Abstract
Introduction
There is a clinical need to improve the outcomes of peripheral nerve regeneration and repair after injury. In addition to its immunosuppressive effects, FK506 (tacrolimus) has been shown to have neuroregenerative properties. To determine biologically relevant local FK506 and growth factor concentrations, we performed an in vitro bioassay using dorsal root ganglion (DRG) from chicken embryos.
Methods
Neurite elongation and neurite branching were analyzed microscopically after addition of FK506, glial cell line-derived neurotrophic factor (GDNF), and nerve growth factor (NGF), each alone and in combination.
Results
FK506 induced modest neurite elongation (~500–800 μm) without improving neurite branching significantly. The combination of FK506 with NGF, GDNF, or both, exerted a potentiating or competitive effect on neurite elongation (~700–1100 μm) based on dosage and competitive effect on neurite branching (~0.2–0.4).
Conclusions
These results strongly suggest that the interaction of FK506 with GDNF and NGF mediates distinct enhancement of neurite growth.
Keywords: dosage, FK506, GDNF, nerve regeneration, Neurite growth in vitro, NGF, potentiation
Peripheral nerve injuries have been a clinical challenge over the years.1 The extensive network of nerve tissue throughout the body makes peripheral nerves prone to external trauma.2 Peripheral nerve injuries frequently produce weakness, chronic pain, and neuropathies that lead to severe disability.3 Nerve autograft has been the gold standard for treatment of injuries that result in a nerve gap.1 Autograft involves harvesting donor nerve tissue and implanting the graft across the nerve gap.3 Nerve autografts have been comparatively successful, but there are problems associated with donor tissue availability,2 obtaining a donor nerve by means of single or multiple surgeries, multiple scarring sites, neuroma formation, and limited success at repairing gaps larger than 10 mm.2 These issues led to fabrication of hollow, tube-like nerve guiding conduits to span gaps in nerves and provide structural support for the transected nerve.4 These nerve guides, although structurally helpful, do not effectively replicate a nerve autograft in terms of support cells and neurotrophism and thus are not as effective at repairing large nerve gaps.4 Following a peripheral nerve injury, neurites will tend to regrow and cross short gaps, but they need guidance and the ability to overcome the scarring process.5
To improve the repair process, in addition to providing guidance, therapeutic agents need to be provided at the site of transection or injury.6 Several drugs and growth factors have been shown to be effective in enhancing neurite outgrowth across nerve gaps.6 A study of nerve growth factor (NGF)-loaded microspheres has shown potential for repair of nerve gaps.7 Collagen tubes loaded with NGF alone or in combination with glial cell line-derived neurotrophic factor (GDNF) have produced neurite elongation in vitro.8 Use of NGF or GDNF within a specified concentration range has resulted in improved neurite growth in chick dorsal root ganglia (DRG).9 Several papers suggest that improved peripheral nerve regeneration can be expected by supplying neurotrophins.5,10
The data obtained from several in vivo studies have indicated that NGF and GDNF synthesis is also upregulated during an inflammatory process.11,12 After a peripheral nerve injury, proliferating and reactive Schwann cells produce growth factors, cytokines, and growth-associated proteins, which play key roles in axon regeneration and nerve repair.13,14 It has been observed that exogenously administered NGF and GDNF increase both the number and myelination of regenerating axons.15,16 This is due to effects of NGF and GDNF signaling both on regenerating nerve fibers and on Schwann cells and inflammatory cells,17 and Schwann cell migration is thought to precede and promote axon elongation into repair sites.17
FK506 was approved by the FDA in 1994 for liver transplants and is one of the main systemic immunosuppressants used to prevent nerve allograft rejection.18 It has also been observed to have neuroregenerative properties when administered after peripheral nerve injuries.19,20 It can enhance the activity of NGF by increasing the sensitivity of cells toward smaller concentrations of the growth factor.21,22 The complex of FK506 with FK506-binding protein-12 inhibits the phosphatase activity of calcineurin, resulting in accumulation of phosphorylated substrates, including nuclear factor of activated T-cells. This phosphorylated nuclear factor of activated T-cells functions as a regulator of the transcription of numerous genes, including interleukin-2 and, therefore, induces the immunosuppressive effects of FK506.23
There is increasing evidence that the beneficial effects of FK506 on neuroregeneration are unrelated to calcineurin inhibition and thus immunosuppression.19,21 This first became evident as studies began to demonstrate that cyclosporine A, another immunosuppressant that works through calcineurin inhibition, does not have the same neuroregenerative properties.23 Several research groups have demonstrated that nonimmunosuppressive FK506-binding protein ligands lack the ability to bind calcineurin but promote neurite growth in vitro and stimulate regeneration of peripheral nerves in rats.24 Some studies have suggested that the neuro-regenerative effects of FK506 might result from interactions with FK506-binding protein 52.25 While several in vivo small animal studies have demonstrated improved rates of nerve regeneration with systemic FK506,26–30 not many detailed studies have confirmed the dosage activity profile of FK506 treatment alone or in combination with other growth factors. For this purpose, we have developed an in vitro assay using chicken embryonic DRGs which exhibit similarities to other animal neuronal systems.
The study we report here also assessed the regulation of protein kinase B (Akt) and phosphorylated Akt (pAkt) in the process of neurite growth after single and combined treatment with FK506 and neurotrophins. Akt is a multifunctional regulator of cell survival, growth, and glucose metabolism.31–33 It functions as a major downstream target of phosphatidylinositol 3-kinase (PI3-K), and the PI3-K pathway is involved in nerve growth factor-dependent neuronal survival.33–35 Data suggest that FK506 treatment enhances nerve growth through activation of the Ras/Raf/MAP kinase signaling pathway downstream of PI3K-Akt.28 GDNF also activates the Ras/MAP kinase and PI3K/Akt pathways.36 The goal of this study was to determine how neurite growth is affected by FK506, NGF, GDNF, and the combinations of FK506 with either NGF or GDNF or both in terms of neurite length and branching.
MATERIALS AND METHODS
DRG-Explant Cultures
Fertilized chicken eggs (Merrills Poultry, Paul, Idaho) were incubated at ~39 °C under 100% relative humidity for 12 days. The eggs were first cleaned with 70% ethanol and then opened to collect the embryos. DRGs were dissected from the embryos under a stereomicroscope using a standard dissection procedure.37 They were separated carefully from connective tissue for culturing in 24-well plates coated with laminin (1 μg/ml). Dulbecco Modified Eagle Medium (DMEM) F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% Antimycotic/Antibiotic solution were added to each well. DRGs were plated at a density of 1 per well, and growth factors were added as specified below. Cultures were maintained in a humid atmosphere at 37 °C and 5% CO2 for 72 h. Unless specified otherwise, all reagents for cell culture were purchased from Fisher Scientific (Pittsburgh, Pennsylvania).
Drug Dosing
For each experimental condition including control, 4 DRGs of similar size were used, and the experiments were repeated 3 to 4 times. In the single growth factor experiments, the DRGs were treated with increasing concentrations of either NGF (Sigma Aldrich, St. Louis, Missouri) (0.1, 1, 5, 10, 100 ng/ml), GDNF (R&D systems, Minneapolis, Minnesota) (0.1, 1, 5, 10, 100 ng/ml), or FK506 (Astellas Pharma, Northbrook, Illinois) (1, 5, 10, 20, 50 ng/ml). For control, DRG-explants were incubated in cell culture medium matrix (DMEM+10% FBS+1% Antimycotic) without any growth factor or drug. In the experiments using combined growth factors (NGF/GDNF, NGF/FK506, GDNF /FK506, and NGF/GDNF/FK506), the DRGs were treated with random dosages chosen using the statistical software Minitab 17. All possible combinations for each group were subjected to a fractional factorial design in the software, which both randomly and systematically selected dosages that were tested to predict the response of remaining combinations.38
We used a fractional factorial design, because for experiments with many factors and levels, full factorial designs can lead to large amounts of data. Fractional factorial designs use a fraction of the runs required by full factorial designs. A subset of experimental treatments is selected based on an evaluation (or assumption) of which factors and interactions have the most significant effects.39 The neurotrophic factor dilutions were prepared with the cell culture medium matrix (DMEM+10% FBS+1% Antimycotic).
Quantification of Neurite Outgrowth: Neurite Length and Branching
After 72 h in culture, DRGs were fixed in methanol, imaged using a widefield microscope (Nikon Spinning disk) with a phase contrast lens, and images were captured with a digital camera at 4 × magnification. Phase contrast images of DRGs were taken after 3 days of incubation, and the average neurite length and branching were measured.
Neurite length and branching measurements were done using a previously discussed procedure.9 The area of the ganglion body (ADRG) and the total area of the DRG with the growing neurites (Atot) were measured using ImageJ 1.31v software (National Institutes of Health, Bethesda, USA). Average neurite length (lavg) was calculated by: lavg = (Atot/π)1/2 − (ADRG/π)1/2. Neurite branching was calculated from the area occupied by the neurites (Aneurite), which was determined using the threshold function (range of 100 ± 25 to 230 ± 25 pixels) available in ImageJ. Neurite branching = Aneurite/(Atot − ADRG)
DRG Neurite Growth Rate
Images of DRGs were taken every 24 h during 3 days of incubation, and average neurite length was measured at each time point as described above. The cultured explants were not fixed for the growth rate study. From these measurements, the average neurite growth rate was determined.
DRG Lysate and Western Blot
Six DRGS were plated per well in a 6-well plate and treated with different single and combined dosages for 72 h. Cells were then lysed in Cell Extraction Buffer (Bio-source) according to the manufacturer’s protocol. Cell lysates suspended in Laemmli sample buffer (Bio-Rad, Hercules, California) were heated at 95 °C for 5 min, separated by SDS-PAGE gel (Bio-Rad), and transferred to a polyvinylidene difluoride membrane (EMD Millipore, Massachusetts). Membranes were incubated with the following primary antibodies: AKT (1:1,000, Cell Signaling Technology) and pAKT (1:2,000, Cell Signaling Technology). After washing with phosphate buffered saline (PBS) containing 0.1% Tween, membranes were incubated with a 1:5,000 dilution of horseradish peroxidase conjugated Anti-Rabbit secondary antibody (Cell Signaling Technology). Antibody was detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Massachusetts). To control for protein loading, membranes were stripped and probed with a 1:5,000 dilution of mouse anti-GAPDH (Novus Biologicals, Littleton, Colorado). Scanned autoradiographic blot images were quantified using the NIH ImageJ densitometry software.
Immunocytochemistry and Fluorescence Microscopy
DRGs were cultured for 72 h and then fixed with methanol for 10 min. Cells were washed with PBS, incubated with PBS containing Triton X-100, treated with blocking buffer containing 1% BSA (bovine serum albumin) at room temperature for 30 min, and incubated with primary antibody (β tubulin 1:200 dilution in 1% BSA in PBS-Triton X100) overnight at 4 °C. Cells were then washed and incubated for 1 h at room temperature with a secondary antibody (Rabbit 568 red 1:500 dilution in 1% BSA). Image acquisition was accomplished using Nikon Spinning disk widefield microscope and NIS Elements software.
Statistical Analysis
The data were analyzed using a multivariable linear regression model in StataIC 13. The outcome variable was neurite length and neurite branching. Specific doses of a given drug or drug combination were included in the model as indicator variables rather than assuming a specific form of dose-response, such as a linear increase across the range of doses. Interaction terms were used to test if the effects of drug combinations were greater than the sum of the effects of individual drugs. Comparisons between specific drug–dose combinations were made by varying the referent drug–dose combination in the included indicator variables, or using Wald posttest comparisons, where either approach produced comparisons analogous to independent sample t-tests. All reported P-values are for 2-sided comparisons. P-values ≤0.05 were considered significant.
In our experiments, the goal was to identify the drug(s)-dose(s) combination that provided the greatest response or to observe some range of effective doses. Statistical comparisons are provided merely descriptively to indicate where the maximum or plateau of the curve separates from the ineffective drug(s)-dose(s) combinations.
RESULTS
Neurite Elongation
Single Growth Factor
FK506 was observed to induce neurite elongation in the lower concentration range, and the average neurite length of DRGs exposed to 1 (633.1 ± 65.3 μm), 5 (682.0 ± 61.0 μm), and 10 (751.3 ± 38.0 μm) ng/ml had statistically longer neurite extensions than the control group (0 ng/ml; 469.7 ± 22.1 μm; P < 0.05) [Figs. (1 and 2)a]. In contrast, there was no difference in neurite length between DRGs exposed to 20 ng/ml FK506, 50ng/ml FK506, or control. Treatment of the DRGs with NGF concentrations of 0.1 (910.5 ± 39.8 μm), 1 (993.7 ± 53.6 μm), 5 (1063.5 ± 57.8 μm), 10 (1117.6 ± 55.6 μm) ng/ml, and 100 ng/ml (755.7 ± 57.8 μm) enhanced neurite elongation compared with control (469.7 ± 22.1 μm; P < 0.05) (Fig. 2b).
FIGURE 1.

Phase contrast images of DRGs treated with different concentrations of FK506: control (left), 1 ng/ml (center), 10 ng/ml (right) (a); NGF: control (left), 1 ng/ml (center), 10 ng/ml (right) (b); GDNF: control (left), 1 ng/ml (center), 10 ng/ml (right) (c). Neurite outgrowth can be seen based on different doses of FK506, NGF, and GDNF. Scale = 500 μm.
FIGURE 2.

Plots showing effect of single drug treatments on axonal elongation. (a) FK506 Dosage/length. (b) NGF Dosage/length. (c) GDNF Dosage/length. The bars represent mean ± SD for n = 4 (* statistically different from 0 ng/ml; P < 0.05).
Combination Treatment
The combined response of 2 drugs was compared with control and with their individual doses to determine the response. The response was found to be potentiating when the combination treatment resulted in a better response than what was obtained individually. Certain treatment responses were found to be competitive when the combined dosage resulted in reduction of growth compared with the individual response. If there was no significant change in growth with the combination treatment when compared with single treatments, the response was listed as noncompetitive.
The combined treatment of FK506 and NGF showed increased neurite elongation compared with their corresponding individual doses (Fig. 3b and 3c). The combination of NGF with FK506 treatment was potentiating and showed increasing neurite elongation with increasing NGF concentration except when NGF was combined with 1 ng/ml FK506, which was competitive (Fig. 4a). Higher FK506 concentrations (20–100 ng/ml), while inhibitory on their own, exhibited significantly increased elongation in combination with NGF and GDNF (seen in shaded region of Fig. 4a). The best combinations were found to be 1 ng/ml NGF with 5– 10 ng/ml FK506, 100 ng/ml NGF with 50 ng/ml FK506, and 10 ng/ml NGF with 20 ng/ml FK506 (P < 0.05).
FIGURE 3.
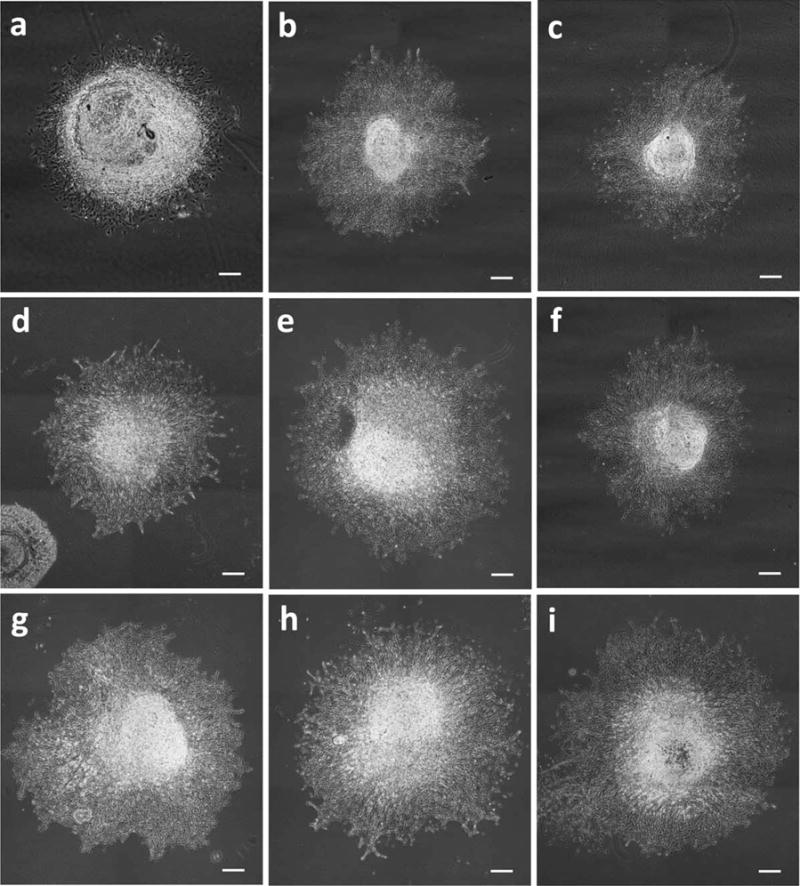
Phase contrast images of DRGs treated with growth factor combinations (starting from top left). (a) control (0 ng/ml). (b) NGF 1 ng/ml + FK506 1 ng/ml. (c) NGF 1 ng/ml + FK506 10 ng/ml. (d) GDNF 1 ng/ml + FK506 1 ng/ml. (e) GDNF 1 ng/ml + FK506 10 ng/ml. (f) NGF 1 ng/ml + GDNF 1 ng/ml + FK506 1 ng/ml. (g) NGF 1 ng/ml + GDNF 1 ng/ml + FK506 10 ng/ml. (h) NGF 10 ng/ml + GDNF 10 ng/ml + FK506 10 ng/ml. (i) NGF 1 ng/ml + GDNF 1 ng/ml. Neurite outgrowth is increased with combined treatment of FK506 with growth factors. Scale = 500 μm.
FIGURE 4.
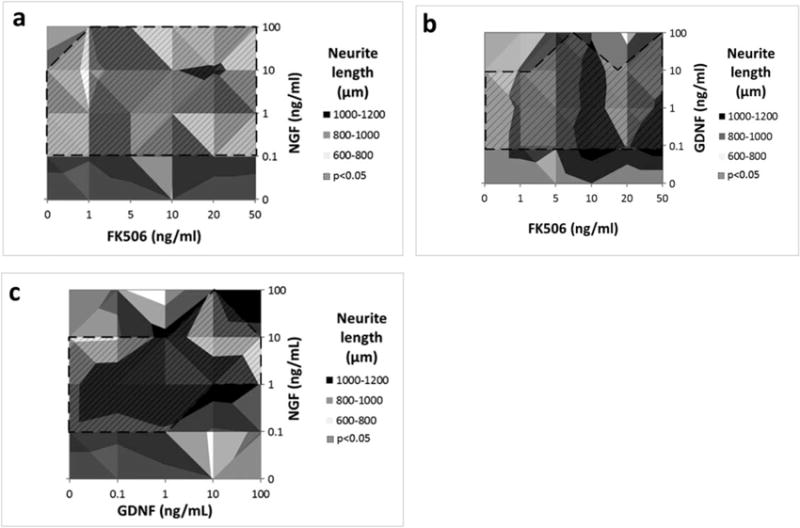
Surface plots showing effect of combined drug treatments on axonal elongation. (a) NGF+FK506 combined dosage. (b) GDNF+FK506 combined dosage. (c) NGF+GDNF combined dosage. (Shaded region with dashed lines specifies the concentration range which produced significantly different axonal elongation response from 0 ng/ml, P < 0.05).
The combined treatment of FK506 and GDNF showed significant increased neurite elongation (shaded region of Figure 4b) as compared with their individual treatments (Figs. 2a,b). The combined treatment of FK506 and GDNF was potentiating for combinations of 1–10 ng/ml of GDNF with 1–10 ng/ml of FK506 (Fig. 3d,e and 5c), and for combination of GDNF 0.1 ng/ml with FK506 50 ng/ml (P < 0.05). All other combinations showed a noncompetitive response (P > 0.05).
FIGURE 5.
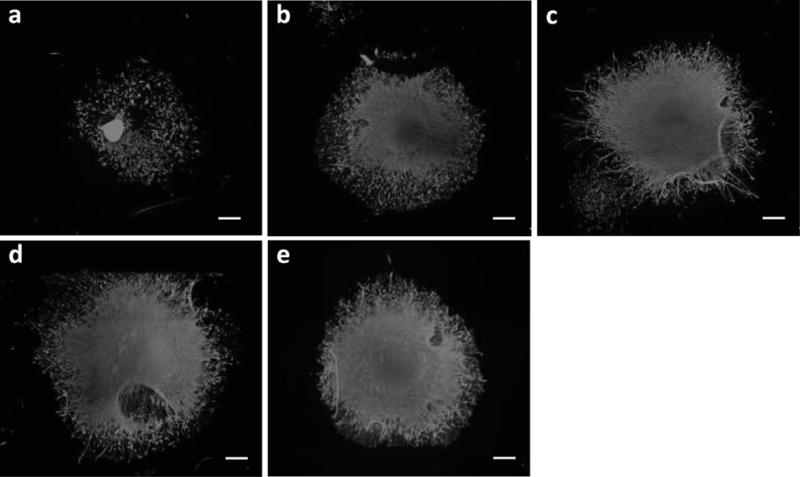
Fluorescence images of DRGs stained with β-tubulin. (a) control (0 ng/ml). (b) NGF 1 ng/ml + FK506 10 ng/ml. (c) GDNF 1 ng/ml + FK506 10 ng/ml. (d) NGF 1 ng/ml + GDNF 1 ng/ml + FK506 1 ng/ml. (e) NGF 1 ng/ml + GDNF 1 ng/ml. The images show enhanced neurite elongation with the specified treatments.
Combined GDNF and NGF had a potentiating effect and produced significant enhancement of neurite outgrowth as compared with individual treatments, shown in shaded regions of Figure 4c. The plots suggest a strong effect of increasing NGF concentration (0.1, 1, and 10 ng/ml). The average neurite length at the optimal GDNF+NGF concentrations (1 ng/ml NGF combined with 1 or 0.1 ng/ml GDNF) (P < 0.05) was 1,000–1,100 μm, which is higher than the 800–950 μm neurite elongation observed with NGF or GDNF alone (Fig. 3i and 5e). Altogether, the results indicate a strong interaction of both growth factors. Statistically, combinations of 1 ng/ml NGF with either 0.1 ng/ml, 1 ng/ml, or 10 ng/ml of GDNF were found to produce the best neurite growth response (P < 0.05 when compared with control).
Combination treatment with FK506, NGF, and GDNF produced both potentiating and competitive responses on neurite elongation (Table 1). This triple combination group mediated the greatest neurite elongation when compared with all other treatments with 1 ng/ml of each NGF, GDNF, and FK506 enhancing growth to the greatest extent (1282.4 ± 25.1 μm) (P < 0.05). All combinations had a potentiating response except for the combinations with 100 ng/ml of NGF and 100 ng/ml of GDNF (P > 0.05), which showed a competitive response.
Table 1.
Effect of NGF+GDNF+FK506 combined dosage on neurite elongation.*
| FK506 (ng/ml) | NGF (ng/ml) | GDNF (ng/ml) | Neurite length (μm) |
|---|---|---|---|
| 0 | 0 | 0 | 417.4 |
| 1 | 1 | 1 | 1282.4 |
| 1 | 5 | 10 | 834.1 |
| 1 | 10 | 5 | 765.3 |
| 5 | 5 | 100 | 966.6 |
| 5 | 10 | 1 | 889.1 |
| 5 | 100 | 5 | 781.6 |
| 10 | 1 | 1 | 905.7 |
| 10 | 5 | 1 | 843.8 |
| 10 | 10 | 10 | 1025.7 |
| 10 | 10 | 10 | 1036.1 |
| 20 | 1 | 10 | 1005.7 |
| 20 | 100 | 100 | 743.8 |
All of the combinations listed were significantly different from 0 ng/ml, P < 0.05.
Neurite Branching
Single Growth Factors
FK506 did not promote neurite branching, because there was no statistical difference in neurite branching between the different concentrations tested (P ≥ 0.05) (Fig. 6a). NGF and GDNF induced significant neurite branching in the concentration range of 0.1–100 ng/ml (P < 0.05) (Figs. 6b,c). Finally, the neurite branching of the NGF or GDNF treated DRG was generally approximately 50% above that observed with the FK506 treated DRGs.
FIGURE 6.
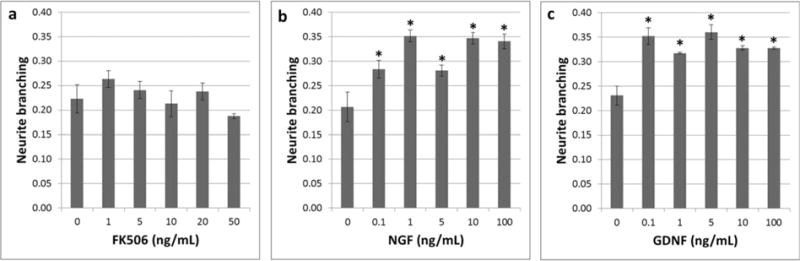
Plots showing the effect of single drug treatment on axon density. (a) FK506 Dosage/density. (b) NGF dosage/density. (c) GDNF dosage/density. The bars represent mean ± SD for n = 4 (*statistically different from 0 ng/ml; P < 0.05).
Combined Growth Factors
As seen in Figure 7a, the average neurite branching due to combined NGF+FK506 treatment was not dose-dependent, and the optimum neurite branching response was observed at the combinations of 0.1 ng/ml and 100 ng/ml of NGF with 10 ng/ml of FK506 (0.3 ± 0.01–0.33 ± 0.01) (P < 0.05) which was the same as seen with NGF alone (0.34–0.35) (P < 0.05). There was a noncompetitive interaction for combinations of NGF 0.1–100 ng/ml with 1– 50 ng/ml of FK506 (0.28–0.33).
FIGURE 7.
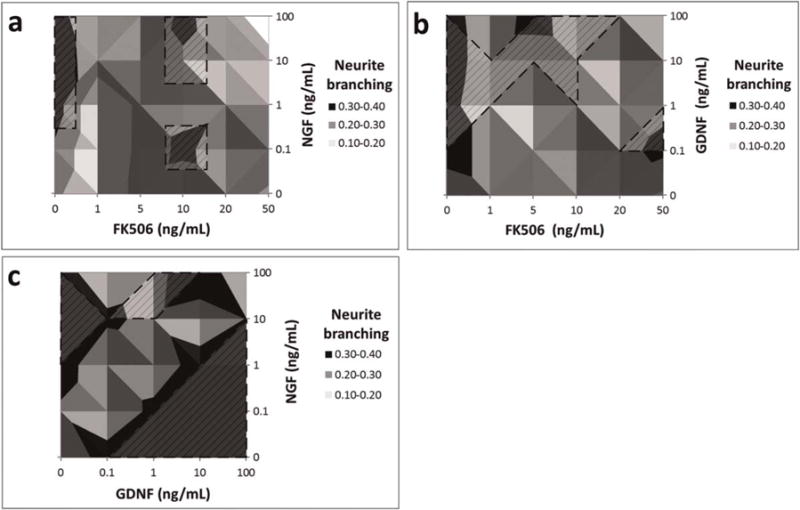
Surface Plots showing the effect of combined drug treatment on axon density. (a) NGF+FK506 combined dosage. (b) GDNF+FK506 combined dosage. (c) NGF+GDNF combined dosage. Shaded region with dashed lines specifies the concentration range that produced significantly different axonal branching responses when compared with 0 ng/ml, P < 0.05.
Generally, GDNF+FK506 treatment (Fig. 7b) produced a competitive response and reduced neurite branching (0.2–0.3) as compared with those obtained with GDNF treatment alone (0.3–0.4), except when 5 ng/ml of FK506 was combined with 100 ng/ml of GDNF (0.32 ± 0.01) (P < 0.05), which had a noncompetitive response.
Figure 7c shows that the optimum neurite branching (0.34–0.35) for combined GDNF+NGF treatment was obtained with 1 ng/ml NGF plus 10 or 100 ng/ml GDNF or with 10 ng/ml NGF plus 10 or 100 ng/ml GDNF (P < 0.05). Neurite branching measurements were more NGF dose-dependent than GDNF dose-dependent. Neurite branching displayed a competitive interaction at lower GDNF concentrations and a potentiating interaction at GDNF values >1 ng/ml.
Combined treatment of NGF+GDNF+FK506 did not produce enhancement in neurite branching as compared to individual treatments (Table 2). The combinations of NGF/GDNF (1–10 ng/ml) and FK506 (10 ng/ml) resulted in a noncompetitive response (0.3–0.35) (P < 0.05) while the combinations involving higher concentrations of NGF and GDNF (100 ng/ml) caused a competitive interaction (0.15–0.22) (P > 0.05). The response obtained was not dose-dependent.
Table 2.
Effect of NGF+GDNF+FK506 combined dosage on neurite branching
| FK506 (ng/ml) | NGF (ng/ml) | GDNF (ng/ml) | Neurite branching |
|---|---|---|---|
| 0 | 0 | 0 | 0.21 |
| 1 | 1 | 1 | 0.35 |
| 1 | 5 | 10 | 0.36 |
| 1 | 10 | 5 | 0.30 |
| 5 | 5 | 100 | 0.33 |
| 5 | 10 | 1 | 0.33 |
| 5 | 100 | 100 | 0.25 |
| 5 | 100 | 5 | 0.31 |
| 10 | 1 | 1 | 0.16 |
| 10 | 5 | 1 | 0.34 |
| 10 | 10 | 10 | 0.33 |
| 10 | 10 | 10 | 0.30 |
| 20 | 1 | 10 | 0.35 |
Neurite Growth Rate
The rate of neurite growth was measured over 3 days (Fig. 8). The concentrations of 10 ng/ml of FK506, NGF, and GDNF were chosen from our analysis of neurite growth at 72 h, because they consistently enhanced neurite growth to a greater extent than controls. The growth rate of the control group slowed down by day 2, such that there was no significant difference in neurite length between days 2 and 3 (P > 0.05) (Fig. 8). There was a close to linear increase in neurite length over the 72-h time frame, when DRGs were exposed to FK506, NGF, or a combination of the 2 (Fig. 8a). However, the combination of FK506 and NGF had a steeper growth rate compared with single treatment exposure, as evidenced by the longer neurites at 72 h (933.2 ± 21.2 μm) compared with the other experimental groups (NGF: 789.5 ± 23.4 μm, GDNF: 723.1 ± 22.4 μm). When DRGs were exposed to GDNF, there was initially a steep increase in neurite growth, but the rate decreased dramatically between days 2 and 3 (Fig. 8b). In contrast, there was a more linear increase in neurite growth when DRGs were exposed to the combination of FK506 and GDNF; this combination had the longest neurites by 72 h 811.3 ± 37.4 μm (Fig. 8b). For the different groups, the overall daily growth rate over 3 days of incubation was approximately 188.1 ± 21.1, 228.6 ± 33.2, 263.2 ± 42.1, 241.0 ± 37.5, 316.2 ± 23.4, 311.1 ± 21.2, 270.4 ± 25.3, and 320.4 ± 30.1 μm/d for the control group, the FK506, NGF, GDNF, NGF+GDNF, NGF+FK506, GDNF+FK506, and NGF+GDNF+FK506 treatment groups, respectively.
FIGURE 8.

Kinetics of axonal elongation of DRGs. (a) treated with FK506 alone (10 ng/ml), NGF alone (10 ng/ml), combined NGF/FK506 (10 ng/ml each), combined NGF/GDNF/FK506 (10 ng/ml each), and untreated (control). (b) treated with GDNF alone (10 ng/ml), FK506 alone (10 ng/ml), combined GDNF/FK506 (10 ng/ml each), combined NGF/GDNF/FK506 (10 ng/ml each), and untreated (control). Bars = mean ± SD of n = 3. All treatment groups were significantly different (P < 0.05) from control (not indicated with asterisks).
Western Blot
Based on our neurite growth data, we tested some of the optimum dosages from the single and combined groups for protein expression using Western blot analysis (Fig. 9). The protein expression levels shown represent the ratio of target protein to the GAPDH internal control. Western blots showed Akt expression of 14.2% by DRGs treated with a combination of 1 ng/ml NGF and 10 ng/ml FK506. This interaction was potentiating (P < 0.05) when compared with the individual treatments of 1 ng/ml NGF and 10 ng/ml FK506, which resulted in 7.7% and 5% expression of Akt, respectively. Similar results were seen for pAkt expression by the NGF+FK506 treated DRGs (~12%) as compared with 10% pAkt expression by single treatment of 1ng/ml NGF or ~9% pAkt expression by 10 ng/ml FK506 dose (P < 0.05). This result was in agreement with DRG growth readings (Figs. 2a,b, 4a), which showed that combined treatment of NGF+FK506 enhanced neurite growth more than either drug alone.
FIGURE 9.
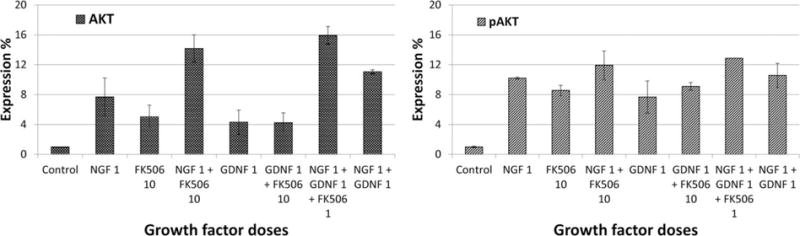
Plots showing protein expression of DRG lysates treated with different drug treatments. The bars indicate mean ± SD for n = 3 obtained from densitometry of western blot scans.
The optimum GDNF+FK506 (1 ng/ml GDNF+10 ng/ml FK506) combined treatment for neurite elongation had a slightly competitive interaction resulting in AKT expression of ~4%, which was slightly lower than what was observed from a single treatment of 10 ng/ml FK506 (AKT 5%; pAkt 8.5%) or 1 ng/ml GDNF (Akt ~4%; pAkt ~7.7%). This result is similar to what we observed from DRG neurite elongation data for this treatment (Figs. 2a,c, 4b).
The overall response of the optimal combined treatment of NGF+GDNF+FK506 (1 + 1+1 ng/ml) was the highest with Akt expression of ~16% and pAkt expression ~13% when compared with dual combinations or the single treatments (P < 0.05). This result was consistent with the neurite growth readings obtained from DRGs (Table 1).
DISCUSSION
The main findings of this study are that: (1) FK506 primarily enhances neurite elongation; (2) NGF and GDNF promote both neurite elongation and neurite branching; (3) neurite outgrowth is dose-dependent, with optimal concentrations falling in the range of 1–10 ng/ml for FK506, NGF, and GDNF; (4) combining FK506, GDNF, and NGF exerts a potentiating effect on neurite growth; (5) significant increases in neurite growth are observed in the first 48 h of culture; and (6) combinations of FK506, GDNF, and NGF increased protein expression of AKT and phosAKT when compared with individual treatments.
NGF and GDNF are important neurotrophins.40 They have been shown to promote neuronal growth both in vitro and in vivo9 and have been studied extensively.41,42 They are the 2 most commonly used neurotrophins when selecting or designing a delivery system for nerve growth and have been used in numerous neurorestorative studies.40–43 NGF has been found to be essential for development and phenotypic maintenance of neurons in the peripheral nervous system.44 NGF has been considered to be a very powerful and selective growth factor for sympathetic and sensory neurons, especially for survival and axonal outgrowth of sensory neurons.41,45 GDNF has shown pronounced effects on motor axonal regeneration and has been identified as a key factor for promoting axonal elongation.40,41,46,47 More recent work has shown that GDNF ligands can regulate pain responses by modulating sensitization of sensory neurons to different stimuli.48–50 GDNF was also shown recently to elicit different effects on motoneuron axons versus sensory cell bodies.51 Delivery of GDNF in a cell therapy model has been shown to prolong lifespan and control disease progression in a rat model of amyotrophic lateral sclerosis.52
This study analyzed the potential effects of different dosages of FK506 in combination with other growth factors on nerve growth. In vivo tests have shown that systemically delivered FK506 has nerve regenerative properties in addition to being an immunosuppressant.20,29,30 There is, however, insufficient information regarding the optimal local dose of FK506 for nerve regeneration after injury. To improve this knowledge, we used a bio-activity test system based on DRG explants from chicken embryos. We have tested the effects of FK506, GDNF, and NGF, alone and in combination, on neurite elongation (Figs. 1–4), branching (Figs. 6 and 7), growth kinetics (Fig. 8), and protein expression of AKT and pAKT (Fig. 9). Chicken DRG explants were selected for this testing process because of their availability, low cost, and their ganglion structure associated with Schwann cells. Due to their structure, DRG explants more closely mirror the situation of neurite growth in vivo than single neurons.42 Chicken DRG explants are a widely used model for neurite growth. Chicken embryonic DRGs are readily available and have also been shown to exhibit growth similarities with other animal neural systems.9,37 DRGs have been used for testing effects of neurotrophic factors and drugs in several animal models.53 Neurite outgrowth and survival studies have been done using DRGs.53 We expected that the use of DRGs for testing should provide relevant information on bio-activity and the useful dose of selected drugs. FK506, GDNF, or NGF induced readily visible neurite outgrowth when compared with control cells. FK506 and GDNF primarily promoted neurite elongation, whereas NGF induced extensive neurite branching with neurite elongation. We found that 1–10 ng/ml of FK506, NGF, and GDNF showed the highest level of elongation of DRG neurites.
The dramatic neurotrophic action of combined doses of FK506 and other growth factors on DRGs is extraordinary in terms of sensitivity, because as little as 1 ng/ml FK506 combined with 1 ng/ml NGF/GDNF was neurotrophic. The effect of NGF/GDNF in promoting neurite sprouting and elongation has been shown earlier.9 On a molecular level, NGF is known to stimulate neurite extension in embryonic DRG neurons by binding to tyrosine kinase A (TrkA) receptors and activating intracellular signaling pathways,54 and GDNF promotes primarily motor neuron survival and neurite growth.47 The optimal NGF/GDNF dose of 0.1–10 ng/ml for maximum neurite outgrowth we observed is in agreement with previous data.9
Although neurite growth did not differ significantly between treatments with 1 and 10 ng/ml of GDNF or NGF, it declined at higher doses (100 ng/ml). This result is in agreement with findings in rats, where high doses of NGF delayed nerve regeneration by retarding GAP43 production in the early phase (7 days) after axotomy.15 We observed that combinations of FK506 with high doses of NGF produced a potentiating response in neurite elongation, which suggests that FK506 may help to enhance neurite growth even with higher doses of neurotrophic factors. In certain cases of combined doses, such as combinations of 1 ng/ml or 5 ng/ml FK506 with GDNF, we see a competitive interaction which could reflect negative feedback resulting from the interaction of different protein expressions and signaling pathways used by FK506 and the neurotrophic factors.55
No difference in the rate of neurite outgrowth was found between FK506, GDNF, and NGF treatment during the first 2 days of treatment, but NGF yielded a higher outgrowth rate than others between days 2 and 3 of the experiment. Interestingly, the combination of FK506 with GDNF and/or NGF enhanced the rate of neurite outgrowth at all time points over the other treatment groups. The potentiating effect of FK506 with GDNF and/or NGF on initial neurite growth rate should be considered when developing new therapeutic drug delivery systems.
The potentiating effect of FK506 with GDNF and/or NGF on both neurite growth and, to a lesser extent, on neurite branching is an interesting observation. NGF and GDNF have been found to activate Akt and pAkt expression in cells in neuroprotection and neuro-regeneration pathways.41,44 Akt is a serine-threonine protein kinase. Induction of the serine-threonine kinase activity is critical for cell survival, as well as cell proliferation. Akt is also regulated by growth factor and serum factor signaling through PI3 kinase.32,36 Data suggest that FK506 potentiates neurite outgrowth through the Ras/Raf/MAP kinase signaling pathway downstream of PI3K-Akt.28 The phosphatidylinositol-3 kinase–Akt (PI3K-Akt) pathway is one of the major growth factor pathways that has been studied and shows that Akt/pAkt expression is associated with accelerated neurite regeneration after axotomy.56,57
Also, Akt has been found to be necessary for neurotrophin-stimulated neurite growth.36,56 With Western blot analysis, we found that Akt/pAkt activation occurs during neurite growth, and the percentage of protein expression can be related to neurite elongation due to different dosage combinations. In this study, we evaluated whether FK506 individually or in combination with other neurotrophic factors would upregulate the protein expression of Akt and phosAkt. We found that combined application of neurotrophic factors with FK506 enhanced co-expression of both receptors. These results strongly suggest that the interaction of FK506 with GDNF and NGF mediates distinct neurite growth enhancement. Activation of the Akt signaling pathway has been associated with increased neurite growth58 and would explain the increased expression of receptors.
In conclusion, these results indicate that there is a dramatic increase in nerve growth activity when FK506 is combined with NGF and/or GDNF. NGF and FK506 combined treatments showed potentiating responses for certain dose combinations and had significant effects on neurite growth when compared with their individual doses. Combined GDNF and FK506 treatments exhibited a slightly potentiating effect, but to a lesser extent when compared with FK506+NGF. Combined treatments of all 3 drugs were highly potentiating and resulted in extraordinary effects on neurite growth and branching. However, further investigations are required to elucidate the mechanisms that support the enhanced neurite elongation by combined FK506 and GDNF/NGF treatment. These neurotrophic actions have positive therapeutic ramifications. Numerous neurotrophic proteins, such as brain derived neurotrophic factor, GDNF, and NGF, are being evaluated clinically in various disease states. Difficulties in manufacturing of these large proteins and delivery to target sites create serious barriers to therapeutic application. Drugs such as FK506 are readily synthesized and are thermally stable. Both NGF and GDNF are synthesized endogenously in low amounts at the site of nerve injury, and their efficacy may be enhanced with exogenous delivery of FK506.
The authors thank Greg Stoddard for his consultation in this work. The authors acknowledge Rainey Cornaby for her assistance in this study. The authors also thank their colleagues at the State of Utah Center of Excellence for Biomedical Microfluidics and the Department of Surgery Research Laboratory for their assistance in this work.
Acknowledgments
Funding: This work is funded by the DOD Award number W81XWH1310363.
Abbreviations
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified eagle medium
- DRG
dorsal root ganglion
- FBS
fetal bovine serum
- FK506
tacrolimus
- GDNF
glial cell line derived neurotrophic factor
- NGF
nerve growth factor
- PBS
phosphate buffered saline
References
- 1.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caneva Soumetz F, Giacomini M, Phillips JB, Brown RA, Ruggiero C. A drug delivery system for the treatment of peripheral nervous system injuries. Conf Proc IEEE Eng Med Biol Soc. 2004;2:5047–5049. doi: 10.1109/IEMBS.2004.1404395. [DOI] [PubMed] [Google Scholar]
- 3.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 4.Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133:1420–1430. doi: 10.1097/PRS.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 5.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63–68. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- 6.Madduri S, Gander B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release. 2012;161:274–282. doi: 10.1016/j.jconrel.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Hadlock TA, Sheahan T, Cheney ML, Vacanti JP, Sundback CA. Biologic activity of nerve growth factor slowly released from microspheres. J Reconstr Microsurg. 2003;19:179–184. doi: 10.1055/s-2003-39831. [DOI] [PubMed] [Google Scholar]
- 8.Madduri S, Feldman K, Tervoort T, Papaloizos M, Gander B. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J Control Release. 2010;143:168–174. doi: 10.1016/j.jconrel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Madduri S, Papaloizos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65:88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 11.Saab CY, Shamaa F, El Sabban ME, Safieh-Garabedian B, Jabbur SJ, Saade NE. Transient increase in cytokines and nerve growth factor in the rat dorsal root ganglia after nerve lesion and peripheral inflammation. J Neuroimmunol. 2009;208:94–103. doi: 10.1016/j.jneuroim.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Gougeon PY, Lourenssen S, Han TY, Nair DG, Ropeleski MJ, Blennerhassett MG. The pro-inflammatory cytokines IL-1beta and TNFalpha are neurotrophic for enteric neurons. J Neurosci. 2013;33:3339–3351. doi: 10.1523/JNEUROSCI.3564-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fregnan F, Muratori L, Simoes AR, Giacobini-Robecchi MG, Raimondo S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen Res. 2012;7:2259–2266. doi: 10.3969/j.issn.1673-5374.2012.29.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 16.Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci U S A. 2001;98:14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci U S A. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner MJ, Mackinnon SE, Rickman SR, Jaramillo A, Tung TH, Hunter DA, et al. FK506 and anti-CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci. 2005;23:237–249. [PubMed] [Google Scholar]
- 19.Jost SC, Doolabh VB, Mackinnon SE, Lee M, Hunter D. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17:39–44. [PubMed] [Google Scholar]
- 20.Navarro X, Udina E, Ceballos D, Gold BG. Effects of FK506 on nerve regeneration and reinnervation after graft or tube repair of long nerve gaps. Muscle Nerve. 2001;24:905–915. doi: 10.1002/mus.1088. [DOI] [PubMed] [Google Scholar]
- 21.Lyons WE, George EB, Dawson TM, Steiner JP, Snyder SH. Immuno-suppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc Natl Acad Sci U S A. 1994;91:3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Zhang Z, Wang S, Lv D. Combined treatment with FK506 and nerve growth factor for spinal cord injury in rats. Exp Ther Med. 2013;6:868–872. doi: 10.3892/etm.2013.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson A, Moller E. Evidence that the immunosuppressive effects of FK506 and cyclosporine are identical. Transplantation. 1990;50:1001–1007. doi: 10.1097/00007890-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Gold BG, Densmore V, Shou W, Matzuk MM, Gordon HS. Immuno-philin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J Pharmacol Exp Ther. 1999;289:1202–1210. [PubMed] [Google Scholar]
- 25.Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15:285–306. doi: 10.1007/BF02740664. [DOI] [PubMed] [Google Scholar]
- 26.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995;15:7509–7516. doi: 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Doolabh VB, Mackinnon SE, Jost S. FK506 promotes functional recovery in crushed rat sciatic nerve. Muscle Nerve. 2000;23:633–640. doi: 10.1002/(sici)1097-4598(200004)23:4<633::aid-mus24>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. Br J Pharmacol. 2003;140:825–829. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udina E, Ceballos D, Verdu E, Gold BG, Navarro X. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve. 2002;26:348–355. doi: 10.1002/mus.10195. [DOI] [PubMed] [Google Scholar]
- 30.Yang RK, Lowe JB, III, Sobol JB, Sen SK, Hunter DA, Mackinnon SE. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast Reconstr Surg. 2003;112:1832–1840. doi: 10.1097/01.PRS.0000091167.27303.18. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Chen Y, Ikonomovic MD, Nathaniel PD, Kochanek PM, Marion DW, et al. Increased phosphorylation of protein kinase B and related substrates after traumatic brain injury in humans and rats. J Cereb Blood Flow Metab. 2006;26:915–926. doi: 10.1038/sj.jcbfm.9600238. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 33.Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb Perspect Biol. 2010;2:a001925. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM. Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J Cell Biol. 2001;154:995–1005. doi: 10.1083/jcb.200101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dajas-Bailador F, Bantounas I, Jones EV, Whitmarsh AJ. Regulation of axon growth by the JIP1-AKT axis. J Cell Sci. 2014;127:230–239. doi: 10.1242/jcs.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilsland J, Rigby M, Young L, Harper S. A rapid method for semi-quantitative analysis of neurite outgrowth from chick DRG explants using image analysis. J Neurosci Methods. 1999;92:75–85. doi: 10.1016/s0165-0270(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 38.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14:202–224. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14:202–224. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giger RJ, Hollis ER, Jr, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Ramburrun P, Kumar P, Choonara YE, Bijukumar D, du Toit LC, Pillay V. A review of bioactive release from nerve conduits as a neurotherapeutic strategy for neuronal growth in peripheral nerve injury. Biomed Res Int. 2014;2014:132350. doi: 10.1155/2014/132350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 45.Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 48.Ibanez CF, Andressoo JO. Biology of GDNF and its receptors - relevance for disorders of the central nervous system. Neurobiol Dis. 2016 doi: 10.1016/j.nbd.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 49.Ohnaka M, Miki K, Gong YY, Stevens R, Iwase T, Hackett SF, et al. Long-term expression of glial cell line-derived neurotrophic factor slows, but does not stop retinal degeneration in a model of retinitis pigmentosa. J Neurochem. 2012;122:1047–1053. doi: 10.1111/j.1471-4159.2012.07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. J Neurophysiol. 2012;108:2545–2553. doi: 10.1152/jn.00576.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahavi EE, Ionescu A, Gluska S, Gradus T, Ben-Yaakov K, Perlson E. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J Cell Sci. 2015;128:1241–1252. doi: 10.1242/jcs.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther. 2013;21:1602–1610. doi: 10.1038/mt.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melli G, Hoke A. Dorsal root ganglia sensory neuronal cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin Drug Discov. 2009;4:1035–1045. doi: 10.1517/17460440903266829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229:460–470. doi: 10.1016/j.expneurol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Steiner JP, Connolly MA, Valentine HL, Hamilton GS, Dawson TM, Hester L, et al. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclospor-in A. Nat Med. 1997;3:421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- 56.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 57.Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, et al. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg JL. How does an axon grow? Genes Dev. 2003;17:941–958. doi: 10.1101/gad.1062303. [DOI] [PubMed] [Google Scholar]


