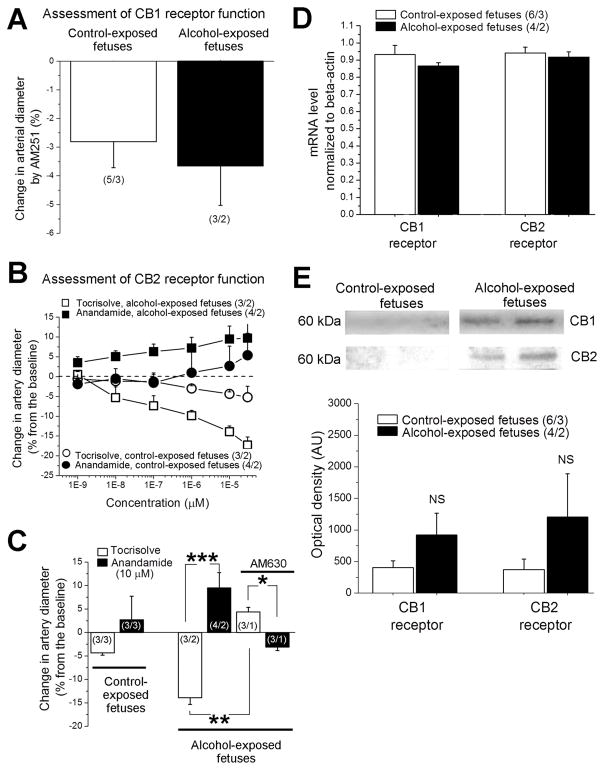
To address possible subcellular mechanisms mediating ethanol-induced dilation of fetal arteries, we focused on the eCBS. First, eCB signaling has been implicated in the development of ethanol-induced neurodegeneration in central neurons and FASD (Basavarajappa, 2015). Second, eCBS have been reported to regulate vascular diameter both in vitro and in vivo (Stanley & O’Sullivan, 2014). Third, a drop in brain perfusion may lead to cognitive dysfunction that resembles one observed with FASD (Amemiya et al., 2016). To begin to explore the involvement of the eCB’s in ethanol-induced dilation of fetal cerebral arteries, we first used pharmacological means to demonstrate any possible involvement of cannabinoid receptors in alcohol action. Indeed, ethanol-induced in vitro dilation of pressurized fetal cerebral arteries was completely prevented by the mixture of AM251 and AM630, which are antagonists of cannabinoid receptors CB1 and CB2, respectively (Fig. 3B). Next, we used selective pharmacological ligands (agonists and antagonists) to assess the functional contribution of endocannabinoid receptors CB1 and CB2 to the regulation of fetal cerebral artery diameter and its modulation by alcohol. Selective block of CB1 receptor with AM251 resulted in fetal cerebral artery constriction, which was similar in cerebral arteries from control- vs. alcohol-exposed fetuses (Fig. 4A). To assess CB2 receptor function, we perfused fetal cerebral arteries with the CB receptor agonist AEA. AEA was tested in the presence of AM251 (to block any possible effect of AEA via CB1 receptors) on arteries that were pre-constricted with high potassium (60 mM KCl) solution. Soybean oil-based vehicle Tocrisolve™ was used to deliver AEA to cerebral arteries. Thus, AEA-free Tocrisolve™ served as control for AEA effects. In cerebral arteries from control-exposed fetuses, AEA (sub μM) failed to evoke dilation when compared to Tocrisolve™ alone (Fig. 4B). Remarkably, AEA caused a concentration-dependent (EC50≈100 nM) dilation in arteries from alcohol-exposed fetuses, in spite of the constriction evoked by the solvent (Fig. 4B). Moreover, AEA-induced dilation of fetal cerebral arteries disappeared when AM251 was complemented with the CB2 receptor blocker AM630 (Fig. 4C). Although highly consistent (observed in three different arterial segments), this outcome must be interpreted with some caution, as the three segments were isolated from the same donor. Collectively, our data indicate that ethanol-induced in vitro dilation involves CB receptors, and strongly suggest that fetal cerebral artery CB2 receptor function is enhanced by alcohol exposure in utero.
Fig. 4. Evaluation of CB1 and CB2 receptor function in pressurized fetal cerebral arteries in vitro.
A. Averaged data showing similar degree of artery constriction in response to CB1 receptor blocker AM251; data were compared using unpaired t test. B. Concentration-response curves for anandamide in vehicle Tocrisolve™ and corresponding curves for anandamide-free Tocrisolve™ obtained from cerebral arteries of control- vs. alcohol-exposed fetuses in presence of AM251 and pre-constricted with KCl. C. Averaged data reflecting anandamide-induced cerebral dilation in arteries from alcohol-exposed fetuses; this dilation disappears when AM251 is complemented with CB2 receptor blocker AM630. Artery data from control-exposed fetuses were compared using unpaired t test. For alcohol-exposed fetuses, three sets of data were compared using independent unpaired t tests: Tocrisolve- versus anandamide-induced changes in artery diameter in absence and in presence of AM630, and Tocrisolve-induced changes in artery diameter in absence versus presence of AM630. *Significantly different from Tocrisolve-induced changes in diameter, p < 0.05. **Significantly different from Tocrisolve effect, p < 0.01. ***Significantly different from Tocrisolve effect in absence of AM630, p < 0.001. (n/N) = number of artery segments/number of fetuses. D. Averaged values of mRNA concentration normalized to beta-actin show no significant differences in gene expression of CB1 and CB2 receptors between control- and alcohol-exposed fetuses. In D and E, data for CB1 and CB2 receptors were compared using independent unpaired t tests. E. Representative images of Western blot data (top panels) in cerebral arteries from control- and alcohol-exposed fetuses. Artery homogenate samples contained equal amount of total protein. Bar graph shows averaged values of optical density corresponding to CB1 and CB2 protein levels. NS: not significant.
The increase in CB2 receptor function that mediates enhanced AEA-induced dilation of cerebral arteries in alcohol-exposed fetuses may be driven by several non-mutually exclusive mechanisms. They include increased gene expression or the receptor protein level, increased receptor affinity to the agonist, or functional changes driven by intracellular signaling downstream of cannabinoid receptor activation. We first found that CB1 and CB2 mRNA transcripts did not differ in cerebral arteries from control vs. alcohol-exposed fetuses (Fig. 4D). In addition, protein levels probed by Western blot did not show statistically significant differences between cerebral arteries of control- vs. alcohol-exposed fetuses (Fig. 4E). These results indicate that the differences in transcription or translation of CB1 and CB2 cannot explain the functional changes observed in arteries from alcohol-exposed fetuses. Rather, these changes should be attributed to modifications of ligand-receptor affinity and/or modified downstream signaling (see Discussion).
Discussion
In the current study, we identified for the first time that in vivo and in vitro exposure of fetal cerebral arteries from non-human primates (baboons) to ethanol at a period corresponding to the second trimester of human pregnancy evokes dilation of fetal cerebral arteries. This ethanol action is mediated by subcellular effectors located in the fetal cerebral artery itself: our study indicates that endocannabinoid receptors are the mediators of alcohol-induced dilation of fetal cerebral arteries. Moreover, based on a pharmacological approach with agonists and blockers of CB receptors, complemented with Western blotting and quantitative PCR, our study advances the idea that CB2 receptor function is increased in the cerebral arteries from fetuses exposed to alcohol in utero.
About a third of women who consume alcohol during pregnancy engage in binge drinking, i.e., drinking that results in a rapid rise of BAC reaching 80 mg/dL within 2 h (http://www.cdc.gov/media/releases/2015/p0924-pregnant-alcohol.html). Often, episodic moderate-to-heavy alcohol intake (such as during binge drinking) may produce much higher BACs (Diamond, 1991). As documented by numerous studies, a BAC at or above 80 mg/dL produces the most negative effects on the developing fetus (Maier & West, 2001; May & Gossage, 2011). Thus, it comes as no surprise that the FASD-affected births are most frequent in geographic areas with a high prevalence of alcohol binge drinking (May & Gossage, 2011; May et al., 2005; Viljoen et al., 2005). Importantly, our findings on the fetal cerebral circulation and eCBS were obtained in a model of alcohol exposure that rendered 80 mg/dL alcohol in maternal blood (Fig. 1).
Among all organs, the brain is the most sensitive to prenatal alcohol exposure, as the latter results in a wide range of structural and functional brain abnormalities (Nunez, Roussotte, & Sowell, 2011; Ramadoss, Lunde, Piña, Chen, & Cudd, 2007). Considering that the cerebral circulation provides key nutrients for effective neuronal function, the idea that alterations in fetal cerebral blood flow may accompany maternal alcohol consumption has been considered by several groups. Experimental work in sheep showed an attenuated adaptation of cerebral blood flow to neuronal damage in fetuses exposed to maternal binge drinking. This attenuated adaptation could not be explained by changes in vessel density but has been interpreted as arising from malfunctioning of cerebral blood vessels (Simon, Mondares, Born, & Gleason, 2008). Indeed, a recent study on a mouse model of pregnancy demonstrated that maternal binge drinking during the second trimester of pregnancy significantly reduced acceleration and the velocity time integral in fetal MCA (Bake, Tingling, & Miranda, 2012). Furthermore, our study demonstrates that alcohol administration to non-human primates during mid-pregnancy leads to a decreased PSV, as determined by Doppler ultrasound (Fig. 2). Collectively, these findings seem to indicate that maternal binge alcohol exposure may cause dilation of fetal cerebral arteries. The latter poses a substantial threat to fetal development (Hernandez-Andrade, Serralde, & Cruz-Martinez, 2012). Indeed, cerebral vasodilation may cause a drop in perfusion pressure, likely resulting in cognitive deficiency and skull malformation in children and young adults (Gordon, 2009; Yoon, Parsa, & Horton, 2013). Remarkably, these symptoms are similar to major features of FASD (Nunez et al., 2011).
However, our in vivo findings should be interpreted with caution – in our experimental model, the effect of alcohol was observed in the presence of anesthesia (i.e., induction with ketamine followed by maintenance using isoflurane). In rodents, however, ethanol was shown to promote a similar increase in cerebral blood volume in awake rats when compared to isoflurane-anesthetized animals (Luo et al., 2007). Moreover, a recent study of fetal cerebrovascular function in a mouse model also employed ethanol administration under isoflurane anesthesia (Bake et al., 2012). Because the control group in our study received anesthesia identical to that administered to alcohol-receiving baboons, a likely explanation for our in vivo findings is that ethanol itself is responsible for the changes in hemodynamics of fetal cerebral circulation. However, we cannot rule out that the final alcohol effect may be conditioned by the presence of ketamine (Silvestre, Pallarés, Nadal, & Ferré, 2002), with this fact adding a layer of complexity to the interpretation of our in vivo data.
A very critical finding from the current study is the demonstration that ethanol is able to evoke vasodilation in isolated in vitro pressurized fetal cerebral arteries (Fig. 3) – we documented alcohol-induced fetal artery dilation in the absence of complex neuronal circuitry and vasoactive factors from systemic circulation. Our findings may help to explain previously reported human data that demonstrated an alcohol-induced decrease in metabolic activity in some regions of human brain (Volkow et al., 1988; Wang et al., 2000). It should be underscored, however, that studies on arteries pressurized in vitro have their limitations. In particular, we cannot exclude the possibility that the complex neuronal integrity that is present in the intact neurovascular unit, or maternal vasoactive circulating factors, contribute to the in vivo response of fetal cerebral arteries to ethanol administration. Identification of all possible factors is daunting, yet deserves a thorough consideration and future experimental testing.
Our work also revealed that alcohol-induced dilation of fetal arteries disappeared in the presence of CB receptor blockers (Fig. 3). To our knowledge, this finding is the first ever to identify specific mechanisms of vasoactive effects of maternal alcohol on fetal cerebral arteries. Outside the vascular system, eCB components are known to be targeted by alcohol. For example, the neuronal eCB signaling system represents an important target for alcohol, and pharmacological modulation of neuronal eCBS has been proposed as an emerging therapy for treatment of alcohol-use disorders (Pacher, Bátkai, & Kunos, 2006; Pava & Woodward, 2012; Serrano et al., 2012; Sidhpura & Parsons, 2011). With regard to episodic drinking (such as binge drinking), CB1 receptor-mediated agonism of the endocannabinoid system has been implicated in modulating binge-like alcohol intake in mouse models (Agoglia, Holstein, Eastman, & Hodge, 2016; Linsenbardt & Boehm, 2009; Zhou, Huang, Lee, & Kreek, 2016).
In the current work, we failed to detect changes in transcript or protein levels of CB1 and CB2 receptors within cerebral arteries from alcohol-exposed fetuses when compared to control fetuses (Fig. 4D–E). However, alcohol exposure in utero enhanced fetal cerebral artery dilation in response to the pharmacological agonist AEA when CB1 receptor was selectively blocked by AM251. The increased response of the CB2 receptor to constantly perfused AEA in the absence of increased CB2 protein level strongly suggests an enhanced function of CB2 receptors. This enhanced functionality may result from several not mutually exclusive pathways, such as increased affinity of CB2 receptor to AEA, enhanced efficiency of translating AEA binding into the receptor activation, and increased downstream signaling effectors of CB2 activation. The possible role of endocannabinoid production within the fetal cerebral artery and its modulation by the exogenously introduced pharmacological agents should also be explored. These studies are currently under way.
Noteworthy, the CB2 receptor’s upregulation was shown to contribute to the delayed neuroprotective effect against cerebral ischemia induced by MCA occlusion in rats (Ma et al., 2011). It is tempting to speculate that enhanced CB2 receptor function observed in cerebral arteries of alcohol-exposed fetuses might represent a protective mechanism against a repeated drop in fetal artery PSV in the presence of alcohol.
In synthesis, our study presents the first robust evidence that the key components of the fetal cerebral artery eCBS (CB2) are targeted by alcohol exposure in utero, underscoring that elements of this system, the EC receptors in particular, are major determinants of the vasodilatory effect of alcohol on fetal cerebral arteries. These findings may pave the road to a better understanding of the pathophysiology of FAS/FASD as a result of hemodynamic dysregulation in the fetal brain, as well as to open up a potential therapeutic application of CB receptor inverse agonists (such as rimonabant) as a rescue therapy to normalize fetal cerebral circulation caused by maternal alcohol consumption. Rimonabant blocks psychoactive effects and several cardiovascular effects of Δ9-tetrahydrocannabinol (THC) in humans (Huestis et al., 2001). Interestingly, our data show that ethanol dilation of fetal cerebral arteries is totally blunted by the combination of AM251 and AM630, which are both structurally close to rimonabant. Further in vivo studies are imperative to develop endocannabinoid antagonists/inverse agonists with no major side effects to consider their potential use in ameliorating negative consequences of maternal alcohol consumption that involves disruption of fetal arterial function.
Highlights.
Mechanisms of fetal brain damage by alcohol are largely unclear.
Baboons were used to study alcohol’s effect on fetal cerebral arteries.
Alcohol dilates fetal cerebral arteries via cannabinoid receptors.
Alcohol exposure enhances cerebral artery CB2 receptor responses to anandamide.
Alcohol exposure does not change CB1 and CB2 mRNA or protein levels.
Acknowledgments
The authors deeply thank Dr. Natalia Schlabritz-Lutsevich for her contribution to the conceptual design of the work and development of the binge alcohol infusion protocol in the baboon model of pregnancy at UT HSC. The authors also thank Shivantika Bisen and Jennifer Chang for their assistance in preparation of experimental saline solutions. This work was supported by NIH R21 AA022433 (ANB), and in part by the Office of the Director, National Institutes of Health, under Award Number P40OD010988. The LC-MS/MS instrument was acquired via shared instrumentation grant S10OD016226, Office of the Director, National Institutes of Health. The authors would like to dedicate the findings of this work to the memory of Terry Costello, who passed away unexpectedly during the revision of this manuscript.
Footnotes
Author contributions
ANB, AMD, and OS designed the research. AT, JSJ, RS, DM, PS, SD, YA, DLT, TC, SB, WL, OS, and ANB acquired data. AT, DM, OS, and ANB analyzed data. OS, AT, JSJ, RS, DM, GM, and ANB wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoglia AE, Holstein SE, Eastman VR, Hodge CW. Cannabinoid CB1 receptor inhibition blunts adolescent-typical increased binge alcohol and sucrose consumption in male C57BL/6J mice. Pharmacology, Biochemistry, and Behavior. 2016;143:11–17. doi: 10.1016/j.pbb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya S, Takahashi K, Mima T, Yoshioka N, Miki S, Ohtomo K. Reversible alterations of the neuronal activity in spontaneous intracranial hypotension. Cephalalgia. 2016;36:162–171. doi: 10.1177/0333102415585085. [DOI] [PubMed] [Google Scholar]
- Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcoholism: Clinical and Experimental Research. 2012;36:748–758. doi: 10.1111/j.1530-0277.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Farro J, Gonzales S, Helms C, Grant KA. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcoholism: Clinical and Experimental Research. 2014;38:2835–2843. doi: 10.1111/acer.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS. Fetal Alcohol Spectrum Disorder: Potential Role of Endocannabinoids Signaling. Brain Sciences. 2015;5:456–493. doi: 10.3390/brainsci5040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyó Z, Ruisanchez É, Leszl-Ishiguro M, Sándor P, Pacher P. Endocannabinoids in cerebrovascular regulation. American Journal of Physiology Heart and Circulatory Physiology. 2016;310:H785–H801. doi: 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:2410–2423. doi: 10.1161/ATVBAHA.111.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Wood E, Jabbour L. Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Research Part C, Embryo Today: Reviews. 2016;108:174–180. doi: 10.1002/bdrc.21129. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Bowden DM. Fetal alcohol syndrome: a new primate model for binge drinking and its relevance to human ethanol teratogenesis. The Journal of Pediatrics. 1982;101:819–824. doi: 10.1016/s0022-3476(82)80340-5. [DOI] [PubMed] [Google Scholar]
- Czikora Á, Lizanecz E, Boczán J, Daragó A, Papp Z, Édes I, et al. Vascular metabolism of anandamide to arachidonic acid affects myogenic constriction in response to intraluminal pressure elevation. Life Sciences. 2012;90:407–415. doi: 10.1016/j.lfs.2011.12.016. [DOI] [PubMed] [Google Scholar]
- de Winter JC. Using the Student’s t-test with extremely small sample sizes. Practical Assessment, Research & Evaluation. 2013;18:1–12. [Google Scholar]
- Diamond I. Alcoholism and Alcohol Abuse. In: Wyngaarden JB, Smith LH, Bennett JC, editors. Cecil’s Textbook of Medicine. 19. Philadephia: WB Saunders Co; 1991. pp. 44–47. [Google Scholar]
- Engeli S, Blüher M, Jumpertz R, Wiesner T, Wirtz H, Bosse-Henck A, et al. Circulating anandamide and blood pressure in patients with obstructive sleep apnea. Journal of Hypertension. 2012;30:2345–2351. doi: 10.1097/HJH.0b013e3283591595. [DOI] [PubMed] [Google Scholar]
- Gauthier TW. Prenatal Alcohol Exposure and the Developing Immune System. Alcohol Research. 2015;37:279–285. doi: 10.35946/arcr.v37.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. Spontaneous intracranial hypotension. Developmental Medicine and Child Neurology. 2009;51:932–935. doi: 10.1111/j.1469-8749.2009.03514.x. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacology & Therapeutics. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Stafford J, Thiede A, Kiley C, Odagiri M, Ferguson B. Who is at risk? Population characterization of alcohol self-administration in nonhuman primates helps identify pathways to dependence. Alcohol Research & Health. 2008;31:289–297. [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Andrade E, Serralde JA, Cruz-Martinez R. Can anomalies of fetal brain circulation be useful in the management of growth restricted fetuses? Prenatal Diagnosis. 2012;32:103–112. doi: 10.1002/pd.2913. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of General Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Castro C, Davis DM, Dudley D, Wey HY, Purdy D, et al. Fetal brain during a binge drinking episode: a dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport. 2010;21:716–721. doi: 10.1097/WNR.0b013e32833b5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lípez-Miranda V, Herradón E, Martín MI. Vasorelaxation caused by cannabinoids: mechanisms in different vascular beds. Current Vascular Pharmacology. 2008;6:335–346. doi: 10.2174/157016108785909706. [DOI] [PubMed] [Google Scholar]
- Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biological Psychiatry. 2016;79:516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, et al. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. Journal of Magnetic Resonance Imaging. 2007;26:557–563. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhu Z, Zhao Y, Hou L, Wang Q, Xiong L, et al. Cannabinoid receptor type 2 activation yields delayed tolerance to focal cerebral ischemia. Current Neurovascular Research. 2011;8:145–152. doi: 10.2174/156720211795495394. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Research & Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Research & Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Research & Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Research & Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais AS, Hendricks LS, et al. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. American Journal of Public Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Moore EM, Thomas JD, Riley EP. Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies. Alcohol Research. 2015;37:97–108. doi: 10.35946/arcr.v37.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez CC, Roussotte F, Sowell ER. Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Research & Health. 2011;34:121–131. [PMC free article] [PubMed] [Google Scholar]
- Olivier L, Curfs LM, Viljoen DL. Fetal alcohol spectrum disorders: Prevalence rates in South Africa. South African Medical Journal. 2016;106(6 Suppl 1):S103–S106. doi: 10.7196/SAMJ.2016.v106i6.11009. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcoholism: Clinical and Experimental Research. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Haskó G, Huffman JW, Mackie K, Pacher P. CB2 cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. British Journal of Pharmacology. 2008;153:347–357. doi: 10.1038/sj.bjp.0707569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Piña KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcoholism: Clinical and Experimental Research. 2007;31:1252–1258. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Serrano A, Rivera P, Pavon FJ, Decara J, Suárez J, Rodriguez de Fonseca F, et al. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcoholism: Clinical and Experimental Research. 2012;36:984–994. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. 2011;61:1070–1087. doi: 10.1016/j.neuropharm.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Pallarés M, Nadal R, Ferré N. Opposite effects of ethanol and ketamine in the elevated plus-maze test in Wistar rats undergoing a chronic oral voluntary consumption procedure. Journal of Psychopharmacology. 2002;16:305–312. doi: 10.1177/026988110201600404. [DOI] [PubMed] [Google Scholar]
- Simon KE, Mondares RL, Born DE, Gleason CA. The effects of binge alcohol exposure in the 2nd trimester on the estimated density of cerebral microvessels in near-term fetal sheep. Brain Research. 2008;1231:75–80. doi: 10.1016/j.brainres.2008.06.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C, O’Sullivan SE. Vascular targets for cannabinoids: animal and human studies. British Journal of Pharmacology. 2014;171:1361–1378. doi: 10.1111/bph.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VC. Some reproductive studies in the baboon. Human Reproduction Update. 1997;3:533–540. doi: 10.1093/humupd/3.6.533. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Grimsrud KN, Midic U, Mtango N, Latham KE. Transgenerational effects of binge drinking in a primate model: implications for human health. Fertility and Sterility. 2015;103:560–569. doi: 10.1016/j.fertnstert.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, et al. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. Journal of Studies on Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, et al. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Research. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, et al. Regional brain metabolism during alcohol intoxication. Alcoholism: Clinical and Experimental Research. 2000;24:822–829. [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcoholism: Clinical and Experimental Research. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Yoon MK, Parsa AT, Horton JC. Skull thickening, paranasal sinus expansion, and sella turcica shrinkage from chronic intracranial hypotension. Journal of Neurosurgery. 2013;11:667–672. doi: 10.3171/2013.2.PEDS12560. [DOI] [PubMed] [Google Scholar]
- Zabirnyk O, Liu W, Khalil S, Sharma A, Phang JM. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis. 2010;31:446–454. doi: 10.1093/carcin/bgp299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang T, Lee F, Kreek MJ. Involvement of Endocannabinoids in Alcohol “Binge” Drinking: Studies of Mice with Human Fatty Acid Amide Hydrolase Genetic Variation and After CB1 Receptor Antagonists. Alcoholism: Clinical and Experimental Research. 2016;40:467–473. doi: 10.1111/acer.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]