Abstract
BACKGROUND
Chronic rhinosinusitis (CRS) is a common inflammatory disease of the upper airways that is often categorized into subtypes including “with” and “without” nasal polyps. However, the influence of multiple important epidemiologic factors, including race, on CRS has not been investigated.
OBJECTIVE
The present study assessed various phenotypic characteristics of CRS in patients, living in the United States, with different racial backgrounds.
METHODS
We performed a large retrospective cohort study of patients with CRS treated at a large urban tertiary care referral center in Chicago.
RESULTS
African American (AA) patients with CRS living in Chicago were more likely to report hyposmia as a symptom of CRS. Furthermore, AA patients with CRS who failed medical therapy and required surgical intervention had a significantly higher frequency of nasal polyposis and aspirin-exacerbated respiratory disease, and a higher disease severity index on computed tomography imaging than did white patients with CRS. The increased polyposis in AAs was associated with increased hospitalization for asthma. There were no differences in the prevalence of atopy, asthma, atopic dermatitis, food allergy, duration of disease, or number of surgeries between different races.
CONCLUSIONS
AAs with refractory CRS are at increased risk for nasal polyposis, smell loss, aspirin-exacerbated respiratory disease, and a greater severity of disease based on imaging, resulting in increased health care utilization.
Keywords: Chronic rhinosinusitis (CRS), Race, African American, Asthma, Nasal polyp
Chronic rhinosinusitis (CRS) is a chronic disease affecting approximately 5% to 15% of the general population.1–3 CRS has a significant negative effect on patients’ quality of life.1 Despite an increasing knowledge base regarding the disease in recent decades, there remain multiple unknown factors regarding its complex pathophysiology. Indeed, there are phenotypic differences among patients with CRS that are likely based on the underlying pathophysiology, thus hindering our understanding of this disease.4 This also adds to the dilemma of selecting the most appropriate treatment strategies. Several international/national guidelines and consensus studies are available that present the currently available treatment strategies for CRS.1,5,6 However, despite these guidelines and medical treatment options, many patients with CRS continue to suffer from refractory symptoms.2
Understanding of the heterogeneous nature of CRS has promoted the concept that CRS consists of multiple biological subtypes or “endotypes.”7 Endotypes are defined by distinct pathophysiologic mechanisms that might be identified by bio-markers. Classifying diseases on the basis of endotypes has been used for other chronic respiratory diseases such as asthma.8 Subclassifying CRS according to the presence and absence of nasal polyps—CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP)—has been useful in selecting the best treatment option. However, consideration of diverse clinical phenotypes so far has not provided a complete understanding of the underlying pathophysiologic mechanisms of CRS.
Genetic differences are major factors in categorizing disease according to endotypes, and race is one marker of such differences. For example, genetic alterations have been identified in individuals of Latino or African American (AA) background in association with asthma, alterations that are not as common in patients of European origins.9,10 This is indicative of genetic differences in susceptibility to asthma.
At present, very little is known regarding the epidemiology of CRS as it relates to racial and ethnic differences. A few studies have previously investigated CRS phenotypes with regard to the racial background of patients, but these are limited in size or focus only on specific disease subtypes. A study based on national health surveys suggests that CRS is a significant health issue in all racial groups in the United States.11 CRS accounted for an estimated 20.3 million of 902 million (2.3%) physician visits in 2006. The frequency of visits to physicians for CRS was similar across all racial groups. In contrast, AAs accounted for about 34% of the emergency room (ER) visits related to this disease, despite comprising only 12.6% of the US population.11 Whether this discrepancy is partially due to lower access to primary care or higher severity of disease in this group is unclear and merits further investigation. Bush et al12 showed that both whites and AAs had a significant improvement in symptoms at 6 months after functional endoscopic sinus surgery, although AA subjects had an earlier relapse. Thus, determining the patient-related factors that result in increased health care utilization among AAs is an important public health concern.
In addition to the increased health care utilization, the effect of CRS on quality of life (QOL) in different groups is unevenly distributed. A study focusing on QOL in patients of different racial backgrounds with CRS found that Hispanic patients had a worse Rhinosinusitis Disability Index rating, a score indicative of QOL.11
Our knowledge of the impact of race on CRS health care utilization, QOL, and disease severity is limited to the above studies. No studies to date have determined whether CRS phenotypic features, severity of disease, or frequency of comorbidities have associations with race. To address the impact of race on severity and outcomes in CRS, we conducted a large-scale study of the association of CRS-related variables with different races in a tertiary care center.
METHODS
Subjects
The study was approved by the Institutional Review Board of Rush University Medical Center. An electronic medical records database search was performed by the Information Technology Department to identify patients with a diagnosis of CRS evaluated in the allergy or otolaryngology clinics between January 2008 and December 2014. All encounters of the selected patients were reviewed to ensure that they had a diagnosis of CRS based on the guidelines of the American Academy of Otolaryngology—Head and Neck Surgery Chronic Rhinosinusitis Task Force.13 To be eligible for our study, patients were required to have continuous symptoms of rhinosinusitis for more than 12 weeks documented as part of the history in the chart, with objective findings of sinusitis on either computed tomography (CT) scan or nasal endoscopy documented in the medical records. All patients with allergic fungal rhinosinusitis and sinonasal malignancies were excluded from the study. Clinical and demographic factors related to CRS were recorded in a REDcap database for statistical analysis. The definition used for each condition is detailed in Table E1 in this article’s Online Repository at www.jaci-inpractice.org. All personal health information was deidentified for the analysis.
The terminology and categories used for race are based on the National Institutes of Health recommendation of reporting 5 racial categories (American Indian or Alaska Native, Asian, black or AA, Native Hawaiian or Other Pacific Islander, and white) and 2 ethnic categories (Hispanic or Latino, and Not Hispanic or Latino) (National Institutes of Health policy notice no. NOT-OD-01-053).
To simplify the reporting of data, individuals who had identified themselves as white race plus Hispanic ethnicity were referred to as Latino and the non-Hispanic whites were referred to as whites in this study.
Database of refractory CRS cases
Patients who had failed initial medical treatment and had undergone functional endoscopic sinus surgery were selected for the refractory CRS cohort. These recalcitrant CRS cases were sub-classified using the aforementioned race groups for statistical analyses.
Statistical analysis
Comparisons between groups were first performed using either the χ2 test or ANOVA and Newman-Keuls multiple comparison tests. Logistic and linear regressions adjusting for the covariates were used to test whether demographic factors influenced the findings. Subgroup analyses were performed by logistic regression for some of the variables to further explore the findings. For statistical analyses, SPSS version 21 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY) and GraphPad Prism version 6.00 for Windows were used (GraphPad Software, La Jolla, Calif; www.graphpad.com). Differences were considered statistically significant at P ≤ .05.
RESULTS
A total of 1344 patients received a diagnosis of CRS during the study period. All charts were reviewed twice for diagnostic accuracy. Of the initial 1344 cases, 995 were confirmed to have CRS on the basis of inclusion criteria outlined in the Methods section; 919 were given the diagnosis of CRS by an otolaryngologist or allergist on the basis of symptoms longer than 12 weeks and a positive CT scan. An additional 76 cases were included on the basis of positive endoscopic findings or evidence of polyps clearly documented on an anterior rhinoscopy examination. The demographics of these cases are detailed in Table I. We excluded 173 cases because they had a normal CT scan that ruled out sinusitis, and an additional 176 more patients were excluded because they did not have objective CT or endoscopic findings in the chart to support the diagnosis.
TABLE I.
Demographic and clinical characteristics of 995 patients with CRS visited at Rush University Medical Center between 2008 and 2014
| Characteristic | Number or mean |
|---|---|
| Sex | |
| Male | 582 |
| Female | 397 |
| Age (y), mean ± SD | 49.61 ± 16.27 |
| BMI (kg/m2), mean ± SD | 28.97 ± 6.74 |
| Nasal polyp | |
| CRSsNP | 655 |
| CRSwNP | 304 |
| Smell loss | |
| Report of smell loss | 173 |
| No reported smell loss | 787 |
| Asthma | |
| Yes | 301 |
| No | 659 |
| AERD | |
| Yes | 40 |
| No | 779 |
| Atopy | |
| Negative skin test result | 67 |
| Positive skin test result | 284 |
| No skin test | 644 |
| Eczema | |
| Yes | 72 |
| No | 687 |
| Food allergy | |
| Yes | 121 |
| No | 826 |
| LMS, mean ± SD | 5.23 ± 5.50 |
| No. of surgeries, mean ± SD | 0.68 ± 1.12 |
| Duration of disease (y), mean ± SD | 6.73 ± 7.8 |
Of the 995 confirmed CRS cases, in 960 cases the patient’s race was identified in the medical chart as AA, white, or Asian. Patients of other races or mixed backgrounds were excluded from the analysis because of low numbers. There were 231 (24.05%) black or AA, 606 (63.05%) white or non-Hispanic white, 109 (11.4%) Hispanic-white or Latino, and 14 (1.5%) Asian cases. AAs had the highest male prevalence, which was significantly higher compared with whites, but not different among other groups. AAs (with CRS) also had a higher body mass index compared with whites, Latinos, and Asians. The mean age was similar among AAs, whites, and Latinos, with the exception that Asian patients were significantly younger than AAs (Table II).
TABLE II.
Demographic variables among different races
| Race group | Whites (n = 606) (63.1%) | AAs (n = 231) (24.1%) | Asians (n =14) (1.4%) | Latinos (n = 109) (11.4%) | P value |
|---|---|---|---|---|---|
| Sex: male, n (%) | 337 (58.3) | 152 (68.5) | 9 (64.5) | 68 (64.2) | <.05* |
| Age (y), mean ± SD | 50.34 ± 16.5 | 51.57 ± 15.3 | 42.36 ± 12.23 | 46.48 ± 15.9 | <.05† |
| BMI, mean ± SD | 27.58 ± 6.08 | 32.65 ± 7.69 | 25.94 ± 6.93 | 29.38 ± 6.44 | <.05‡ |
BMI, Body mass index.
Male sex was analyzed using the χ2 test. Age and BMI were analyzed using ANOVA.
The male percentage was significantly higher in AAs than in whites and Asians. There was no difference in sex among other racial groups.
Asian CRS cases were younger compared with AAs there was no difference in age among other racial groups. Analyses between groups were performed by using Newman-Keuls multiple comparison tests.
AA cases had a significantly higher BMI compared with whites and Asians. The difference was not significant when comparing other groups. Analyses between groups were performed by using Newman-Keuls multiple comparison tests.
Composite data from all CRS cases
Among CRS cases, there was no statistically significant difference among races in the prevalence of polyposis, meaning that the distribution of cases between CRSsNP and CRSwNP was similar in all races. However, AAs had a significantly higher frequency of smell loss than did other groups. The prevalence of aspirin-exacerbated respiratory disease (AERD), as defined by the presence of asthma, CRSwNP, and a documented history of a respiratory reaction to COX-1 inhibitor, was significantly higher in AAs than in white and Asian patients. Although among the different races AA patients had the highest prevalence of asthma, these differences were not statistically significant (Table III).
TABLE III.
Disease characteristics among different races in all patients with CRS visited at Rush
| Characteristic | Race group | |||
|---|---|---|---|---|
| Whites | AAs | Asians | Latinos | |
| Nasal polyp* | — | 1.14 (0.80–1.62) | 0.82 (0.25–2.69) | 0.79 (0.49–1.27) |
| Smell loss | — | 1.84 (1.22–2.76)† | 0.73 (0.04–2.90) | 1.37 (0.80–2.35) |
| Asthma | — | 1.10 (0.77–1.57) | 0.39 (0.08–1.80) | 0.72 (0.44–1.16) |
| AERD | — | 2.56 (1.22–5.36)† | 0.21 (0.05–1.10) | 1.93 (0.74–5.03) |
| Atopy | — | 1.09 (0.83–1.41) | 0.58 (0.27–1.28) | 0.87 (0.47–1.98) |
| Eczema | — | 0.81 (0.42–1.54) | 0.88 (0.39–1.96) | 1.04 (0.13–8.40) |
| Food allergy | — | 1.03 (0.64–1.67) | 0.63 (0.08–4.97) | 1.20 (0.66–2.19) |
| Refractory CRS; needed FESS | — | 0.74 (0.53–1.04) | 0.07 (0.10–0.60)† | 0.95 (0.62–1.45) |
| LMS, mean ± SD | 5.34 ± 5.3 | 5.26 ± 5.7 | 6.36 ± 5.6 | 4.44 ± 5.6 |
| Regression coefficients (95% CI)‡ | 0.41 (−0.57 to 1.40) | 0.90 (−1.95 to 3.77) | 0.63 (−1.88 to 0.52) | |
| No. of surgeries, mean ± SD | 0.72 ± 1.1 | 0.62 ± 1.1 | 0.14 ± 0.5 | 0.70 ± 1.5 |
| Regression coefficients (95% CI) | −0.025 (−0.21 to 0.16) | −0.063 (−1.23 to −0.04)† | −0.03 (−0.28 to 0.21) | |
| Duration of disease, mean ± SD | 7.18 ± 9.1 | 6.07 ± 4.9 | 3.75 ± 1.4 | 6.35 ± 6.5 |
| Regression coefficients (95% CI) | −1.43 (−2.65 to 0.02) | −2.9 (−7.25 to 1.34) | −0.75 (−3.03 to 1.52) | |
BMI, Body mass index; FESS, functional endoscopic sinus surgery.
Values are odds ratio (95% CI) except where indicated otherwise.
The reported odds ratios (95% CIS) for all categorical variables in different races were calculated in comparison to whites (the largest group) by logistic regression adjusting for age, sex, and BMI.
P < .05.
Regression coefficient for all numeric variables in different races were calculated in comparison to whites (the largest group) by linear regression adjusting for age, sex, and BMI.
The prevalence of reported atopic dermatitis and IgE-mediated food allergy was similar among all races. Three hundred seventy-three patients had been evaluated for allergic rhinitis by skin testing or serum-specific IgE measurements for aeroallergens. There was no statistical difference among the racial groups in terms of evaluation for allergic rhinitis. Among these patients, the prevalence of allergic rhinitis was similar in all races (Table III). There were no differences in severity of CRS based on Lund-Mackay score (LMS) between all race groups. The duration of disease was similar among all races as well. The percentage of patients who needed surgery was similar among AA, white, and Latino cases; however, Asian cases had a significantly lower rate of surgery (7.1%) compared with all other groups.
Refractory CRS group
Between 2009 and 2014, 433 patients at Rush had undergone functional endoscopic sinus surgery after they had failed medical treatment options. There were 88 (20.4%) AA, 292 (67.8%) white, 51 (11.8%) Latino, and 2 (0.5%) Asian cases. Because of the low number of Asians among refractory CRS cases, that group was excluded from the analysis. The prevalence of surgery, including primary and revision sinus surgeries, was not different among AA, white, and Latino cases.
Among the refractory cases, there were no significant differences with regard to age and sex distribution among AAs, whites, and Latinos. AAs had a significantly higher body mass index compared with whites.
AA cases had a significantly higher frequency of nasal polyposis; CRSwNP cases comprised 51.1% of AA cases compared with 36.9% and 31.4% in whites and Hispanics, respectively. Similar to findings for the general CRS group, AAs with refractory CRS had significantly higher rates of subjective smell loss and AERD compared with whites and Latinos. In addition, AA patients had a higher LMS compared with whites. There were no significant differences in the number of revision surgeries and the duration of disease among different races. The prevalence rates of asthma, atopy, atopic dermatitis, and food allergy were similar among the 3 groups as well.
Adjusting above analyses for age, sex, and body mass index by logistic and linear regressions did not change any of the results. The odds ratio for each variable in AAs and Latinos compared with whites is detailed in Table IV.
TABLE IV.
Disease characteristics among different races in patients with refractory CRS
| Characteristic | Race group | ||
|---|---|---|---|
| Whites | AAs | Latinos | |
| Nasal polyp* | — | 1.58 (1.02–2.68)† | 0.72 (0.34–1.40) |
| Smell loss | — | 2.13 (1.16–3.90)† | 1.05 (0.47–2.34) |
| Asthma | — | 0.97 (0.56–1.67) | 0.62 (0.21–1.23) |
| AERD | — | 2.94 (1.25–6.91)† | 1.93 (0.66–5.62) |
| Atopy | — | 0.87 (0.32–2.38) | 0.50 (0.19–1.30) |
| Eczema | — | 0.32 (0.09–1.04) | 0.66 (0.20–2.13) |
| Food allergy | — | 0.72 (0.33–1.58) | 1.45 (0.65–3.24) |
| LMS, mean ± SD | 6.64 ± 6.3 | 8.88 ± 7.2 | 6.34 ± 7.1 |
| Regression coefficients (95% CI)‡ | 2.02 (0.4–4.01)† | 1.19 (−2.37 to 2.33) | |
| No. of surgeries, mean ± SD | 1.52 ± 1.1 | 1.64 ± 1.4 | 1.58 ± 2.1 |
| Regression coefficients (95% CI) | −1.69 (−4.01 to 0.62) | 0.52 (−2.97 to 3.07) | |
| Duration of disease, mean ± SD | 10.14 ± 11.9 | 8.90 ± 5.9 | 9.96 ± 9.0 |
| Regression coefficients (95% CI) | 0.22 (−0.10 to 0.56) | 0.007 (−0.44 to 0.42) | |
Values are odds ratio (95% CI) except where indicated otherwise.
Odds ratios (95% CIs) of each variable in different races compared with the white group (the largest group) calculated by logistic regression.
P < 05.
Regression coefficient calculated in different races compared with the white group (the largest group) by linear regression.
Association of polyps with severity of disease
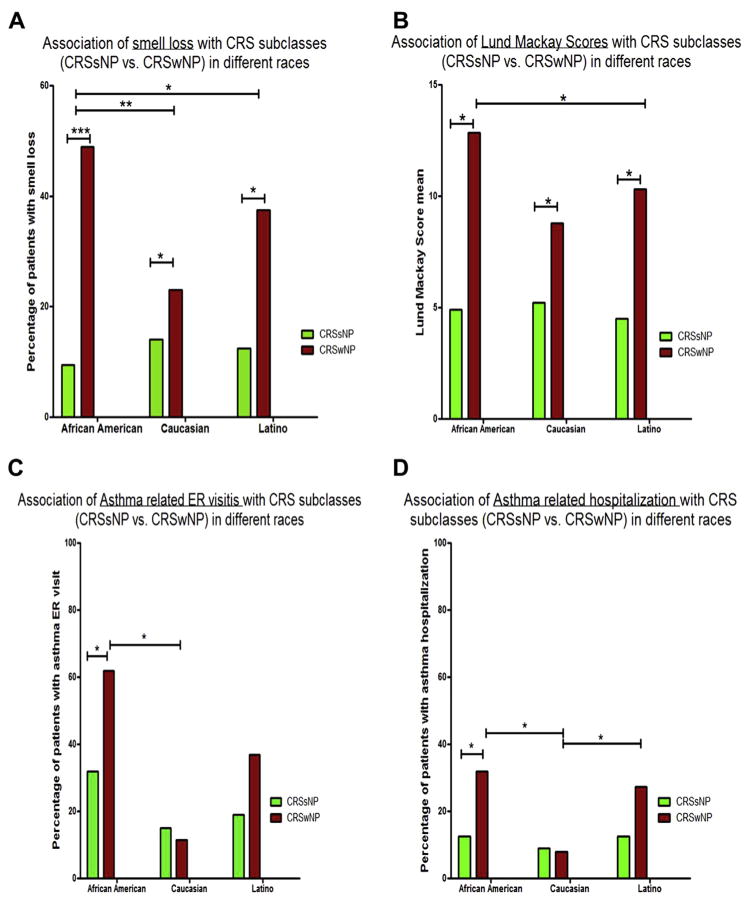
Next, we analyzed the association of polyps with other clinical outcomes in patients with CRS. These clinical outcomes included smell loss, LMS, and asthma-related emergency department visits and hospitalizations in different races (Figure 1).
FIGURE 1.
CRSwNP is associated with higher risk of smell loss (A) and higher LMSs (B) in all races, and is associated with increased ER visits (C) and hospitalizations (D) for asthma only in AAs.
Nasal polyps were significantly associated with higher LMS and higher number of surgeries in all races compared with CRSsNP. The presence of polyps was significantly associated with smell loss in all races. To determine whether polyposis was the only explanation for this observed increase in olfactory dysfunction in AAs, we carried out a logistic regression analysis, testing the association of smell loss with race after adjusting for the presence of polyps. After adjusting for polyps, AA patients still had more frequent smell loss than did other races: the odds ratio (95% CI) was 1.83 (1.2–2.7) when comparing AAs with whites.
The presence of polyps was also associated with an increase in the rate of asthma hospitalizations in AA patients with CRS. For this analysis, we included the data from 278 patients with CRS and asthma who had been evaluated and followed in the Rush Allergy or Otolaryngology clinics for at least 2 years and seen in the Allergy or Pulmonary clinic at least 2 times in that time span. The history of hospitalization and ER visits for asthma at Rush or any other hospital was asked routinely by the pulmonary and allergy physician as part of a template used in asthma visits. Although the prevalence of asthma was not different among races as detailed above, AAs had a higher rate of ER visits and hospitalizations for asthma compared with whites. Interestingly, the presence of polyps was associated with increased ER visits and hospitalizations for asthma in AAs, but not in other races (Figure 1).
DISCUSSION
Our analyses showed that AA patients with refractory CRS who needed surgery had a higher frequency of nasal polyposis, subjective hyposmia, and increased extent of disease by imaging in comparison to whites and Latinos with refractory CRS. Furthermore, AA patients had a higher prevalence of AERD compared with whites. We found no significant differences in the prevalence of asthma, allergic rhinitis, atopic dermatitis, or food allergy in the various races; therefore, other atopic disorders do not seem to be contributing factors to the increased polyposis and AERD seen in AA cases.
The increased rate of nasal polyposis in AAs is an important clinical finding. We demonstrated that CRSwNP in general is associated with increased smell loss, greater extent of disease, and increased need for surgery. In addition, among AA cases, patients with CRSwNP were at increased risk of hospitalization for asthma. Soler et al11 reported that AAs accounted for almost one-third of the national ER visits related to CRS. This high percentage of ER visits could be due, at least partially, to the observed phenotypic differences that we report here in AAs; however, more limited access of AA patients to primary care and routine outpatient care could also play a role.
Multiple studies have shown that AAs are at increased risk for other chronic inflammatory diseases of the airways. Few studies have shown an association between allergic fungal rhinosinusitis (AFRS) and race. Wise et al14 have shown that AFRS is associated more with AA, uninsured, and Medicaid patients (P < .001) compared with the CRS group.14 AAs with AFRS also had significantly higher preoperative LMS and endoscopy score (P < .05) than did whites.15 However, AAs had similar rates of bone erosion compared with other races in another study.16 The association of AFRS,14 a more severe subtype of CRS, with AA race, along with our findings, could indicate that this group of patients is more prone to inflammatory diseases of the upper airways manifested by polyposis or severe allergic inflammation in response to fungus. The increased risk of asthma in AAs17–19 is another indicator that AAs are at increased risk for chronic inflammatory airway diseases. Moreover, multiple genetic alterations have been found to be associated with asthma in this racial group.10,20 Nonetheless, at this point it is not clear whether this finding is due to genetics or socioeconomic disparities or a combination of these factors.20
Despite the greater severity of CRS in AAs, the number of patients who underwent surgery was similar for all races. This could potentially be explained by the finding that in the general CRS database, the markers of disease severity were similar among races and the differences are limited to refractory surgical disease. However, we cannot eliminate the possibility that AAs have more limited access to advanced surgical patient care, which could contribute to the similar rate of surgery in the treatment of patients with CRS across different races. In this scenario, once they get to surgery, AAs would have more advanced disease. Data from the Racial and Ethnic Approaches to Community Health Across the U.S. Risk Factor Survey have shown that residents in most of the minority communities, including AAs, have lower socioeconomic status and greater barriers to health care access. This could result in greater risks for and increased burden of chronic diseases such as CRS.21
A major symptom in patients with CRS that affects their QOL tremendously is olfactory dysfunction.22,23 Smell loss is reported to be associated with eosinophilia24 and polyposis in CRS.25 Olfactory dysfunction in CRS is an important marker of the severity of inflammation in this disease, and could be a permanent disability. It is common that the smell loss either does not improve or rapidly recurs after surgical treatment of CRS.26 This symptom is also associated with greater age, asthma, and smoking.25 In the present study, we found that AA cases have a significantly higher prevalence of subjective hyposmia. This greater smell loss reported by AA patients could be partially due to the higher frequency of nasal polyposis (CRSwNP) in this race. However, after adjusting the analysis for polyposis and age, there was still a significant association between AA race and smell loss, and this difference was observed in both CRSwNP and CRSsNP cases. Although olfactory dysfunction has been shown to be associated with eosinophilia,24 a previous report did not show a difference among AA, Latino, and white patients with CRSwNP in terms of eosinophilic cationic protein levels in polyps and in uncinate tissues as measured by ELISA.27 Furthermore, the authors did not find any significant differences in the frequency of reported eosinophilia in sinus tissue among AAs, Latinos, and whites.27 This indicates that at least in CRSwNP cases, there is no difference in eosinophilia among these 3 races. The observed difference in olfactory dysfunction might be due to other elements of inflammation in this group of patients, and further histopathological studies are required to evaluate this issue. It is worth mentioning that the olfactory loss data in our study are based on self-report of smell loss. Future studies with objective measurements of sense of smell are needed to confirm these findings and investigate the underlying mechanism of smell loss in this group of patients.
Our results suggest that Asian patients have a distinct phenotype of CRS with younger age of onset and significantly reduced need for surgical treatment. This is in line with previous studies comparing Asian and white patients with CRS28,29 and the recently reported findings in second-generation Asians with CRSwNP in the United States.27 These studies have shown major histopathologic and phenotypic differences between Asian and non-Asian patients with CRSwNP.27 Nasal polyps in Asian patients are predominantly noneosinophilic, and there are differences in TH cells involved in tissue inflammation between Asians and whites.29 These findings for Asian patients suggest a role for genetic factors in CRS. Our present study has further found distinct phenotypic features in patients of African ancestry with CRS. Although these findings, as detailed above, may be due to multiple environmental factors, genetic elements could at least partially contribute to them.
The major limitation of this study is its retrospective designs. However, performing a longitudinal study of this volume and extent is extremely difficult. To overcome this limitation, we have placed strict inclusion criteria for the study and double-checked all the data points by 2 investigators who reviewed the charts.
In conclusion, our results show that AA patients with CRS are more likely to report hyposmia. In addition, AA patients with CRS who failed medical therapy and needed surgical intervention had a significantly higher frequency of nasal polyposis, which was associated with increased asthma hospitalizations. Furthermore, AAs with refractory CRS, compared with white cases, were at increased risk for AERD, subjective hyposmia, and more extensive CRS based on imaging. Multiple factors, including greater barriers to health care access or genetic elements, could be playing a role in these observations. Future prospective studies are needed to investigate the underlying mechanism of these observations.
Supplementary Material
What is already known about this topic?
Our current knowledge about effect of race on chronic rhinosinusitis is very limited. In this study, we have for the first time evaluated the impact of race on severity and outcomes in chronic rhinosinusitis.
What does this article add to our knowledge?
Our results showed that African Americans with refractory chronic rhinosinusitis are at increased risk for nasal polyposis, smell loss, aspirin exacerbated respiratory disease, and a greater severity of disease based on imaging. Furthermore, in African Americans, chronic rhinosinusitis with nasal polyps was associated with increased asthma-related emergency room visits and hospitalization, hence increased healthcare utilization.
How does this study impact current management guidelines?
Our findings indicate that African Americans need to be followed more closely. More importantly, the effect of nasal polyposis on their asthma need to be emphasized and addressed during the care of these patients.
Acknowledgments
M.M. is supported by Cohn Scholarship from Rush University Mentoring office. R.P.S. is supported in part by the Ernest S. Bazley Foundation, the National Institute of Allergy and Infectious Diseases (grant no. U19 AI106683), and the National Heart, Lung, and Blood Institute (grant no. R37 HL068546). A.K. is supported by the National Institutes of Health (grant nos. R01 AT007143-05, R01 AA023417-02, and R01 AA020216-05).
Abbreviations used
- AA
African American
- AERD
aspirin-exacerbated respiratory disease
- AFRS
allergic fungal rhinosinusitis
- CRS
chronic rhinosinusitis
- CRSwNP
CRS with nasal polyps
- CRSsNP
CRS without nasal polyps
- CT
computed tomography
- ER
emergency room
- LMS
Lund-Mackay score
- QOL
quality of life
Footnotes
Conflicts of interest: A. T. Peters has received consultancy fees from Greer and Baxter. R. P. Schleimer has received research support from the National Institutes of Health; has received consultancy fees from Intersect ENT, GlaxoSmithKline, Allakos, Aurasense, Merck, BioMarck, and Sanofi; and has stock/stock options in Allakos, Aurasense, and BioMarck. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012: a summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, et al. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7:25. doi: 10.1186/1939-4551-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe—an underestimated disease: a GA(2) LEN study. Allergy. 2011;66:1216–23. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 4.Tomassen P, Van Zele T, Zhang N, Perez-Novo C, Van Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc. 2011;8:115–20. doi: 10.1513/pats.201005-036RN. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan A. Canadian guidelines for chronic rhinosinusitis: clinical summary. Can Fam Physician. 2014;59:1275–81. e528–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Scadding GK, Durham SR, Mirakian R, Jones NS, Drake-Lee AB, Ryan D, et al. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy. 2008;38:260–75. doi: 10.1111/j.1365-2222.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–60. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Igartua C, Myers RA, Mathias RA, Pino-Yanes M, Eng C, Graves PE, et al. Ethnic-specific associations of rare and low-frequency DNA sequence variants with asthma. Nat Commun. 2015;6:5965. doi: 10.1038/ncomms6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torgerson DG, Capurso D, Mathias RA, Graves PE, Hernandez RD, Beaty TH, et al. Resequencing candidate genes implicates rare variants in asthma susceptibility. Am J Hum Genet. 2012;90:273–81. doi: 10.1016/j.ajhg.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012;26:110–6. doi: 10.2500/ajra.2012.26.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush CM, Jang DW, Champagne JP, Kountakis SE. Epidemiologic factors and surgical outcomes in patients with nasal polyposis and asthma. ORL J Otorhinolaryngol Relat Spec. 2013;75:320–4. doi: 10.1159/000354804. [DOI] [PubMed] [Google Scholar]
- 13.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise SK, Ghegan MD, Gorham E, Schlosser RJ. Socioeconomic factors in the diagnosis of allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2008;138:38–42. doi: 10.1016/j.otohns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Champagne JP, Antisdel JL, Woodard TD, Kountakis SE. Epidemiologic factors affect surgical outcomes in allergic fungal sinusitis. Laryngoscope. 2010;120:2322–4. doi: 10.1002/lary.21127. [DOI] [PubMed] [Google Scholar]
- 16.Ghegan MD, Wise SK, Gorham E, Schlosser RJ. Socioeconomic factors in allergic fungal rhinosinusitis with bone erosion. Am J Rhinol. 2007;21:560–3. doi: 10.2500/ajr.2007.21.3082. [DOI] [PubMed] [Google Scholar]
- 17.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4:58–68. doi: 10.1513/pats.200607-146JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith LA, Hatcher-Ross JL, Wertheimer R, Kahn RS. Rethinking race/ethnicity, income, and childhood asthma: racial/ethnic disparities concentrated among the very poor. Public Health Rep. 2005;120:109–16. doi: 10.1177/003335490512000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearlman DN, Zierler S, Meersman S, Kim HK, Viner-Brown SI, Caron C. Race disparities in childhood asthma: does where you live matter? J Natl Med Assoc. 2006;98:239–47. [PMC free article] [PubMed] [Google Scholar]
- 20.Joubert BR, Reif DM, Edwards SW, Leiner KA, Hudgens EE, Egeghy P, et al. Evaluation of genetic susceptibility to childhood allergy and asthma in an African American urban population. BMC Med Genet. 2011;12:25. doi: 10.1186/1471-2350-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Bang D, Cosgrove S, Dulin R, Harris Z, Taylor A, et al. Surveillance of health status in minority communities—Racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) Risk Factor Survey, United States, 2009. MMWR Surveill Summ. 2011;60:1–44. [PubMed] [Google Scholar]
- 22.Katotomichelakis M, Simopoulos E, Zhang N, Tripsianis G, Danielides G, Livaditis M, et al. Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. Am J Rhinol Allergy. 2013;27:293–8. doi: 10.2500/ajra.2013.27.3903. [DOI] [PubMed] [Google Scholar]
- 23.Katotomichelakis M, Simopoulos E, Tripsianis G, Balatsouras D, Danielides G, Kourousis C, et al. Predictors of quality of life outcomes in chronic rhinosinusitis after sinus surgery. Eur Arch Otorhinolaryngol. 2014;271:733–41. doi: 10.1007/s00405-013-2626-6. [DOI] [PubMed] [Google Scholar]
- 24.Haruna S, Otori N, Moriyama H, Nakanishi M. Olfactory dysfunction in sinusitis with infiltration of numerous activated eosinophils. Auris Nasus Larynx. 2006;33:23–30. doi: 10.1016/j.anl.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118:2225–30. doi: 10.1097/MLG.0b013e318184e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka H, Tsuzuki K, Takebayashi H, Kojima Y, Daimon T, Sakagami M. Olfactory changes after endoscopic sinus surgery in patients with chronic rhinosinusitis. Auris Nasus Larynx. 2013;40:452–7. doi: 10.1016/j.anl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Mahdavinia M, Suh LA, Carter RG, Stevens WW, Norton JE, Kato A, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2015;135:576–9. doi: 10.1016/j.jaci.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–8–968.e1–6. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.