Abstract
Thiols (-SH) play various roles in biological systems. They are divided into protein thiols (PSH) and non-protein thiols (NPSH). Due to the significant roles thiols play in various physiological/pathological functions, numerous analytical methods have been developed for thiol assays. Most of these methods are developed for glutathione, the major form of NPSH. Majority of these methods require tissue/cell homogenization before analysis. Due to a lack of effective thiol specific fluorescent/fluorogenic reagents, methods for imaging and quantifying thiols in live cells are limited. Determination of an analyte in live cells can reveal information that cannot be revealed by analysis of cell homogenates. Previously, we reported a thiol specific thiol-sulfide exchange reaction. Based on this reaction, a benzofurazan sulfide thiol specific fluorogenic reagent was developed. The reagent was able to effectively image and quantify total thiols (PSH+NPSH) in live cells through fluorescence microscopy. The reagent was later named as GUALY’s reagent.
Here we would like to report an extension of the work by synthesizing a novel benzofurazan sulfide triphenylphosphonium derivative [(((7,7′-thiobis(benzo[c][1,2,5]oxadiazole-4,4′-sulfonyl))bis(methylazanediyl))bis(butane-4,1-diyl))bis(triphenylphosphonium) (TBOP)]. Like GUALY’s reagent, TBOP is a thiol specific fluorogenic agent that is non-fluorescent but forms fluorescent thiol adducts in a thiol-specific fashion. Different than GUALY’s reagent, TBOP reacts only with NPSH but not with PSH. TBOP was effectively used to image and quantify NPSH in live cells using fluorescence microscopy. TBOP is a complementary reagent to GUALY’s reagent in determining the roles of PSH, NPSH, and total thiols in thiol-related physiological/pathological functions in live cells through fluorescence microscopy.
Keywords: Non-protein thiol, thiol specific fluorogenic agent, live cell thiol imaging, fluorescence microscopy
Graphical abstract
Live cell imaging and quantification of non-protein thiols by TBOP
Introduction
Thiols (-SH) play important roles in various aspects of cellular functions which include enzyme activity, signal transduction, cell division, cell protection against reactive oxygen and nitrogen species, and removal of reactive electrophiles [1]. Thiols are also the major contributor to cellular redox buffers [2]. Thiol concentration in biological systems can be affected by various normal and abnormal conditions including oxidation of thiols by oxidants such as reactive oxygen or nitrogen specie, glutathionylation or nitrosylation of thiols under conditions of oxidative stress, and reaction of thiol groups with a reactive electrophile [3]. A decrease in thiol concentration has been linked to a number of cellular dysfunctions including changes in enzyme activities, membrane permeability, and energy production [4–7]. Consequently, disturbances in thiol homoeostasis have been associated with aging and neuron degeneration diseases [8–11]. Monitoring thiol status provides valuable information in understanding a thiol-related biochemical process or disease.
Structurally, thiols in biological systems can be divided into protein thiols (PSH) and non-protein thiols (NPSH). Although PSH are the predominant thiols in biological systems with a ratio of PSH to NPSH of approximately 3:1 [12], NPSH are most commonly measured to reflect thiol status. The major form of NPSH is glutathione (GSH). GSH is present in mM concentration under normal physiological conditions. Accordingly, GSH has been commonly measured to reflect thiol status in biological systems. Due to the significant roles thiols play in physiological functions and pathological conditions, numerous analytical methods have been developed to measure thiol concentrations [12–18], especially the concentration of GSH [12]. Most of these analytical methods require tissue/cell homogenization before sample analysis. Methods available to monitor thiols in live cells using fluorescence microscopy are limited due to a lack of effective thiol specific fluorogenic or fluorescent reagents.
Monitoring an analyte in live cells through fluorescence microscopy has been extensively used in biomedical and pharmaceutical research. Compared with most conventional analytical methods, imaging methods have the advantage of allowing visualization of the analyte in its intact and native physiological environment [19] and are able to reveal information that cannot be revealed by most conventional methods. The key in the successful imaging of an analyte in live cells is a reagent that can turn the analyte into a fluorescent compound. Quantification of the analyte through imaging is even more challenging since it requires the reagent to be highly selective (preferably specific) and able to completely turn all the analyte into the fluorescent compound for quantification. A limited number of reagents have been reported to image thiols in live cells through fluorescence microscopy. These reagents include monochlorobimane [20], chloromethyl fluorescein, O-phthaldialdehyde, mercury orange [21, 22], rosamine-based probes [22], disulfide-based analogs [23–25], and rhodamine-based porbes [26, 27] for thiol or GSH imaging. However, none of these reagents can be used to quantify thiols in live cells by fluorescence microscopy due to either a slow reaction rate leading to incomplete thiol derivatization or a lack of specificity towards thiols [21, 26, 28].
We reported previously a thiol specific thiol-sulfide exchange reaction [29]. Based on this reaction, a series of benzofurazan sulfide analogs were developed as thiol specific fluorogenic reagents [29]. These reagents were not fluorescent and reacted specifically with thiols in a rapid and complete fashion to form fluorescent thiol adducts. One of these reagents, bis(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)sulfane, was demonstrated to effectively image and quantify total thiols (PSH+NPSH) in live cells through fluorescence microscopy [29]. The reagent was later named as GUALY’s reagent [30].
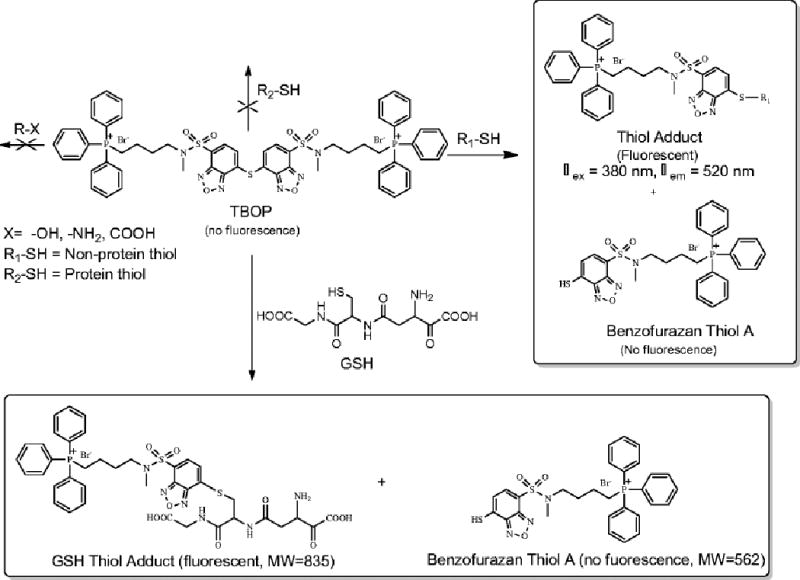
Here, we report an extension of the work by synthesizing a novel benzofurazan sulfide triphenylphosphonium derivative [(((7,7′-thiobis(benzo[c][1,2,5]oxadiazole-4,4′-sulfonyl))bis(methylazanediyl))bis(butane-4,1-diyl))bis(triphenylphosphonium) (TBOP)]. TBOP was initially designed as a fluorogenic reagent for thiol imaging in mitochondria but abandoned due to insufficient fluorescence intensity derived from mitochondrial thiols. Like GUALY’s reagent, TBOP was found to be non-fluorescent and formed fluorescent thiol adducts only after reacting specifically with thiols (Scheme 1); therefore it is a thiol-specific fluorogenic reagent. The excitation and emission wavelengths of various TBOP-thiol adducts were found to be 380 nm and 520 nm respectively. Different than GUALY’s reagent, TBOP was found to react only with NPSH and not with PSH. TBOP was effectively used to image and quantify NPSH in live cells through fluorescence microscopy. The NPSH quantification was validated by GUALY’s reagent and NPSH obtained from the cell homogenates. Together with GUALY’s reagent, TBOP will be a valuable tool in exploring the roles of PSH, NPSH, and total thiols in thiol-related physiological/pathological functions in live cells using fluorescence microscopy.
Scheme 1.
TBOP as a thiol specific NPSH selective fluorogenic reagent
Materials and Methods
Materials and Solutions
TBOP was synthesized in four steps from a commercially available starting materials. The synthesis of TBOP is presented as Electronic Supplementary Material (ESM). Sulfosalicylic acid (SSA) cell lysis solution was prepared as a 5% (w/v) solution in deionized water containing 0.1% (v/v) Triton X-100 [31]. TBOP’s derivatizing solution was prepared from its stock solution (1 mM in acetonitrile) as a 0.1 mM solution in phosphate buffer (0.45 M, pH 7.9) containing 1.8% SDS. The 3% (w/v) SSA solution and stock solutions of GSH (10 mM) were prepared in deionized water. GUALY’s reagent was synthesized according to a literature reported procedure [29]. GUALY’s derivatizing solution was prepared from its stock solution (1 mM in acetonitrile) as a 0.1 mM solution in a phosphate buffer (0.45 M, pH 7.9) containing 1.8% SDS.
Instrumentation
Low resolution mass spectra (LRMS) was obtained from a Thermoquest Finnigan LCQ Deca Mass Spectrometer. Fluorescence properties were obtained on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, California) or Perkin Elmer LS50B spectrometer (Perkin Elmer Ltd, UK). Fluorescence microscopy was obtained on an upright fluorescence microscope (Zeiss AXIO Imager A1) connected to a camera (AxioCam MRc5) (Zeiss, United States). HPLC analysis was carried out on a Beckman HPLC system equipped with a system gold 125 pumping module, a 508 autosampler, a 168 photodiode array detector and Agilent 1100 series fluorescent detector. The HPLC condition employed an Alltech C4 column (150 mm×3.0 mm i.d., 3 μm), solvent A (acetonitrile) and solvent B [aqueous solution with 0.1% (v/v) trichloroacetic acid]. Unless specified otherwise, the initial mobile phase started with solvent B with 5% solvent A for 2 min. Solvent A was then linearly increased to 70% in 11 min and then to 80% in 5 min. Solvent A was kept at 80% for an additional 3 min before being returned to 5% in 2 min. All flow rates were 0.5 mL/min. The injection volume was 30 μL. 254 nm were employed as the detection wavelength for UV detection, 380 nm and 520 nm as the excitation and emission wavelengths for fluorescent detection.
Cell Culture
NCI-H226 cells (human lung cancer) were obtained from the National Cancer Institute and grown in the RPMI 1640 growth medium supplemented with 10% FBS, 100 units/mL penicillin (Mediatech, Inc., Herndon, VA) and 100 μg/mL streptomycin (Mediatech, Inc., Herndon,VA) in a humidified atmosphere containing 5% CO2 at 37 °C.
Chemical stability TBOP’s thiol adducts
TBOP (250 µM) mixed with a thiol molecule (GSH, cysteine, or homocysteine, 50 µM) in the RPMI medium with 1% acetonitrile was stirred at 37 °C in a water bath. Aliquots (20 µL) at different time were withdrawn for HPLC analysis.
Determination of TBOP as a thiol specific reagent
The reaction of TBOP with NPSH and non-thiol amino acids was carried out in a Tris buffer (0.1 M, pH 7.4) with 2 mM EDTA and monitored by HPLC with a photodiode array detector or with a fluorescence detector. TBOP was mixed with an NPSH or a non-thiol amino acid at various concentrations and at different ratios for 20 min except serine which was mixed with TBOP for 8 h. Aliquots were withdrawn for HPLC/UV (20 µL) analysis or fluorescence analysis (100 µL) using the excitation and emission wavelengths determined below.
Determination of TBOP as a fluorogenic agent
The fluorescence property of TBOP and its thiol adducts with various thiol-containing amino acids were determined on a Perkin Elmer LS50B spectrometer. TBOP (20 µM) was mixed with 50 equiv of GSH, N-acetylcysteine (NAC), NAC methyl ester, cysteine, or homocysteine in a Tris buffer (0.1 M, pH 7.4) with 2 mM EDTA at 37 °C for 20 min. The excitation and emission wavelengths were scanned using the spectrometer. Aliquots from the reaction of TBOP with GSH were further checked by HPLC with a photodiode array detector and HPLC with a fluorescence detector based on the excitation and emission wavelengths obtained from the spectrofluorometer.
Reactivity of TBOP with PSH
Reactivity of TBOP with thiols in bovine serum albumin (BSA)
TBOP (2 µL, 5 mM) was mixed with BSA (98 µL, 1 mM) at room temperature for 20 min. BSA was precipitated with acetonitrile (300 µL). The supernatant was used for HPLC analysis for the released benzofurazan thiol A (Scheme 1).
Reactivity of TBOP with thiols in proteins from cell homogenates
NCI-H226 cells (5×106) were washed once with DPBS and homogenized by sonication with 3% SSA (1 mL) for 10 min. The cell homogenate was centrifuged at 14,000 rpm at 4 °C for 5 min. The supernatant was removed, and the protein precipitates were washed once with 3% SSA (1 mL) and re-suspended in 1 mL 3% SSA solution. One third of the cell suspension was centrifuged at 14,000 rpm at 4 °C for 5 min. The protein precipitates were added with 130 µL of a Tris buffer (0.1 M, pH 7.4) and TBOP (50 µM). The mixture was allowed to react at room temperature for 20 min. Proteins were removed by precipitation using acetonitrile, and the supernatant was subjected to HPLC analysis for the released benzofurazan thiol A (Scheme 1).
NPSH imaging and quantification in live cells with TBOP
NCI-H226 cells were plated at a density of 50,000 cells/well on a 15 mm diameter microscope cover glass placed in a well of a 12-well plate in RPMI 1640 growth medium. The cells were allowed to attach at 37 °C in a humidified atmosphere of 5% CO2. After 24 h attachment, cells were treated with different concentrations of N-ethylmaleimide (NEM), a thiol-depleting reagent [32], to modulate intracellular thiol concentration. The NEM-containing medium was removed and the cells were washed once with PBS, followed by the addition of the growth medium containing 10 µM TBOP (1 mL/well). After a 2 h incubation at 37 °C in a humidified atmosphere of 5% CO2, the TBOP containing medium was removed, and the cells were washed once with 1 mL of PBS. The cover glasses were carefully removed from the well with forceps and placed on a microscope slide, which were mounted with a solution of 9:1 glycerol-PBS. The samples were protected from light with aluminum foil during the experiment. The fluorescence images were obtained using a 20x objective and DAPI filter set on an upright fluorescence microscope. The images were captured at an exposure of 150 ms and saved as 16-bit gray scale files. The fluorescence images were processed by ImageJ (http://rsbweb.nih.gov/ij/) [33]. The fluorescence intensity was measured by the “analyze/measure” function of ImageJ with the threshold segmentation. Fluorescence intensity for each sample was obtained using an average of 6 different locations per slide and a minimum of 20 cells/location. The fluorescence from the sample treated with 300 µM NEM was used as a background and subtracted from that of each sample since 300 µM NEM was found to block all thiols in cells.
Validation of NPSH imaging with TBOP by GUALY’S reagent
NPSH imaging and quantification with TBOP in live cells were validated by a literature reported method using GUALY’s reagent with minor modification [30]. Briefly, NCI-H226 cells were treated with NEM in the same way as described above in a 12 well plate except no cover glass was used. After NEM treatment for 3 h, the NEM-containing medium was removed and the cells were washed once with DPBS; SSA cell lysis solution (0.2 mL) was added to each well, and the plate was shaken on a microplate shaker at room temperature at speed 6 for 10 min. The plate was then centrifuged for 10 min at 4000 rpm at 4 °C to remove protein precipitates. The supernatant (40 µL/well), which contained NPSH, was transferred to a 96-well plate and added with GUALY’s derivatizing solution (10 µL/well). The plate was covered with aluminum foil and shaken for an additional 10 min on a microplate shaker at speed 6 before the fluorescence intensity was read on a SpectraMax M2 microplate reader using 430 and 520 nm as λex and λem with a cutoff wavelength of 495 nm for NPSH quantification as reported [30].
Results and discussion
Characterization of TBOP as a thiol specific reagent for NPSH
Characterization of TBOP as a thiol specific reagent for NPSH was achieved through determination of TBOP’s reactivity with NPSH (specifically GSH, cysteine, and homocysteine) vs the reactivity toward common biologically relevant nucleophilic functional groups other than thiols. These common biologically relevant nucleophilic groups mainly include -OH, -NH2, and COOH. The employed model compound with these common nucleophilic functional groups was serine. The reactivity was further checked with various non-thiol amino acids. TBOP was demonstrated to react only with thiols. The details are presented below.
Reactivity of TBOP toward NPSH
The reactivity of TBOP (50 µM) with NPSH was initially determined with GSH at a 1:1 molar ratio and monitored by HPLC/UV. TBOP completed the reaction in 5 h at room temperature (Fig. 1a). The rate was much slower than GUALY’s reagent, which completes the same type of reaction in 5 min [29]. To check whether the reaction could be facilitated by using an excessive amount of TBOP, we conducted a reaction by using a 5:1 molar ratio of TBOP:GSH [TBOP (250 µM):GSH (50 µM)] and found the reaction was completed in 20 min.
Fig. 1.
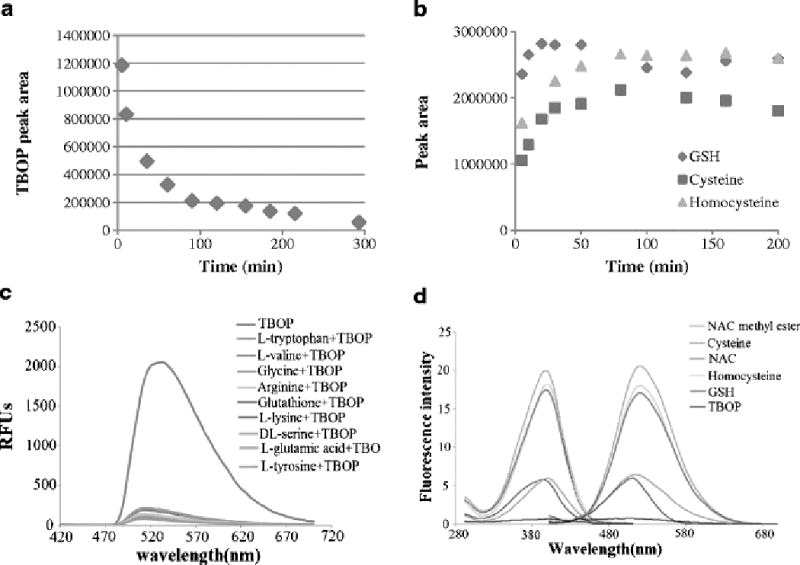
TBOP - a thiol specific and fluorogenic agent for NPSH. (a). A plot of TBOP’s disappearance against time when TBOP (50 µM) reacted with GSH (50 µM). The disappearance was monitored by HPLC/UV; (b). Formation of thiol adducts from a reaction of TBOP (250 µM) with GSH (50 µM), cysteine (50 µM) or homocysteine (50 µM) at room temperature. The reaction was monitored by HPLC/UV; (c). Emission spectra of TBOP and the reaction mixtures of TBOP with various non-thiol amino acids or GSH. TBOP (50 µM) was mixed with 50 equiv of a non-thiol amino acid or GSH in a Tris buffer (pH 7.4, 0.1 M) containing 2 mM EDTA in a 96-well plate at 37 °C for 10 min. The emission spectra were obtained with the excitation wavelength at 380 nm on a SpectraMax M2 Microplate Reader; (d). Excitation and emission spectra of thiol adducts derived from a reaction of TBOP with various thiol compounds. The thiol adducts’ maximum excitation and emission wavelengths for all tested compounds were 380 and 520 nm, respectively. The spectra were obtained by mixing TBOP (20 µM) with a thiol compound (1 mM) in a Tris buffer (0.1 M, pH 7.0) for 20 min.
Next, the products of the reaction of TBOP with GSH were characterized. As shown in Scheme 1, TBOP is expected to react with GSH to form a GSH thiol adduct and to release benzofurazan thiol A. A reaction was conducted in which GSH (2.5 mM) was mixed with TBOP (50 µM) in a 50:1 molar ratio for 20 min to ensure a completion reaction. At the end of the reaction, an aliquot was analyzed by HPLC. The HPLC chromatogram demonstrates that the peak for TBOP disappeared and two major new peaks formed (17.558 min and 19.942 min, Fig. 2A). Mass characterization of the fractions collected from these two peaks revealed that they matched the molecular weight of the expected GSH thiol adduct (m/z 835) (17.558 min) and benzofurazan thiol A (m/z 562) (19.942 min) (insert of Fig. 2Aii). The results confirmed that the reaction of TBOP with a thiol was a clean reaction that yielded the expected thiol adduct and benzofurazan thiol A; the same type of reaction as observed in the reaction of a thiol with GUALY’s reagent [29].
Fig. 2.
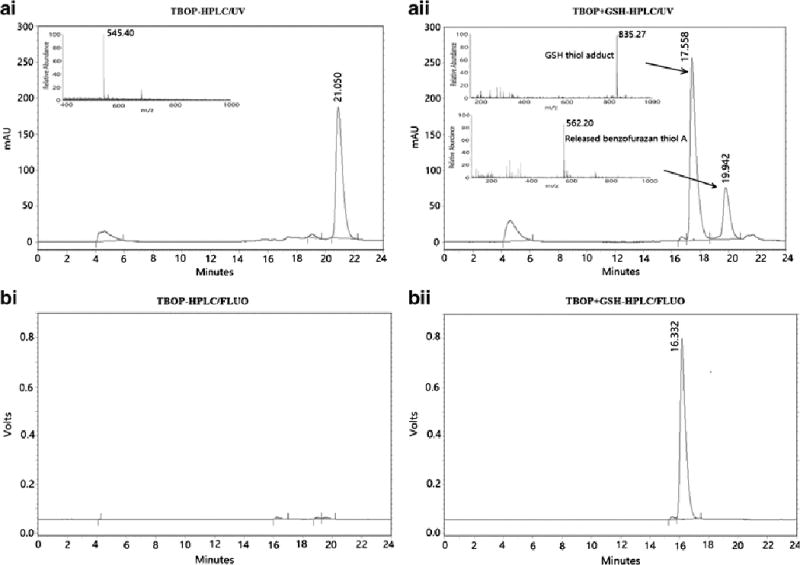
The chromatograms of HPLC/UV (A) and HPLC/fluorescence (B) of TBOP (Ai and Bi) and the reaction mixture of TBOP (50 µM) with GSH (2.5 mM) for 20 min (Aii and Bii).
We then proceeded with the reaction of TBOP with cysteine and homocysteine. Both cysteine and homocysteine reacted with TBOP in a similar manner to yield the cysteine thiol adduct and homocysteine thiol adduct, respectively, but at a slower rate. At a TBOP:thiol ratio of 5:1, the reaction was completed in about 20 min for GSH and 80 min for both cysteine and homocysteine as monitored by HPLC/UV (Fig. 1b). The thiol adducts of TBOP derived from GSH, cysteine, and homocysteine were stable over the 200 min period (Fig. 1b). It is not clear why cysteine and homocysteine reacted more slowly than GSH. One possible explanation is that GSH contains two carboxylic acid groups while cysteine and homocysteine contain only one carboxylic acid. This additional carboxylic acid in GSH provides an additional anion to attract the positively charged TBOP which may help facilitate the reaction.
Reactivity of TBOP toward common biologically relevant nucleophilic functional groups other than thiol
The reactivity of TBOP with other nucleophilic groups (-OH, -NH2, and –COOH) was checked by employing the amino acid serine as a model compound. When TBOP was mixed with 50 equiv of serine at 37 °C, no reaction was observed by HPLC/UV for up to 8 h revealing that TBOP did not react with -OH, -NH2, and -COOH. The reactivity was further checked with various non-thiol amino acids. These amino acids included acidic, basic, and neutral amino acids as well as amino acids containing a hydroxyl group or phenol group. When TBOP was mixed with 50 equiv of these amino acids (tryptophan, lysine, valine, glycine, glutamic acid, arginine, and tyrosine) for 20 min at 37 °C, no disappearance of TBOP was observed by HPLC/UV, and no new peaks appeared either confirming that TBOP did not react with these non-thiol amino acids (data not included). An additional piece of evidence for no reaction between TBOP and these non-thiol amino acids was obtained through fluorescence monitoring. No fluorescence was observed when TBOP was mixed with 50 equiv excess of these non-thiol amino acids in a 96 well plate while strong fluorescence was observed when mixed with GSH (Fig. 1c). These data confirm that TBOP is a thiol specific derivatizing agent.
Characterization of TBOP as a fluorogenic reagent
The fluorogenic nature of TBOP was determined by checking the fluorescence properties of TBOP and its thiol adducts derived from GSH, NAC, NAC methyl ester, cysteine, and homocysteine. TBOP exhibited minimal fluorescence while its thiol adducts were all fluorescent with different intensities confirming that TBOP is a fluorogenic reagent (Fig. 1d). The thiol adduct derived from NAC methyl ester exhibited the strongest fluorescence, followed by homocysteine and GSH. The thiol adducts derived from cysteine and NAC exhibited relatively weak fluorescence (Fig. 1d). The excitation and emission wavelengths for all the thiol adducts were the same with λex and λem at 380 nm and 520 nm (Fig. 1d), respectively, suggesting these two wavelengths are appropriate for detection of thiols in biological systems.
The fluorogenicity of TBOP was further confirmed by HPLC using GSH as a model thiol compound. When TBOP (50 µM) was mixed with GSH (2.5 mM) for 20 min, the HPLC/UV chromatogram shows a strong peak for TBOP (Fig. 2Ai) and peaks from the GSH thiol adduct and benzofurazan thiol A (Fig. 2Aii). However, the HPLC chromatograms derived from fluorescence detection (λex = 380 nm, λem = 520 nm) showed only one strong peak that is the GSH thiol adduct (Fig. 2Bii) with no peak observed for TBOP (Fig. 2Bi), further confirming that TBOP is a fluorogenic reagent. Benzofurazan thiol A also exhibited no fluorescence (Fig. 2Bii).
Reactivity of TBOP toward PSH
Unexpectedly, we found that TBOP did not react with PSH. Since it is difficult to monitor thiol adducts of PSH, the reactivity of TBOP with PSH was checked through the detection of the released benzofurazan thiol (benzofurazan thiol A, Scheme 1) and the remaining TBOP by HPLC. BSA is known to contain 35 thiol groups/molecule [12] and was used as a model protein. When TBOP (100 µM) was mixed with BSA (1 mM) in a TBOP:BSA thiol ratio of 1:350 in a Tris buffer (0.1 M, pH 7.4) for 20 min, we were surprised to find that there was no release of benzofurazan thiol A. At the same thiol:TBOP ratio (350:1), NPSH (GSH, cysteine, and homocysteine) completed the reaction with TBOP instantly (data not shown). We also reversed the ratio (BSA thiol:TBOP=1:350). No reaction was observed either (data not shown). Fig. 3A presents the HPLC chromatogram of pure TBOP while the chromatogram in Fig. 3B was obtained from a reaction mixture of TBOP with BSA for 20 min. The released benzofurazan thiol A should appear at 14.833 min as shown in Fig. 3C which was obtained from a reaction mixture of GSH with TBOP. As expected, the reaction of GSH and TBOP yielded two products: the GSH thiol adduct (m/z 835.35) and benzofurazan thiol A (m/z 562.26); both were confirmed by the mass spectrum of the reaction mixture (insert of Fig. 3C). The peak for benzofurazan thiol A at 14.833 min was not present in Fig. 3B. The retention time of the major peak at 17.433 min in Fig. 3B appeared slightly different than that of TBOP (17.225 min) in the chromatogram of Fig. 3A. However, the mass spectrum determination of the fraction collected from the peak at 17.433 confirmed that this peak was still the peak of TBOP (m/z 545.54 in the insert of Fig. 3B) suggesting that there was no reaction between BSA and TBOP. The HPLC chromatogram in Fig. 3D was obtained from BSA alone.
Fig. 3.
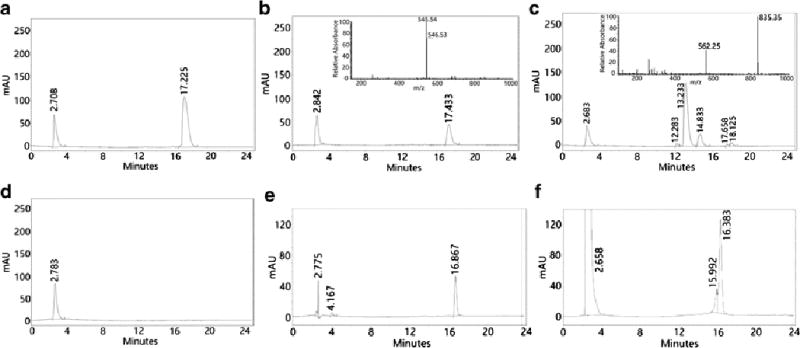
HPLC chromatograms derived from TBOP alone (50 µM) before the reaction of TBOP with BSA (A); reaction mixture of TBOP with BSA (1:10) at room temperature in a Tris buffer (0.1 M, pH 7.4) for 20 min (B); reaction mixture of TBOP with GSH (1:10) at room temperature in a Tris buffer (0.1 M, pH 7.4) for 20 min (C); BSA alone (D). TBOP alone (50 µM) before the reaction of TBOP with protein precipitates from cell homogenates (E); reaction mixture of TBOP with the protein precipitates (F). The HPLC condition is presented in the experimental section except the mobile phase. The mobile phase included solvent A (acetonitrile) and solvent B (0.1% trichloroacetic acid aqueous solution). The initial 2 min was solvent B with 10% solvent A. Solvent A was then linearly increased to 70% in 11 min, and then further increased to 90% in 5 min and kept at 90% for an additional 3 min before back to 10% in 2 min.
To further confirm that TBOP does not react with PSH, protein precipitates separated from cell homogenates were employed. A similar phenomenon was observed. When protein precipitates derived from 5 million NCI-H226 cells were mixed with TBOP (50 µM) for 20 min, no release of benzofurazan thiol A was observed. The HPLC chromatograms in Fig. 3E and 3F were obtained from TBOP alone and the reaction mixture of TBOP with protein precipitates respectively. The TBOP retention time was not the same as that in Fig. 3A due to a slight difference in the HPLC mobile phase. Mass spectrum determination of the fraction collected from the peak at 16.383 min of the chromatogram in Fig. 3F confirmed that this peak was still TBOP. We also checked the possibility of whether a failure to detect the released benzofurazan thiol A was due to protein absorption of the released benzofurazan thiol. Extensive extraction of the protein precipitates with acetonitrile was conducted. Again no benzofurazan thiol A was detected by HPLC. It should be noted that protein thiols from both BSA and protein homogenates were readily detectable by GUALY’s reagent [30]. The possibility of a lack of reaction due to protein absorption of TBOP, which would lead to no free TBOP in the solution and no reaction with PSH, was also ruled out by adding GSH to a reaction solution of BSA and TBOP. The fluorescent GSH adduct was detected the moment GSH was added confirming the presence of free TBOP in the reaction solution.
Based on these results, we concluded that TBOP does not react with PSH. This conclusion was further confirmed by validation using GUALY’s reagent: the NPSH quantified in live cells by TBOP matched well with that determined by GUALY’s reagent (Figure 4 and Table 1). It is not clear why TBOP reacts with NPSH but not PSH at this point. One of the possible explanations is the steric hindrance posted by the large TBOP molecule. This steric hindrance might prevent the molecule from a reaction with proteins.
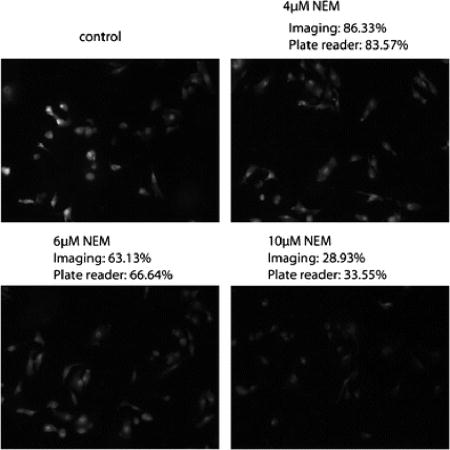
Fig. 4.
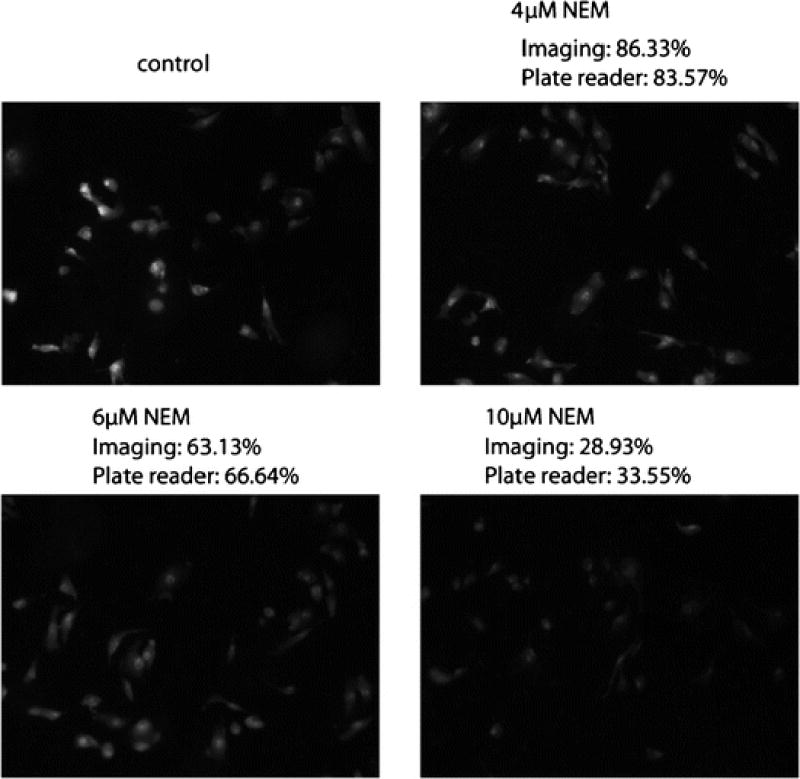
NPSH imaging and quantification by TBOP in live cells using fluorescence microscopy (imaging method) and the validation by GUALY’s reagent (plate reader method). The experimental condition is described in the experimental section.
Table 1.
NPSH quantified by the TBOP imaging assay vs a reported GUALY’s reagent plate reader method.
| NEM concentration (µM) | 0 (control) | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| NPSH determined by TBOP imaging* | 100.00% ±8.90% | 79.65% ±4.76% | 86.33% ±8.51% | 70.22% ±8.43% | 63.13% ±5.10% | 57.62% ±9.35% | 49.36% ±4.91% | 44.05%± 11.13% | 28.93% ±5.25% |
| NPSH determined by GUALY’s reagent** | 100.00% ±4.09% | 83.29% ±1.50% | 83.57% ±2.11% | 81.69% ±6.10% | 66.64% ±2.52% | 71.53% ±7.93% | 54.88% ±4.01% | 47.57%± 3.81% | 33.55% ±5.57% |
n=6 spots from the same sample.
n=3 wells from the same plate. Data are presented as the percentage of control in which cells were treated with no NEM. Data are from one representative experiment of a triplicate.
NPSH imaging and quantification with TBOP in live cells
Optimal TBOP concentration, cell number, and incubation time for NPSH imaging in live cells
NCI-H226 cells, a human lung cancer cell line, were employed as the cell model for the study. This cell line was employed in our previous study for total thiol imaging and quantification in live cells using GUALY’s reagent [29]. The cells were treated with increasing concentrations of TBOP in order to identify an optimal TBOP concentration that would completely derivatize all NPSH in live cells with a minimal cytotoxicity. Trypan blue assay was employed to determine the cell viability. It was found that TBOP caused a significant toxicity to cells at a concentration higher than 20 µM. The cell viability was found to be 94%±6% (n=3) when cells were treated with 10 µM TBOP for 2 h at 37 °C. Later, it was found that this condition was enough to derivatize NPSH in 50,000 cells since an increase of fluorescence continued for 2 h before a fluorescence plateau was observed. Therefore, treatment with TBOP at 10 µM for 2 h at 37 °C was employed to image NPSH in live cells. We have not checked the viability of normal cells treated with TBOP at the same condition. The toxicity probably would vary depending on the division rate and type of cells.
Modulation of intracellular thiol by NEM
To determine whether TBOP can be used to monitor a change in thiol concentration, NEM was employed to modulate intracellular thiol concentration. NEM has been used to modulate intracellular thiols (both PSH and NPSH) through depletion of intracellular thiols by covalent bond formation with a thiol group [32]. Preliminary data revealed that cell detachment occurred when cells were treated with NEM for longer than 4 h. No cell detachment was observed when cells were treated with NEM for 3 h. Also, no further depletion of intracellular thiols was observed once NEM concentration was above 300 µM indicating that NEM at 300 µM was high enough to block all thiols. Therefore, the fluorescence for cells treated with 300 µM NEM was used as the background fluorescence, and NEM at concentrations below 300 µM were employed to modulate intracellular thiols.
Photostability of TBOP thiol adducts in live cells
The fluorescence stability of thiol adducts in live cells was checked by exposing the sample to light under fluorescence microscopy. It was found that the fluorescence intensity of the sample derived from NCI-H226 cells treated with TBOP (10 µM for 2 h at 37 °C) decreased over time and lost ~50% in 120 seconds when exposed to light suggesting that the sample should be protected from light for thiol imaging and quantification. The fluorescence was stable when the samples were covered with aluminum foil. Nevertheless, the photostability is comparable to FITZ [34] that is a commercially available and most widely used fluorescence labeling reagent.
NPSH imaging and quantification in live cells with TBOP
After establishment of the optimal conditions and NEM thiol modulating conditions, experiments were conducted to determine whether TBOP could be used to image and quantify a change of NPSH in live cells. The change in intracellular NPSH was achieved by pre-incubating cells with NEM before NPSH imaged by TBOP. Pretreatment of cells with different concentrations of NEM provided cells with different intracellular thiol concentrations. Fig. 4 presents representative images derived from NPSH imaging by 10 µM TBOP at 37 °C for 2 h in live cells pre-treated with 0, 4, 6, and 10 µM NEM. As shown in the figure, fluorescence intensity decreased in cells pre-treated with an increase in NEM concentration revealing that TBOP could detect a change in intracellular NPSH. Compared with the cells treated with no NEM, the relative fluorescence intensities were 86.3%, 63.1% and 28.9% for cells pretreated with 4, 6, and 10 µM NEM, respectively. Table 1 is the tabular presentation of the relative fluorescence intensity produced from a reaction of TBOP with NPSH in cells pre-treated with eight different concentrations of NEM. As shown in the table, TBOP readily detected changes in NPSH produced by NEM.
Validation of the live cell NPSH imaging method
To validate the quantification of NPSH by TBOP, an experiment was designed to check whether the quantification matched the quantification by GUALY’s reagent. GUALY’s reagent was effectively used to quantify NPSH obtained from cell homogenates in a 96 well plate [30]. In this experiment, a 12-well plate was used in order to be consistent with the plate type used for NPSH imaging with TBOP. As presented in Fig. 4 and Table 1, the percentages of NPSH determined by TBOP through live cell imaging match well (less than 5% difference) with most of the percentages determined by GUALY’s reagent with a plate reader method, though a greater than 10% difference was observed for NEM concentration at 5 and 7 µM (Table 1). These data confirm that the TBOP imaging method is a valid method for NPSH quantification.
Conclusion
In summary, a fluorescent probe TBOP was developed for the detection and quantification of NPSH in live cells through fluorescence microscopy. TBOP is thiol specific, fluorogenic, and exhibits good selectivity toward NPSH. The compound also has an excellent water solubility, and demonstrates good cell permeability and low toxicity at the concentration employed. TBOP serves as a complimentary reagent to GUALY’s reagent that can determine total thiols in live cells through fluorescence microscopy [29]. Together with GUALY’s reagent, TBOP will be a valuable tool in exploring the roles of PSH, NPSH, and total thiols in thiol-related physiological/pathological functions in live cells using fluorescence microscopy.
Supplementary Material
Acknowledgments
The authors would like to thank Professor Adam Hoppe of the Chemistry and Biochemistry Department, Professor Michael Hildreth of Biology and Microbiology, and Professor Hemachand Tummala of Pharmaceutical Sciences for technical assistance in fluorescence microscopy experiments and valuable discussion. The authors also would like to thank Professor Teresa Seefeldt for proofreading the manuscript. This work was supported by grants from the National Institutes of Health (1R15GM093678-01; 1R15GM107197-01A1).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Haugaard N. Reflections on the role of the thiol group in biology. Ann N Y Acad Sci. 2000;899:148–58. doi: 10.1111/j.1749-6632.2000.tb06183.x. [DOI] [PubMed] [Google Scholar]
- 2.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208.. [DOI] [PubMed] [Google Scholar]
- 3.Coulter CV, Kelso GF, Lin TK, Smith RA, Murphy MP. Mitochondrially targeted antioxidants and thiol reagents. Free Radic Biol Med. 2000;28(10):1547–54. doi: 10.1016/s0891-5849(00)00255-0. doi: S0891-5849-(00)00255-0 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol. 2004;39(10):1513–9. doi: 10.1016/j.exger.2004.08.014. doi: S0531-5565(04)00269-4 [pii] 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigobello MP, Folda A, Scutari G, Bindoli A. The modulation of thiol redox state affects the production and metabolism of hydrogen peroxide by heart mitochondria. Arch Biochem Biophys. 2005;441(2):112–22. doi: 10.1016/j.abb.2005.07.007. doi: S0003-9861(05)00289-4 [pii] 10.1016/j.abb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Shipounova IN, Svinareva DA, Petrova TV, Lyamzaev KG, Chernyak BV, Drize I, et al. Reactive oxygen species produced in mitochondria are involved in age-dependent changes of hematopoietic and mesenchymal progenitor cells in mice. A study with the novel mitochondria-targeted antioxidant SkQ1. Mech Ageing Dev. 2010;131(6):415–21. doi: 10.1016/j.mad.2010.06.003. doi: 10.1016/j.mad.2010.06.003S0047637410001144 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Lipton SA. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer’s and Parkinson’s diseases. Apoptosis. 2010;15(11):1354–63. doi: 10.1007/s10495-010-0476-x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11(11):2685–700. doi: 10.1089/ARS.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacciatore I, Cornacchia C, Pinnen F, Mollica A, Di Stefano A. Prodrug approach for increasing cellular glutathione levels. Molecules. 2010;15(3):1242–64. doi: 10.3390/molecules15031242. doi: 10.3390/molecules1503124215031242 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross EK, Gray JJ, Winter AN, Linseman DA. Immunocal(R) and preservation of glutathione as a novel neuroprotective strategy for degenerative disorders of the nervous system. Recent Pat CNS Drug Discov. 2012;7(3):230–5. doi: 10.2174/157488912803252014. doi: RPCN-EPUB-201206214 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Martin HL, Teismann P. Glutathione–a review on its role and significance in Parkinson’s disease. FASEB J. 2009;23(10):3263–72. doi: 10.1096/fj.08-125443. doi: 10.1096/fj.08-125443fj.08125443 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Zhao Y, Seefeldt T, Guan X. Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. J Pharm Biomed Anal. 2008;48(5):1375–80. doi: 10.1016/j.jpba.2008.08.033. doi: 10.1016/j.jpba.2008.08.033S0731-7085(08)004780 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 14.Shimada K, Mitamura K. Derivatization of thiol-containing compounds. J Chromatogr B Biomed Appl. 1994;659(1–2):227–41. doi: 10.1016/0378-4347(93)e0444-u. [DOI] [PubMed] [Google Scholar]
- 15.Fu NNW, H, Li ML, Zheng GJ, Zhang HS, Liang SC. Spectrofluorimetric Determination of Thiols in Biological Samples with a New Fluorescent Probe 3-Maleimidylbenzanthrone. Anal lett. 2005;38:791–802. [Google Scholar]
- 16.Chen SJ, Chang HT. Nile red-adsorbed gold nanoparticles for selective determination of thiols based on energy transfer and aggregation. Anal Chem. 2004;76(13):3727–34. doi: 10.1021/ac049787s.. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Rusin O, Xu X, Kim KK, Escobedo JO, Fakayode SO, et al. Detection of homocysteine and cysteine. J Am Chem Soc. 2005;127(45):15949–58. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durocher S, Rezaee A, Hamm C, Rangan C, Mittler S, Mutus B. Disulfide-linked, gold nanoparticle based reagent for detecting small molecular weight thiols. J Am Chem Soc. 2009;131(7):2475–7. doi: 10.1021/ja808548x. doi: 10.1021/ja808548x101021/ja808548x [pii] [DOI] [PubMed] [Google Scholar]
- 19.Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18(1):17–25. doi: 10.1016/j.copbio.2007.01.003. doi: S0958-1669(07)00004-3 [pii] 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Rice GC, Bump EA, Shrieve DC, Lee W, Kovacs M. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 1986;46(12 Pt 1):6105–10. [PubMed] [Google Scholar]
- 21.Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15(4):349–58. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 22.Ahn YH, Lee JS, Chang YT. Combinatorial rosamine library and application to in vivo glutathione probe. J Am Chem Soc. 2007;129(15):4510–1. doi: 10.1021/ja068230m. [DOI] [PubMed] [Google Scholar]
- 23.Lim CS, Masanta G, Kim HJ, Han JH, Kim HM, Cho BR. Ratiometric detection of mitochondrial thiols with a two-photon fluorescent probe. J Am Chem Soc. 2011;133(29):11132–5. doi: 10.1021/ja205081s. [DOI] [PubMed] [Google Scholar]
- 24.Pullela PK, Chiku T, Carvan MJ, 3rd, Sem DS. Fluorescence-based detection of thiols in vitro and in vivo using dithiol probes. Anal Biochem. 2006;352(2):265–73. doi: 10.1016/j.ab.2006.01.047. doi: S0003-2697(06)00081-9 [pii] 10.1016/j.ab.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 25.Lee MH, Han JH, Kwon PS, Bhuniya S, Kim JY, Sessler JL, et al. Hepatocyte-targeting single galactose-appended naphthalimide: a tool for intracellular thiol imaging in vivo. J Am Chem Soc. 2012;134(2):1316–22. doi: 10.1021/ja210065g. [DOI] [PubMed] [Google Scholar]
- 26.Tang B, Xing Y, Li P, Zhang N, Yu F, Yang G. A rhodamine-based fluorescent probe containing a Se-N bond for detecting thiols and its application in living cells. J Am Chem Soc. 2007;129(38):11666–7. doi: 10.1021/ja072572q. [DOI] [PubMed] [Google Scholar]
- 27.Shibata A, Furukawa K, Abe H, Tsuneda S, Ito Y. Rhodamine-based fluorogenic probe for imaging biological thiol. Bioorg Med Chem Lett. 2008;18(7):2246–9. doi: 10.1016/j.bmcl.2008.03.014. doi: 10.1016/j.bmcl.2008.03.014S0960-894X(08)00288-6 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–34. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Yang Y, Guan X. Benzofurazan sulfides for thiol imaging and quantification in live cells through fluorescence microscopy. Anal Chem. 2012;84(15):6877–83. doi: 10.1021/ac301306s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Guan X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonprotein thiols in cells. Anal Chem. 2015;87(1):649–55. doi: 10.1021/ac503411p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen S, Shea JM, Felmet T, Gadra J, Dehn PF. A kinetic microassay for glutathione in cells plated on 96-well microtiter plates. Methods Cell Sci. 2000;22(4):305–12. doi: 10.1023/a:1017585308255. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Lim CS, Tian YS, Han JH, Cho BR. A two-photon fluorescent probe for thiols in live cells and tissues. J Am Chem Soc. 2010;132(4):1216–7. doi: 10.1021/ja9090676. [DOI] [PubMed] [Google Scholar]
- 33.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43(1 Suppl):25–30. doi: 10.2144/000112517. doi: 000112517 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Longin A, Souchier C, Ffrench M, Bryon PA. Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J Histochem Cytochem. 1993;41(12):1833–40. doi: 10.1177/41.12.8245431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


