Abstract
Resin chemistries for dental composite are evolving as noted by the introduction of silorane-based composites in 2007. This shift in the landscape from methacrylate-based composites has fueled the quest for versatile methacrylate-silorane adhesives. The objective of this study was to evaluate the polymerization behavior and structure/property relationships of methacrylate-silorane hybrid systems. Amine compound ethyl-4-(dimethylamino) benzoate (EDMAB) or silane compound tris(trimethylsilyl) silane (TTMSS) was selected as coinitiators. The mechanical properties of the copolymer were improved significantly at low concentrations (15, 25, or 35 wt %) of silorane when EDMAB was used as coinitiator. The rubbery moduli of these experimental copolymers were increased by up to 260%, compared with that of the control (30.8 ± 1.9 MPa). Visible phase separation appeared in these formulations if the silorane concentrations in the formulations were 50–75 wt %. The use of TTMSS as coinitiator decreased the phase separation, but there was a concomitant decrease in mechanical properties. In the neat methacrylate formulations, the maximum rates of free-radical polymerization with EDMAB or TTMSS were 0.28 or 0.06 s−1, respectively. In the neat silorane resin, the maximum rates of cationic ring-opening polymerization with EDMAB or TTMSS were 0.056 or 0.087 s−1, respectively. The phase separation phenomenon may be attributed to differences in the rates of free-radical polymerization of methacrylates and cationic ring-opening polymerization of silorane. In the hybrid systems, free-radical polymerization initiated with EDMAB led to higher crosslink density and better mechanical properties under dry/wet conditions. These beneficial effects were, however, associated with an increase in heterogeneity in the network structure.
Keywords: dental polymers, heterogeneous polymers, phase separation, photopolymerization, ring-opening polymerization
INTRODUCTION
The traditional methacrylate-based dental composites were first developed in the mid-1960s.1 The properties and handling characteristics have improved greatly since the first introduction of these materials. During the last 20 years, the primary motivation for changing the resin chemistry of restorative composites was to reduce the polymerization shrinkage.2,3 Recently, the silicone-epoxy monomers (or “silorane”) restorative system represents a relatively new category of restorative materials; these materials are marketed commercially as a low shrinkage composite with improved clinical performance.4,5
The silorane is formed by joining siloxane and oxirane, resulting in a monomer that combines the two key advantages of its individual components, that is, low polymerization shrinkage and increased hydrophobicity and stability. These monomers have low toxicity, high reactivity, and the ability to undergo facile and rapid cationic polymerization. Compared to the methacrylate-based composites, the silorane-based composites exhibit lower polymerization shrinkage, higher flexural strength and fracture toughness.6–10 However, silorane composites have relatively lower compressive strength and microhardness.7
In contrast to the promising mechanical properties, contradictory results have been reported with marginal gap formation at the interface between the tooth and silorane composite. An in vitro study11 reported that the silorane restorative systems showed statistically equivalent gap formation compared to a methacrylate-based composite. When marginal adaptation in cylindrical cavities was compared, the silorane-based composites performed better than methacrylate-based materials.12 In contrast to these in vitro investigations, in clinical studies methacrylate-based composites provided better marginal adaptation than silorane-based composites.13 A recent 5 years clinical study showed no clinical advantage to silorane-based composite over the methacrylate-based composite, the clinical results suggest that the low-shrinkage may not be a determinant factor for clinical success in class II cavity.14
Due to the incompatibility between methacrylate and silorane,15 a dedicated adhesive system is required for the silorane restorative composite. D’Alpino et al.15 reported that the dedicated adhesive system exhibited signs of degradation immediately after application. This immediate degradation may be attributed to the hydrophilic composition of the primer. The results suggested that the hydrophilic nature of the dentin adhesive represented the weakest link, despite the hydrophobic nature of the silorane-based composites. The poorer marginal adaptation of the silorane restorative composite was linked to the adhesive system.13 Our interest lies in the development of a hybrid methacrylate/silorane polymer that offers compatibility and hydrolytic stability.
The hybrid resin that contains (meth)acrylates and epoxide monomers undergoes simultaneous free radical polymerization (FRP) and cationic ring-opening polymerization (CROP), respectively. The hybrid system has been shown to reduce the atmospheric16 and/or water/alcohol sensitivity.17 This could be a very attractive benefit for adhesives or coatings that must polymerize under hostile environmental conditions. Phase separation is, however, an inevitable phenomenon in these hybrid resin formulations due to the low conversion and polymerization rate of epoxides and/or chemical incompatibility. To control phase separation, most of the techniques have focused on enhancing the conversion and rate of cationic polymerization by hybrid monomers,17,18 chain transfer agents,19 and accelerators.20–22 The most common approach to decrease phase separation in the hybrid methacrylate-epoxide system is to accelerate the CROP.18,19,21 It is well known that the liquid resin can form a network structure in just a few seconds of light irradiation. The polymerization rate will undergo a significant decrease as the polymer transitions from a viscous to glassy state (vitrification point). It is unclear how to balance the rate of polymerization and mechanical properties in the hybrid system. Considerable effort has been devoted throughout the material science community to quantifying the polymerization process with the goal of predicting the final properties of the polymer network by combining simulations and experimental swelling data.23 In spite of these efforts, a comprehensive description of the relationship between polymerization kinetics and final crosslink structure remains a challenging problem. The complexity of this problem is increased in the methacrylate-silorane hybrid formulations by variables such as polymerization-induced phase separation.
The aim of this research was to study the polymerization behavior of a methacrylate/silorane hybrid system, and to determine the relationship between polymerization behavior and final properties of the network. This is the first step in our efforts to optimize the properties of the hybrid resin. In the present investigation, methacrylate monomers (HEMA and BisGMA) and silorane monomer were chosen to be consistent with the current chemistry used in commercial dentin adhesive/composites. The polymerization behavior, mechanical properties, and network structure of the hybrid formulations prepared by different coinitiators (amine or silane) were compared. Here, we compared the effect of amine and silane coinitiator on the polymerization behavior of methacrylate/silorane hybrid formulations and the mechanical properties of the formed hybrid polymers. The overall research hypotheses were (1) the polymerization-induced phase separation can be depressed by matching the polymerization rates between free radical and cationic ring-opening polymerization, (2) the crosslink density of the hybrid resin can be improved with the addition of silorane, and (3) the free radical polymerization rate will not affect the mechanical properties of the hybrid resin.
MATERIALS AND METHODS
Materials
2,2-Bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]propane (BisGMA, Polysciences, Warrington, PA) and 2-hydroxyethyl methacrylate (HEMA, Acros Organics, NJ) were used as received without further purification. 2,4,6,8-tetramethyl-2,4,6,8-tetrakis[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]-1,3,5,7-tetraoxa-2,4,6,8-tetrasilacyclooxtane (ES-4) was synthesized in-house. Camphoroquinone (CQ), ethyl-4-(dimethyl amino) benzoate (EDMAB), tris(trimethylsilyl)silane (TTMSS), 2,4,6,8-tetramethylcyclotetrasiloxane, tris(triphenylphosphine)rhodium(I) chloride (Wilkinson’s catalyst), and 4-vinyl-1-cyclohexene 1,2-epoxide were obtained from Sigma-Aldrich (St. Louis, MO). p-Octyloxy-phenylphenyl iodonium hexafluoroantimonate (OPPIH) was purchased from Gelest Inc., (Morrisville, PA). All other chemicals were reagent grade and used without further purification.
Synthesis of ES-4
The silorane monomer ES-4 was synthesized following the procedure described by Crivello and coworkers24 with slight modification.25 Briefly, to a 250-mL, round bottom flask, fitted with a magnetic stirrer and reflux condenser, were added 2,4,6,8-tetramethylcyclotetrasiloxane 12.02 g (0.05 mol), 30 mg Wilkinson’s catalyst, and 40 mL dry toluene. After the solution was heated to 60°C, 26.08 g (0.41 mol) 4-vinyl-1-cyclohexene 1,2-epoxide mixed with 40 mL dry hexane was added stepwise over a 2 h period. Next, the reaction mixture was raised to 80°C for another 20 h. The progress of the hydrosilation was followed by FTIR (Spectrum 400 Fourier transform infrared spectrophotometer, Perkin-Elmer, Waltham, MA) by monitoring the disappearance of Si-H at 2165 cm−1. When the reaction was terminated, about 2 g activated carbon was added to remove the catalyst. The unreacted starting materials and solvent were removed by rotary evaporation. ES-4 (35 g, 95% theory) was obtained as viscous, pale yellow oil.
The structure was confirmed using 1H NMR spectroscopy. 1H NMR (CDCl3, FT-400 MHz Bruker Spectrometer, δ ppm): 0.04 (12H, CH3—Si—), 0.46 (8H, —CH2—Si—), 0.86–2.16 (36H, –CH2–cyclohexane ring), and 3.14 (8H, —CH—O epoxy ring).
Preparation of resin formulations
Neat methacrylate monomer mixture was made by mixing 45 wt % HEMA and 55 wt % BisGMA.26–28 Neat methacrylate and neat silorane formulations containing the photoinitiators (PIs) CQ (1 wt %), OPPIH (2 wt %), and EDMAB (1 wt %) were used as the controls (C0-EDMAB, ES-EDMAB), respectively. The hybrid formulations consisted of HEMA, BisGMA, PIs (CQ 1 wt %, OPPIH 2 wt %, EDMAB 1 wt %, or TTMSS 3%), and contained varying concentrations of ES-4. The chemical structures of monomers and PIs are shown in Scheme 1. The composition of the neat resin and hybrid formulations are listed in Tables I and II, respectively. Mixtures of monomers/PIs were prepared in brown glass vials under amber light.
SCHEME 1.
Chemical structures of components used in the hybrid formulations.
TABLE I.
The Composition of Neat Methacrylate and Neat Silorane Formulations
| Runa | Monomers (g)
|
Coinitiators (%)
|
|||
|---|---|---|---|---|---|
| HEMA | BisGMA | ES-4 | EDMAB | TTMSS | |
| C0-EDMABb | 0.45 | 0.55 | – | 1 | – |
| C0-TTMSS 1% | 0.45 | 0.55 | – | – | 1 |
| C0-TTMSS 3% | 0.45 | 0.55 | – | – | 3 |
| C0-TTMSS 5% | 0.45 | 0.55 | – | – | 5 |
| ES-EDMABc | – | – | 1.00 | 1 | – |
| ES-TTMSS 1% | – | – | 1.00 | – | 1 |
| ES-TTMSS 3% | – | – | 1.00 | – | 3 |
In all formulations, the weight percent of CQ and OPPIH is 1 wt % and 2 wt %, respectively.
The control of neat methacrylate formulation.
The control of neat silorane formulation.
TABLE II.
The Composition of Hybrid Resin Formulations
| Runa | Monomers (g)
|
Coinitiators (%)
|
||
|---|---|---|---|---|
| C0b | ES-4 | EDMAB | TTMSS | |
| ES 15%-EDMAB | 0.85 | 0.15 | 1 | – |
| ES 25%-EDMAB | 0.75 | 0.25 | 1 | – |
| ES 35%-EDMAB | 0.65 | 0.35 | 1 | – |
| ES 50%-EDMAB | 0.50 | 0.50 | 1 | – |
| ES 75%-EDMAB | 0.25 | 0.75 | 1 | – |
| ES 15%-TTMSS | 0.85 | 0.15 | – | 3 |
| ES 25%-TTMSS | 0.75 | 0.25 | – | 3 |
| ES 35%-TTMSS | 0.65 | 0.35 | – | 3 |
| ES 50%-TTMSS | 0.50 | 0.50 | – | 3 |
| ES 75%-TTMSS | 0.25 | 0.75 | – | 3 |
In all formulations, the weight percent of CQ and OPPIH is 1 wt % and 2 wt %, respectively.
C0 is made by mixing 45 wt % HEMA and 55 wt % BisGMA.
The preparation of polymer beams for mechanical property characterization using dynamic mechanical analysis (DMA) has been reported.29–31 Briefly, the solutions containing the monomers/PIs were mixed overnight at 25°C to promote complete dissolution and formation of a homogeneous solution. The prepared resins were injected into a glass-tubing mold (Fiber Optic Center, Inc., part no.: ST8100, New Bedford, MA) and light-cured for 40 s at 25°C with an LED light curing unit (LED Curebox, 100 mW cm−2 irradiance; Proto-tech, Portland, OR). The polymerized samples were stored in the dark at 25°C for at least 48 h before being used. The rectangular beam specimens, with a dimension of 1 mm × 1 mm and length 15 mm, were used to determine dynamic mechanical properties.
For thermal behavior analysis, approximately 20 μL of resin was injected into a hermetic lid (900794.90, TA Instruments, New Castle), covered with mylar film, and light-cured for 40 s at 25°C with a commercial visible-light-polymerization unit (Spectrum® 800; Dentsply, Milford, DE), at an intensity of 550 mW cm−2. The polymerized samples were then stored in the dark at 25°C for at least 48 h and thereafter kept in a vacuum oven at 25°C prior to thermal measurement.
Real-time conversion and maximal polymerization rate
The degree of conversion (DC) and polymerization behavior were determined by FTIR as described previously.27,32 Real-time, in situ monitoring of the photopolymerization behavior of the resin formulations was performed using an infrared spectrometer (Spectrum 400 Fourier transform infrared spectrophotometer; Perkin-Elmer) at a resolution of 4 cm−1.
One drop of the resin was placed on the diamond crystal top plate of an attenuated total reflectance (ATR) accessory (PIKE Technologies Gladi-ATR, Madison, WI) and covered with a mylar film to prevent oxygen exposure. Exposure to the commercial visible-light-polymerization unit (Spectrum® 800; Dentsply) at an intensity of 550 mW cm−2 was initiated after 50 infrared spectra had been recorded. The light exposure time was 40 s. Real-time IR spectra were continuously recorded for 600 s after light activation began. A time-based spectrum collector (Spectrum TimeBase; Perkin-Elmer) was used for continuous and automatic collection of spectra during polymerization. A minimum of three measurements (n = 3) were carried out for each formulation. Methacrylic double bond conversion was monitored by the band ratio profile-1637 cm−1 (C=C)/1608 cm−1 (phenyl), and epoxy group conversion was followed by monitoring the decrease in the absorbance of the epoxy group at 884 cm−1. The calculation for epoxy group conversion was based on the published article.16,17,33,34 The degree of conversion (DC) of C=C bond and epoxy were calculated using the following equations.
| (1) |
| (2) |
The average of the last 50 values of the time-based spectra is reported as the DC value. The maximum polymerization rate was determined using the maximum slope of the linear region of the DC versus time plots.26
Dynamic mechanical analysis
In dynamic mechanical tests, a sinusoidal stress is applied, and the resultant strain is measured to obtain the storage and loss moduli and tan δ. The storage modulus (E′) represents the amount of energy recovered during the cyclic loading, and this value is proportional to the elasticity of a viscoelastic solid. The loss modulus (E′′) represents viscous dissipation in the cyclic process. The ratio of loss modulus (E′′) to storage modulus (E′) is referred to as the mechanical damping, or tan δ (that is, tan δ = E′/E′′). The tan δ value reaches a maximum as the polymer undergoes the transition from the glassy state to the rubbery state. The intensity of the maximum tan δ peak reflects the extent of the mobility of the copolymer chain segments. In the current work, DMA tests were performed using a TA instruments Q800 DMA (TA Instruments, New Castle) with a three-point bending clamp. The dynamic mechanical properties of methacrylate-based adhesive formulations have been described previously.32 A sinusoidal stress is applied, and the resultant strain is measured. Rectangular beam specimens (1 mm × 1 mm × 15 mm) were used for DMA measurements and minimum of three specimens were tested for each material. The following testing parameters were used: displacement amplitude of 15 μm, frequency of 1 Hz and preload force of 0.01 N.30,35 In addition to this, temperature was ramped at the rate of 3°C min−1 from 10 to 200°C. The glass transition temperature (Tg) is determined as the position of the maximum on the derivative storage modulus versus temperature plots. For wet testing, specimens were submerged in water at 37°C for 5 days for complete saturation, and tests were obtained using the three-point submersion clamp.36 The test temperature was varied from 10 to 80°C with a ramping rate of 1.5°C min−1.
Modulated differential scanning calorimetry test
The thermal behavior in the Tg region was measured with a TA instruments model Q200 modulated differential scanning calorimetry (MDSC; New Castle, DE). The specimens were weighed (~20 mg) in aluminum DSC pans. The DSC cell was purged with nitrogen gas at 50 mL min−1, and the specimens were heated under nitrogen purge from 220 to 200°C at 3°C min−1, with a modulation period of 60 s and amplitude of ±2°C. The second scans were consistent with the first scan. Only the first cycle of heating was taken into account, and the results are shown as differential reversible heat flow versus temperature graphs. The Tg values were reported as the temperature of the peaks, that is, inflection points of the heat flow curves.
Statistical analysis
The results were analyzed statistically using one-way analysis of variance, together with Tukey’s test at α = 0.05 (Microcal Origin Version 8.0; Microcal Software, Northampton, MA) to identify significant differences in the means.
RESULTS
Real-time photopolymerization kinetic behavior of the neat methacrylate and neat silorane formulations are shown in Figure 1. In neat methacrylate resin, with the increase in TTMSS concentration from 1 to 5%, the degree of conversion of the double bond (at 600s) increased slightly from ~58 to ~65%, which is significantly (p < 0.05) lower than the control (C0-EDMAB 1%). Compared to the control, the maximum polymerization rate (P.R. or Rp(max)/[M]) of the free radical polymerization (FRP) decreased from 0.28 to 0.06 s−1. In the neat silorane resin, with the increase in TTMSS concentration from 1 to 3%, the degree of conversion of the epoxy group is over 50% and is significantly higher (p < 0.05) than the control (ES-EDMAB 1%). At the same time, the P.R. of cationic ring-opening polymerization (CROP) increased from 0.056 ± 0.001 to ~0.087 ± 0.008 s−1. However, the DC of epoxy and P.R. of CROP were not significantly different (p < 0.05) when TTMSS concentration was 1 or 3 wt %.
FIGURE 1.
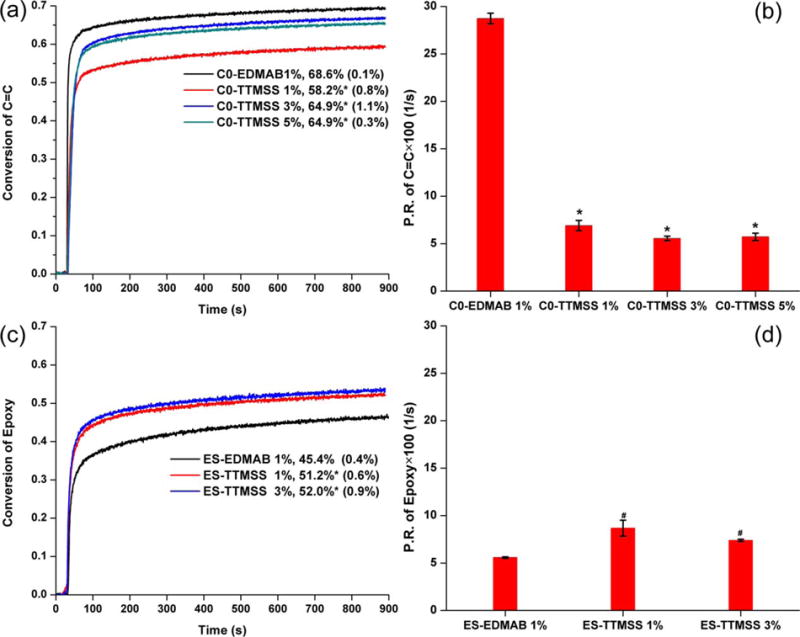
Real-time conversion and polymerization rate of controls (C0-EDMAB 1%, ES-EDMAB 1%) and experimental adhesive formulations. A and B, Neat methacrylate resin, (C and D):neat silorane resin. The adhesives were light-cured for 40 s at 25°C using a commercial visible light lamp (Spectrum® 800; Dentsply, Milford, DE. Intensity is 550 mW cm−2). * Significantly (p < 0.05) different from the control (C0-EDMAB 1%). # Significantly (p <0.05) different from the control (ES-EDMAB 1%). [Color figure can be viewed in the online issue, which is available at wileyonli-nelibrary.com.]
The DC and P.R. of methacrylate-silorane hybrid formulations obtained from the real-time photopolymerization kinetic study are listed in Table III. When the silorane concentration was kept below 35%, the polymerization rates and degree of conversion of silorane were not easy to determine by FTIR due to the characteristic peak overlap. Using EDMAB as coinitiator, the P.R. of FRP was obviously higher than that of CROP, and phase separation was visible in the sample beams (white spots or translucent). Using TTMSS as coinitiator, the difference between the P.R. of FRP and CROP was reduced, and the polymer beams became transparent.
TABLE III.
Results of DCs and Polymerization Rates of Methacrylate-Silorane Hybrid Formulations
| Run | ES-4:C0a (%:%) | Coinitiator (%)b
|
DC (%)c
|
P.R.×100 (s−1)d
|
Appearance of Beam | |||
|---|---|---|---|---|---|---|---|---|
| EDMAB | TTMSS | C=C | Epoxy | C=C | Epoxy | |||
| C0-EDMAB | 0:100 | 1 | 68.6 (0.1) | 28.73 (0.56) | Transparent | |||
| ES 25%-EDMAB | 25:75 | 1 | 71.8 (0.2) | – | 24.92 (1.75) | – | Transparent | |
| ES 50%-EDMAB | 50:50 | 1 | 69.6 (0.6) | 40.1 (0.7) | 22.70 (1.40) | 2.56 (0.38) | Turbid white | |
| ES 75%-EDMAB | 75:25 | 1 | 56.7 (1.1) | 41.4 (0.3) | 12.15 (0.93) | 2.52 (0.41) | Turbid white | |
| ES-EDMAB | 100:0 | 1 | 45.4 (0.4) | 5.58 (0.08) | Transparent | |||
| ES 50%-TTMSS | 50:50 | 3 | 65.6 (0.2) | 39.6 (0.5) | 6.50 (0.29) | 3.14 (0.18) | Transparent | |
| ES 75%-TTMSS | 75:25 | 3 | 62.5 (1.7) | 39.6 (1.6) | 4.95 (0.81) | 2.37 (0.11) | Translucent | |
| ES-TTMSS 3% | 100:0 | 3 | 52.0 (0.9) | 7.38 (0.12) | Transparent | |||
C0 was mixture of HEMA and BisGMA with a weight ratio of 45/55 (w/w).
The weight percent of coinitiator to the total resin.
The degree of conversion was obtained by real-time FTIR method after irradiation 600 s.
P.R.= Rp/[M0], s−1.
The appearance of polymer beams observed after the LED light 40-s irradiation. The value in the () is the standard deviation. – indicated the value cannot be obtained due to the characteristic peak of epoxy was too small.
The dynamic mechanical properties of hybrid formulations in the dry condition at various temperatures are shown in Figure 2. When EDMAB was used as coinitiator, the storage modulus values at 25 or 37°C were not significantly different from the control (C0-EDMAB, p < 0.05), except for the ES-4 35 wt % concentration. The rubbery modulus and tan δ for the experimental copolymers were significantly different from the control (C0-EDMAB, p < 0.05). With the increase in ES-4 content from 0 to 35 wt %, the difference between the two transition temperatures obtained from the derivative storage modulus versus temperature profiles increased from 56.0 ± 0.7 to 74.1 ± 1.8°C. Additionally, the intensity of the maximum tan δ peaks decreased accordingly.
FIGURE 2.
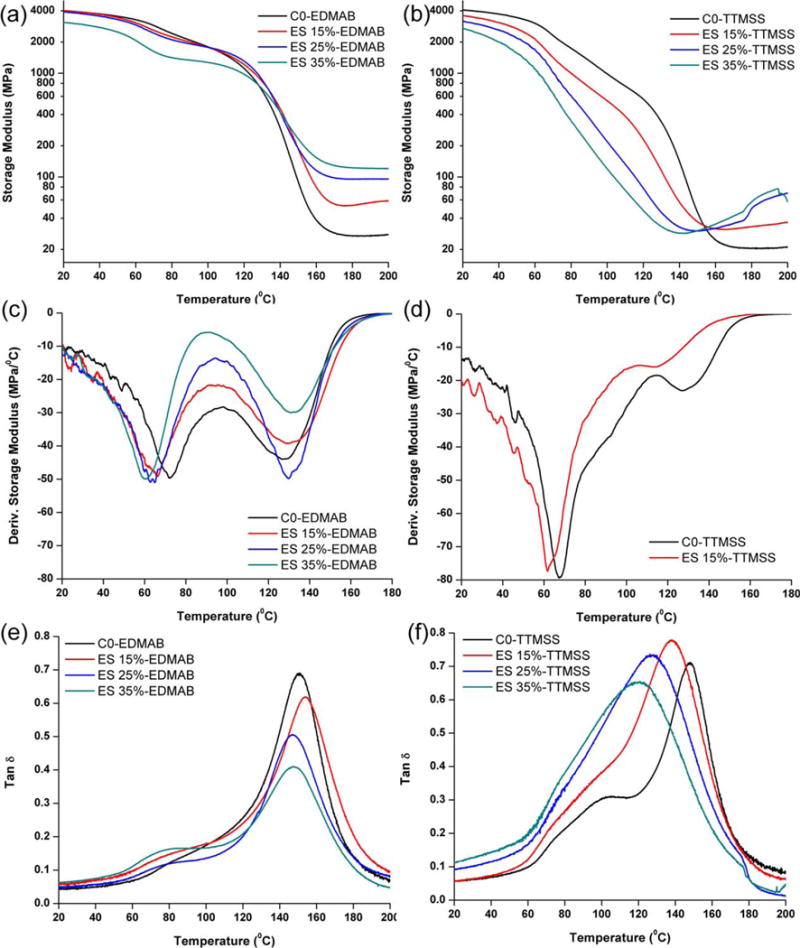
Representative storage modulus, derivative storage modulus, and tan δ versus temperature curves of the controls (C0-EDMAB and C0-TTMSS) and experimental adhesive copolymers in dry conditions using EDMAB (left, A, C, and E) and TTMSS (right, B, D, and F) as coinitiator. [Color figure can be viewed in the online issue, which is available at wileyonli-nelibrary.com.]
When TTMSS was used as coinitiator, both the storage moduli (25 and 37°C) and the intensity of tan δ were significantly different from the control (C0-TTMSS, p < 0.05). The rubbery modulus values were similar in spite of the change in concentration of the ES-4 monomer. From the derivative storage modulus versus temperature profiles, two transition peaks merge gradually with the increase in ES-4 concentration [Figure 2(D)]. The maximum values of the tan δ peaks move toward lower temperatures, and the peaks become broad with the increase in ES-4 content. The storage moduli of methacrylate formulations (C0-EDMAB and C0-TTMSS) were similar at 25 and 37°C. The rubbery modulus of C0-TTMSS (20.4 ± 0.4 MPa) was significantly lower than that of C0-EDMAB (26.8 ± 0.5 MPa).
The dynamic mechanical properties of the copolymers in the wet condition are shown in Figure 3. When EDMAB was used as coinitiator, the storage modulus at 70°C of the experimental formulation (ES-4 35%) was significantly higher than the control (C0-EDMAB, p < 0.05). When TTMSS was used to replace EDMAB as coinitiator, the storage moduli of the experimental formulations were significantly lower than the control (C0-TTMSS, p < 0.05). The storage moduli at 25, 37, and 70°C of methacrylate formulations of C0-EDMAB were significantly higher than that of C0-TTMSS.
FIGURE 3.
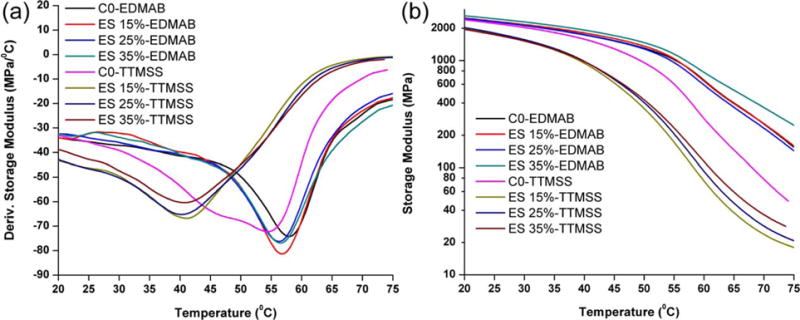
Representative storage modulus and derivative storage modulus versus temperature curves of the controls (C0-EDMAB and C0-TTMSS) and experimental adhesive copolymers in wet conditions using EDMAB or TTMSS as coinitiator. (C0-EDMAB: EDMAB 1 wt %, C0-TTMSS: TTMSS 3 wt %). [Color figure can be viewed in the online issue, which is available at wileyonli-nelibrary.com.]
Figure 4 shows the reversible heat flow signals and derivative reversing heat flow of neat resin or hybrid formulations with EDMAB or TTMSS as coinitiator. In neat methacrylate resin, the difference between the two peaks became smaller with the increase in TTMSS concentration. In neat silorane, the glass transition temperatures of polyether were similar (~64°C) whether EDMAB or TTMSS was used as coinitiator. In the hybrid formulations, there were two transition temperatures for EDMAB and one for TTMSS.
FIGURE 4.
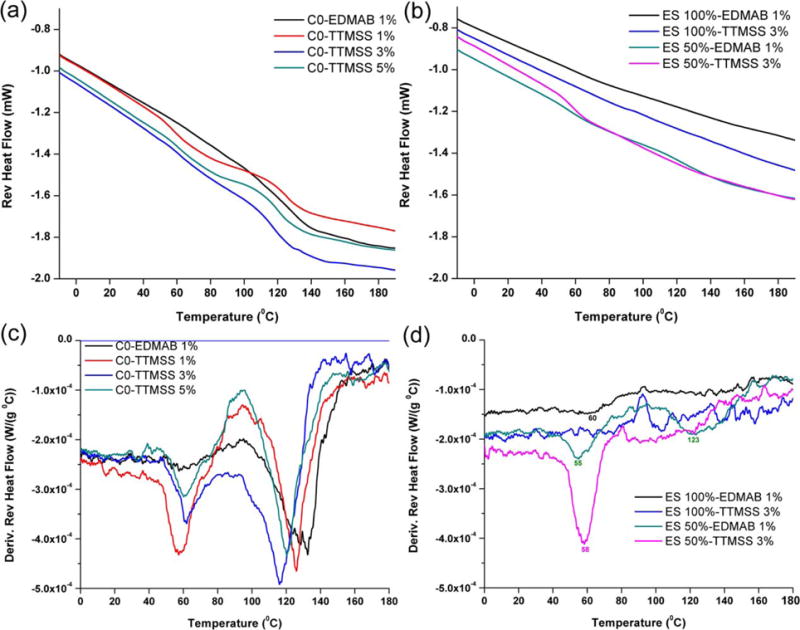
Reversible heat flow signals and derivative reversible heat flow versus temperature of neat methacrylate resin (A and C), methacrylate/silorane hybrid resin (B and D) using EDMAB or TTMSS as coinitiator. [Color figure can be viewed in the online issue, which is available at wileyonli-nelibrary.com.]
DISCUSSION
Polymerization behavior
Amines have been widely used as coinitiators in dental adhesives due to their high reactivity and efficiency. Recently, there has been strong interest in the photopolymerization reaction of silanes. Silane is reported to be more efficient than an amine in FRP or free radical promoted cationic polymerization (FRPCP).37–40 For the TTMSS silyl radical, a high reactivity with addition rate constant (kadd ~ 2:2×107 M−1 s−1) to methylacrylate was noted38. For the EDMAB generated radical, this value was 5 × 105 M−1 s−1.41 These results demonstrate the high potential of the silyl radicals to act as photoinitiating species. In the present work, the maximum polymerization rate of neat methacrylate resin using TTMSS (P.R. ~0.060 s−1) as coinitiator was slower than that of EDMAB (P.R. ~0.287 s−1). It was reported that the hydrogen abstraction rate constants of PI/amines are substantially higher than that of PI/silane (kH ~106–108 M−1 s−1).38 This difference was mainly attributed to a different mechanism, that is, electron/proton transfer for amine versus hydrogen atom transfer for silane.42 The slow CQ/TTMSS hydrogen abstraction could limit the concentration of silyl free radical, which is the crucial step to determine the rate of FRP. In neat methacrylate resin, the polymerization rates were not significantly different when the TTMSS content was varied from 1 to 5 wt %.
In neat silorane formulations, the cationic polymerization rates increased with the addition of TTMSS. This is due to the silyl radicals being reduced efficiently by the iodonium salt (the oxidation rate constant 2.6 × 106 M−1 s−1), and the silylium cation is a particularly accommodating polymerization-initiating structure.43 The FRPCP mechanism was adopted to meet the visible light irradiation in dental adhesive application. The FRPCP is an elegant and fairly flexible way to generate cationic species, and the overall mechanism involves oxidation of photochemically formed radicals by onium salt.44–48 Compared with EDMAB, although the generation of silyl free radical is slower, the generated silylium cation is more efficient in promoting cationic polymerization. At the same time, by increasing TTMSS from 1 to 3 wt %, the DC of the epoxy group can only reach about 50% (measured at t = 600 s after irradiation). This is due to the high functionality of ES-4 (four epoxy groups), that is, the transition from the liquid to vitrified state occurs sooner in this formulation and about 50% of the epoxy groups remain unreacted, presumably leaving the unreacted epoxy groups on the polymer chain as pendent groups.
In the hybrid formulations (methacrylate/silorane), when ES-4 content is below 50 wt %, due to the characteristic peak (884 cm−1) overlap of epoxy with methacrylate, it was difficult to accurately determine the DC and P.R. of epoxy groups by FTIR. When ES-4 content is between 50 and 75 wt % and EDMAB is used as coinitiator, phase separation can be observed visibly after photopolymerization. This phenomenon could be attributed to polymerization-induced phase separation as a result of the chemical thermodynamic incompatibility between methacrylate and siloxane and the significant difference in the polymerization rate using EDMAB as coinitiator (see Table III). After irradiation, methacrylate monomers polymerize quickly and higher crosslink regions can be obtained. Meanwhile, the silorane will polymerize slowly, and polyether-rich regions are formed in the final structure. When the ES-4 concentration is over 50%, the polyether-rich region grows and becomes large enough to allow the phase separation to be observed. When TTMSS was used as coinitiator, the polymerization rate difference between FRP and CROP became smaller, and the polymer specimens were transparent. Thus, the results support the Hypothesis (1) that the polymerization-induced phase separation could be depressed by matching the polymerization rates.
In the hybrid formulations, the free radical centers (EDMAB, TTMSS, or phenyl free radicals) generated during visible-light irradiation are suitable for initiating the FRP. Meanwhile, the generated cations via FRPCP mechanism initiate the CROP of silorane monomers (ES-4). It has been reported that the activated monomer mechanism of cationic polymerization occurs in the presence of a nucleophilic species, such as alcohol.19,49 In our hybrid formulations, the epoxy groups could react with the hydroxyl groups of HEMA or BisGMA and covalently bond the epoxide network to the polymethacrylate network; this reaction would be beneficial in terms of reducing the phase separation and enhancing the crosslink density of the network structure. When using EDMAB as coinitiator, the much faster free radical polymerization created the polymethacrylate domain first, thereby preventing the silorane from achieving high conversion. Therefore, the phase separation phenomenon was observed when the silorane concentration was over 50 wt %. When TTMSS was used as coinitiator, the gap between the rate of polymerization for free radical and cationic ring-opening polymerization was smaller. This decrease in the difference of polymerization rates facilitated the cationic polymerization through the activated monomer mechanism, and the phase separation was depressed. This result indicated that the cationic polymerization mechanism (activated monomer mechanism) and the matching polymerization rates between FRP and CROP can reduce the phase separation in the hybrid formulations. It should be noted that the phase separation phenomenon, especially nanophase separation, is a general feature of crosslinked polymethacrylate-based dentin adhesive.50,51 In the present work, with the addition of TTMSS, only the microphase separation phenomenon was retarded.
Dynamical mechanism analysis
The DMA data can be used to interpret properties of polymer networks because it gives information on the relaxation of molecular motions, which are sensitive to structure. In this study, the DMA tests were carried out using both standard 3-point bending and 3-point bending submersion methods. It was anticipated that the results acquired with the water-submersion clamp would be more representative of the behavior of the polymer in a wet environment.
The polymer specimens of hybrid formulations (ES-4 concentration > 35 wt %) were very brittle and could not be cast into rectangular beam specimens for DMA measurements. Therefore, only specimens cured with lower ES-4 concentration (<35 wt %) were used for the DMA measurements.
Methacrylate polymers containing either EDMAB or TTMSS have similar storage moduli, as shown in Figure 2(A,B). The rubbery modulus of polymer beams with EDMAB (26.8 ± 0.5 MPa) was higher than that of TTMSS (20.4 ± 0.4 MPa), which suggested a lower crosslink density structure with TTMSS. Although the degree of conversion of the double bond is similar (~68% for 1 wt % EDMAB and 65% for 3 wt % TTMSS), the polymerization rates are significantly different (~0.28 s−1 for EDMAB and 0.055 s−1 for TTMSS). Work published by Bowman’s group52–54 has indicated that in highly crosslinked polymers formed by polymerization of multifunctional monomers containing primary and secondary cycles, the primary cyclization can lead to a reduction in the effective crosslink density. The chain flexibility of monomers and the rate of initiation can also affect the primary and secondary cyclization. It was reported that higher initiation rates can decrease the rate of primary cyclization because at high initiation rates radicals have shorter lifetimes and less time to cycle.55 In the current study, the rate of initiation of CQ-TTMSS was slower than that of CQ-EDMAB due to the slow generation of free radicals. This difference may increase the primary cyclization rate in the CQ-TTMSS system which could lead to loosely crosslinked structure.53
When EDMAB was used as coinitiator, an increase in the rubbery modulus was observed as the silorane monomer concentration was increased [Figure 2(A)]. From the derivative storage modulus versus temperature profiles [Figure 2(C)], with the increase in silorane concentration from 15 to 35 wt %, the glass transition temperature increased from 121.9 ± 3.7 to 133.5 ± 0.6°C, and the first transition temperature decreased from 63.9 ± 1.1 to 59.4 ± 1.3°C. A more heterogeneous structure was formed when EDMAB was used as coinitiator. As stated previously, in the hybrid formulations, the silorane monomers prefer to polymerize via the activated monomer mechanism and covalently bond to the polymethacrylate polymer chains. The fast methacrylate polymerization rate and low ES-4 concentration inhibit substantial growth of the polyether phase, and most of the ES-4 monomer acts as a crosslinker, reacting with the hydroxyl groups to increase the crosslink density (see Figure 5). Therefore, the Hypothesis (2), which proposed that the crosslink density of hybrid resins could be improved with the addition of ES-4, was accepted.
FIGURE 5.
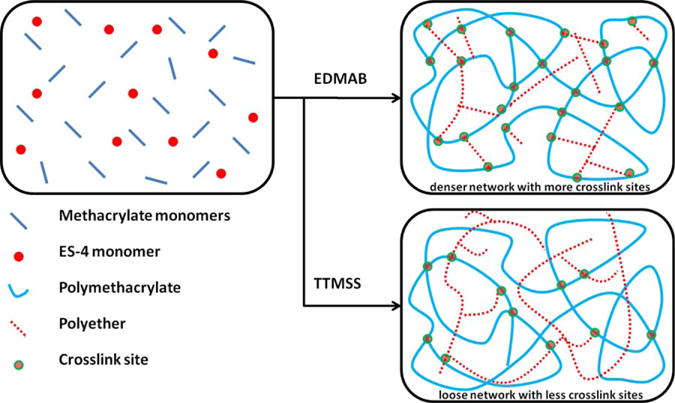
Schematic illustration of the network structure of methacrylate/silorane hybrid dentin adhesive using EDMAB or TTMSS as coinitiator. [Color figure can be viewed in the online issue, which is available at wileyonli-nelibrary.com.]
When TTMSS was used as coinitiator, the storage moduli of hybrid formulations at 25 and 37°C [Figure 2(B)] were lower than that of the control (C0-TTMSS). The Tg decreased to 115.7 ± 2.1°C when the ES-4 concentration was 15 wt %, and eventually the Tg peak merged with the first transition peak when ES-4 concentration was over 25 wt % [Figure 2(D)]. With the decrease in FRP rate, the primary cyclization was dominant, and a loosely crosslinked structure of polymethacrylate was formed. Even though the rate of CROP increased by using TTMSS, it was still slower than that of FRP, and a loosely networked polymethacrylate (lower crosslink density, see Figure 5) might be the dominant structure. Therefore, the Hypothesis (3), which proposed that the free radical polymerization rates will not affect the mechanical properties of the hybrid resin, was rejected.
It is reported that the heterogeneity will increase as the crosslink agent concentration increases within a copolymer system.56 When EDMAB was used as coinitiator, an increase in ES-4 concentration corresponded to a decrease in the intensity of the maximum tan δ peak [Figure 2(E)]. Additionally, a shoulder peak appeared, which may be attributed to the polyether-rich phase. When TTMSS was used as coinitiator, the control (C0-TTMSS) showed two peaks. As discussed previously, the primary cycle became the dominant reaction with the slow initiation rate, and formation of the loosely crosslink region. In contrast, the hybrid formulations showed only one broad peak. With the addition of TTMSS, the rate of CROP increased from 0.056 to 0.087 s−1, and ES-4 not only could bond onto the polymethacrylate main chains via the activated monomer polymerization mechanism through reaction with hydroxyl groups but also the molecular weight of polyether chains could gradually increase. With the increase in ES-4 concentration, the intensity of the tan δ peak decreased, which suggested that a higher crosslink density structure was formed. At the same time, due to the flexibility of polyether chains, the peaks became broad, and the maximum peaks moved toward a lower temperature.
The moduli of the controls (C0-EDMAB and C0-TTMSS) and experimental samples measured by the water-submersion method were significantly lower than those of the dry samples as shown in our previous work.57 The difference is attributed to the plasticization of the copolymer in the water. Water can be attracted to the polar functional groups (such as hydroxyl and ester) of the copolymer to form hydrogen bonds. Water attracted to these functional groups will decrease the intermolecular interaction of the copolymers. Simultaneous water diffusion and mechanical loading can lead to anomalously high creep strain or lower strength in some polymers.58,59 Using EDMAB as a coinitiator, the modulus of the hybrid formulations with ES-4 content up to 35% was significantly higher than that of the control (C0-EDMAB, p < 0.05) at 70°C. This means that with the increase in ES-4 content, the highly crosslinked structure can counteract the effect of water plasticization. Using TTMSS as a coinitiator, the storage moduli of the hybrid formulations at 37 and 70°C are significantly lower than that of the control (C0-TTMSS, p < 0.05). This indicates a loosely crosslinked structure formed with the reduced polymerization rate noted by the inclusion of TTMSS. In our formulation, the storage modulus at 70°C increased according to the increase in ES-4 content. This is because the ES-4 acts as a crosslinker during the photopolymerization.
Thermal analysis
The modulated temperature DSC (MDSC) method has been used to obtain the thermal properties and to provide related information on the degree of crosslinking of polymers.60 Figure 4 shows the results obtained in the MDSC analysis for the neat and hybrid resin formulations cured with different coinitiators. Prior to measurement by MDSC, the samples of neat methacrylate formulation were only partly cured, with the DC at about 60%. As the sample is heated, it could reach the glass transition region, and the thermal energy provides sufficient molecular mobility to facilitate continuation of the curing process, causing a shift in the transition region. The non-reversible components showed an exothermic peak arising from the simultaneous thermal curing (not shown). As the temperature was increased still further, the reaction finally ceased as the system reached full cure. The first transition temperature could be attributed to the side-chains while the second transition temperature could be due to the movement of segments of the main chains (Tg). In neat methacrylate resin, the derivative reversible heat flow could be used to show the glass transition. It was seen that with the increase in TTMSS content from 1 to 3 wt %, the Tg of the formulations decreased about 10°C, and the Tg width became smaller than that of the control (C0-EDMAB). At the same time, the difference between the two temperatures decreased accordingly. This behavior could be attributed to the less heterogeneous structure. As stated before, with the increase in TTMSS content from 1 to 5 wt %, the P.R. of FRP was slightly reduced from 0.069 to 0.057 s−1. The slow initiation rates may promote the primary cyclization, causing a delay in gel point, and reducing the heterogeneity. Thus, the gradual narrowing of the glass transition with increase in TTMSS content may be attributed to a decrease in the heterogeneity of the polymer network.
In neat silorane formulations, the glass transition temperatures were similar (~64°C) whether EDMAB or TTMSS was used as a coinitiator. Because the ES-4 monomer has four epoxy groups, a higher crosslink network formed immediately after the visible-light irradiation, and the lower degree of conversion (<50%). In the hybrid formulations (ES-4 50 wt %–EDMAB 1%), two peaks appeared when EDMAB was used as coinitiator [Figure 4(D)]. The lower temperature peak belonged to a side-chain transition associated with loosely crosslinked region, and a higher temperature peak indicated the main chain transition (higher crosslink region). When using TTMSS as a coinitiator, the higher temperature peak almost disappeared and only one peak (~60°C) could be observed. By decreasing the rate of FRP, the crosslink density of the hybrid polymer decreased and the structure became more homogenous. To clarify the correlations between network structure and physical properties, further experiments with different silorane monomers (varied epoxy functionality), solvents and coinitiators are ongoing.
CONCLUSION
The polymerization behavior of methacrylate/silorane hybrid resins when irradiated with visible light, as well as the mechanical properties of the hybrid formulations using either EDMAB or TTMSS as a coinitiator, were studied. Using EDMAB as a coinitiator, the crosslink density of the resulting polymer was enhanced with the increase in the silorane monomer content from 15 to 35 wt %. Additionally, the polymer showed good performance in dry and wet conditions. The network structure became more heterogeneous with the addition of silorane monomer. Polymerization-induced phase separation appeared when the silorane content exceeded 50 wt %. When using TTMSS as a coinitiator, the cationic ring-opening polymerization rate of silorane was improved and the free radical polymerization rate of methacrylate was decreased. The phase separation was depressed due to the matching of polymerization rates. The mechanical properties of the hybrid resin deteriorated with the increase in ES-4 content due to a loosely crosslinked structure and lower Tg of the hybrid polymer.
Acknowledgments
Contract grant sponsor: National Institute of Dental and Craniofacial Research, National Institutes of Health; contract grant number: R01 DE022054
References
- 1.Bowen RL. Method of preparing a monomer having phenoxy and methacrylate groups linked by hydroxyl glyceryl groups. 3,179,623. US patent. 1965
- 2.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25:435–440. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 3.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21:68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Weinmann W, Luchterhandt T, Guggenberger R, Stippschild A, Then S, Dede K. Comparative testing of volumetric shrinkage and sealing of silorane and methacrylate filling materials. J Dent Res. 2002;81:A417–A417. [Google Scholar]
- 6.Zakir M, Al Kheraif AAA, Asif M, Wong FSL, Rehman IU. A comparison of the mechanical properties of a modified silorane based dental composite with those of commercially available composite material. Dent Mater. 2013;29:E53–E59. doi: 10.1016/j.dental.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Lien W, Vandewalle KS. Physical properties of a new silorane-based restorative system. Dent Mater. 2010;26:337–344. doi: 10.1016/j.dental.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Ilie N, Hickel R. Silorane-based dental composite: Behavior and abilities. Dent Mater J. 2006;25:445–454. doi: 10.4012/dmj.25.445. [DOI] [PubMed] [Google Scholar]
- 9.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moszner N, Hirt T. New polymer-chemical developments in clinical dental polymer materials: Enamel–dentin adhesives and restorative composites. J Polym Sci Part A: Polym Chem. 2012;50:4369–4402. [Google Scholar]
- 11.D’Alpino PHP, Bechtold J, dos Santos PJ, Alonso RCB, Di Hipolito V, Silikas N, Rodrigues FP. Methacrylate- and silorane-based composite restorations: Hardness, depth of cure and interfacial gap formation as a function of the energy dose. Dent Mater. 2011;27:1162–1169. doi: 10.1016/j.dental.2011.08.397. [DOI] [PubMed] [Google Scholar]
- 12.Papadogiannis D, Kakaboura A, Palaghias G, Eliades G. Setting characteristics and cavity adaptation of low-shrinking resin composites. Dent Mater. 2009;25:1509–1516. doi: 10.1016/j.dental.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Kirkevang LL, Horsted-Bindslev P, Poulsen S. Marginal adaptation of a low-shrinkage silorane-based composite: 1-Year randomized clinical trial. Clin Oral Investig. 2011;15:291–295. doi: 10.1007/s00784-010-0446-2. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Dige I, Kirkevang LL, Vaeth M, Horsted-Bindslev P. Five-year evaluation of a low-shrinkage Silorane resin composite material: A randomized clinical trial. Clin Oral Investig. 2015;19:245–251. doi: 10.1007/s00784-014-1238-x. [DOI] [PubMed] [Google Scholar]
- 15.D’Alpino PHP, De Farias NC, Silva MS, de Goes MF, Gonzalez AHM, Di Hipolito V. Compatibility between silorane adhesive and simplified methacrylate-based adhesive systems. Dent Mater J. 2013;32:263–273. doi: 10.4012/dmj.2012-245. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Jessop JLP. Decreased oxygen inhibition in photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer. 2006;47:6560–6566. [Google Scholar]
- 17.Cai Y, Jessop JLP. Effect of water concentration on photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer. 2009;50:5406–5413. [Google Scholar]
- 18.Crivello JV, Liu SS. Synthesis and cationic photopolymerization of monomers based on nopol. J Polym Sci Part A: Polym Chem. 1999;37:1199–1209. [Google Scholar]
- 19.Dillman B, Jessop JLP. Chain transfer agents in cationic photopolymerization of a bis-cycloaliphatic epoxide monomer: Kinetic and physical property effects. J Polym Sci Part A: Polym Chem. 2013;51:2058–2067. [Google Scholar]
- 20.Crivello JV. Synergistic effects in hybrid free radical/cationic photopolymerizations. J Polym Sci Part A: Polym Chem. 2007;45:3759–3769. [Google Scholar]
- 21.Olsson RT, Bair HE, Kuck V, Hale A. Acceleration of the cationic polymerization of an epoxy with hexanediol. J Thermal Anal Calorim. 2004;76:367–377. [Google Scholar]
- 22.Tehfe MA, Gigmes D, Dumur F, Bertin D, Morlet-Savary F, Graff B, Lalevee J, Fouassier JP. Cationic photosensitive formulations based on silyl radical chemistry for green and red diode laser exposure. Polym Chem. 2012;3:1899–1902. [Google Scholar]
- 23.Lattuada M, Del Gado E, Abete T, de Arcangelis L, Lazzari S, Diederich V, Storti G, Morbidelli M. Kinetics of free-radical cross-linking polymerization: Comparative experimental and numerical study. Macromolecules. 2013;46:5831–5841. [Google Scholar]
- 24.Jang M, Crivello JV. Synthesis and cationic photopolymerization of epoxy-functional siloxane monomers and oligomers. J Polym Sci Part A: Polym Chem. 2003;41:3056–3073. [Google Scholar]
- 25.Ortiz RA, Sangermano M, Bongiovanni R, Valdez AEG, Duarte LB, Saucedo IP, Priola A. Synthesis of hybrid methacrylate-silicone-cyclohexanepoxide monomers and the study of their UV induced polymerization. Prog Org Coat. 2006;57:159–164. [Google Scholar]
- 26.Guo X, Wang Y, Spencer P, Ye Q, Yao X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater. 2008;24:824–831. doi: 10.1016/j.dental.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Q, Park J, Topp E, Spencer P. Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dent Mater. 2009;25:452–458. doi: 10.1016/j.dental.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song L, Ye Q, Ge X, Misra A, Laurence JS, Berrie CL, Spencer P. Synthesis and evaluation of novel dental monomer with branched carboxyl acid group. J Biomed Mater Res Part B: Appl Biomater. 2014;102B:1473–1484. doi: 10.1002/jbm.b.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, Ye Q, Singh V, Kieweg SL, Misra A, Spencer P. Synthesis and evaluation of novel dental monomer with branched aromatic carboxylic acid group. J Biomed Mater Res Part B: Appl Biomater. 2012;100B:569–576. doi: 10.1002/jbm.b.31987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parthasarathy R, Misra A, Park J, Ye Q, Spencer P. Diffusion coefficients of water and leachables in methacrylate-based crosslinked polymers using absorption experiments. J Mater Sci: Mater Med. 2012;23:1157–1172. doi: 10.1007/s10856-012-4595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge XP, Ye Q, Song LY, Misra A, Spencer P. Synthesis and evaluation of novel siloxane-methacrylate monomers used as dentin adhesives. Dent Mater. 2014;30:1073–1087. doi: 10.1016/j.dental.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J Biomed Mater Res Part A. 2010;93A:1245–1251. doi: 10.1002/jbm.a.32617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Li GL, Zhang HQ, Wang T. Visible light curing of bisphenol-A epoxides and acrylates photoinitiated by (eta(6)-ben-zophenone)(eta(5)-cyclopentadienyl) iron hexafluorophosphate. J Polym Res. 2011;18:1425–1429. [Google Scholar]
- 34.Kim JY, Patil PS, Seo BJ, Kim TS, Kim J, Kim TH. Photo and thermal polymerization of epoxides and vinyl ethers by novel sulfonium salts. J Appl Polym Sci. 2008;108:858–862. [Google Scholar]
- 35.Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, Spencer P. Dynamic mechanical analysis and esterase degradation of dentin adhesives containing a branched methacrylate. J Biomed Mater Res Part B: Appl Biomater. 2009;91B:61–70. doi: 10.1002/jbm.b.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JG, Ye Q, Topp EM, Misra A, Spencer P. Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dent Mater. 2009;25:1569–1575. doi: 10.1016/j.dental.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Roz M, Lalevee J, Allonas X, Fouassier JP. The silane-ene and silane-acrylate polymerization process: A new promising chemistry? Macromol Rapid Commun. 2008;29:804–808. [Google Scholar]
- 38.Lalevee J, Dirani A, El-Roz M, Allonas X, Fouassier JP. Silanes as new highly efficient co-initiators for radical polymerization in aerated media. Macromolecules. 2008;41:2003–2010. [Google Scholar]
- 39.El-Roz M, Lalevee J, Morlet-Savary F, Allonas X, Fouassier JP. Coinitiators based on group 14 elements in photoinitiating systems for radical and cationic polymerization. Macromolecules. 2009;42:4464–4469. [Google Scholar]
- 40.Chatgilialoglu C, Lalevee J. Recent applications of the (TMS)(3)SiH radical-based reagent. Molecules. 2012;17:527–555. doi: 10.3390/molecules17010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalevee J, Graff B, Allonas X, Fouassier JP. Aminoalkyl radicals: Direct observation and reactivity toward oxygen, 2,2,6,6-tetramethylpiperidine-N-oxyl, and methyl acrylate. J Phys Chem A. 2007;111:6991–6998. doi: 10.1021/jp071720w. [DOI] [PubMed] [Google Scholar]
- 42.Tehfe MA, El-Roz M, Lalevee J, Morlet-Savary F, Graff B, Fouassier JP. Bifunctional co-initiators: A new strategy for the design of efficient systems in radical photopolymerization reactions under air. Eur Polym J. 2012;48:956–962. [Google Scholar]
- 43.Lalevee J, El-Roz M, Allonas X, Fouassier JP. Free-radical-promoted cationic photopolymerization under visible light in aerated media: New and highly efficient silane-containing initiating systems. J Polym Sci Part A: Polym Chem. 2008;46:2008–2014. [Google Scholar]
- 44.Tehfe MA, Lalevee J, Gigmes D, Fouassier JP. Green chemistry: Sunlight-induced cationic polymerization of renewable epoxy monomers under air. Macromolecules. 2010;43:1364–1370. [Google Scholar]
- 45.Lalevee J, Blanchard N, Chany AC, El-Roz M, Souane R, Graff B, Allonas X, Fouassier JP. Silyl radical chemistry and conventional photoinitiators: A route for the design of efficient systems. Macromolecules. 2009;42:6031–6037. [Google Scholar]
- 46.Durmaz YY, Moszner N, Yagci Y. Visible light initiated free radical promoted cationic polymerization using acylgermane based photoinitiator in the presence of onium salts. Macromolecules. 2008;41:6714–6718. [Google Scholar]
- 47.Ge JH, Trujillo-Lemon M, Stansbury JW. A mechanistic and kinetic study of the photoinitiated cationic double ring-opening polymerization of 2-methylene-7-phenyl-1,4,6,9-tetraoxa-spiro 4.4 nonane. Macromolecules. 2006;39:8968–8976. doi: 10.1021/ma061284w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi YB, Neckers DC. A visible-light initiating system for free-radical promoted cationic polymerization. Macromolecules. 1994;27:3683–3693. [Google Scholar]
- 49.Biedron T, Szymanski R, Kubisa P, Penczek S. Kinetics of polymerization by activated monomer mechanism. Makromol Chem: Macromol Symp. 1990;32:155–168. [Google Scholar]
- 50.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J Dental Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Q, Spencer P, Wang Y. Nanoscale patterning in crosslinked methacrylate copolymer networks: An atomic force microscopy study. J Appl Polym Sci. 2007;106:3843–3851. doi: 10.1002/app.27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott JE, Bowman CN. Effect of primary cyclization on free radical polymerization kinetics: Modeling approach. Macromolecules. 2002;35:7125–7131. [Google Scholar]
- 53.Elliott JE, Lovell LG, Bowman CN. Primary cyclization in the polymerization of bis-GMA and TEGDMA: A modeling approach to understanding the cure of dental resins. Dent Mater. 2001;17:221–229. doi: 10.1016/s0109-5641(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 54.Elliott JE, Nie J, Bowman CN. The effect of primary cyclization on free radical polymerization kinetics: Experimental characterization. Polymer. 2003;44:327–332. [Google Scholar]
- 55.Elliott JE, Bowman CN. Kinetics of primary cyclization reactions in cross-linked polymers: An analytical and numerical approach to heterogeneity in network formation. Macromolecules. 1999;32:8621–8628. [Google Scholar]
- 56.Young JS, Kannurpatti AR, Bowman CN. Effect of comonomer concentration and functionality on photopolymerization rates, mechanical properties and heterogeneity of the polymer. Macromol Chem Phys. 1998;199:1043–1049. [Google Scholar]
- 57.Singh V, Misra A, Marangos O, Park J, Ye QA, Kieweg SL, Spencer P. Viscoelastic and fatigue properties of model methacrylate-based dentin adhesives. J Biomed Mater Res Part B: Appl Biomater. 2010;95B:283–290. doi: 10.1002/jbm.b.31712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh V, Misra A, Parthasarathy R, Ye Q, Park J, Spencer P. Mechanical properties of methacrylate-based model dentin adhesives: Effect of loading rate and moisture exposure. J Biomed Mater Res Part B: Appl Biomater. 2013;101:1437–1443. doi: 10.1002/jbm.b.32963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh V, Misra A, Parthasarathy R, Ye Q, Spencer P. Viscoelastic properties of collagen-adhesive composites under water saturated and dry conditions. J Biomed Mater Res Part A. 2015;103A:646–657. doi: 10.1002/jbm.a.35204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res Part A. 2007;80A:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]


