Abstract
Lysosomes are emerging as important players in cellular zinc ion (Zn2+) homeostasis. The series of work on Zn2+ accumulation in the neuronal lysosomes and the mounting evidence on the role of lysosomal Zn2+ in cell death during mammary gland involution set a biological precedent for the central role of the lysosomes in cellular Zn2+ handling. Such a role appears to involve cytoprotection on the one hand, and cell death on the other. The recent series of work began to identify the molecular determinants of the lysosomal Zn2+ handling. In addition to zinc transporters (ZnT) of the solute-carrier family type 30A (SLC30A), the lysosomal ion channel TRPML1 and the poorly understood novel transporter TMEM163 have been shown to play a role in the Zn2+ uptake by the lysosomes. In this review, we summarize the current knowledge on molecular determinants of the lysosomal Zn2+ handling, uptake, and release pathways, as well as discuss their possible roles in health and disease.
Keywords: Mucolipidosis IV, lysosomes, zinc transport, SV31
Introduction
The central paradigm of Zn2+ handling involves its entry in cells via the plasma membrane transporters, chelation by the cytoplasmic proteins, and export into membrane-bound organelles via dedicated transporters (Falchuk et al., 1995; Sensi et al., 1997; Colvin et al., 2000; Balaji and Colvin, 2005). As commonly known, such organelles include components of the Golgi network, mitochondria, and the “secretory vesicles.” The observations in neuronal cells, in breast tissue, and in certain model system points out that lysosomes are also involved in the Zn2+ handling. Such evidence is based on Zn2+ accumulation in the lysosomes of cells exposed to Zn2+, or in cells undergoing processes associated with large Zn2+ transitions, such as cell death at the onset of mammary gland involution and pathological events (Eichelsdoerfer et al., 2010; Kelleher et al., 2011; Seo et al., 2011; McCormick and Kelleher, 2012; Kukic et al., 2013; Cuajungco et al., 2014; Kukic et al., 2014). The lysosomal involvement in Zn2+ handling is interesting from several perspectives. First, it redefines the role of the lysosomes as purely digestive organelles. Second, it uncovers some interesting new biology pertaining to cell death and survival, especially in tissues in which the lysosomal involvement has been under-appreciated. Third, it shows the involvement of new regulatory circuits involving energy sensing, oxidative stress, and organellar biogenesis.
Zn2+ enters the lysosomes through endocytosis of Zn2+-bound proteins, autophagy of Zn2+-rich organelles, and from the cytoplasm via the zinc transporters located in the lysosomal membrane. Proteomic analysis of the lysosomal membrane suggested the presence of ZnT2 and ZnT4 transporters, which was confirmed using confocal microscopy and knockdown studies in several systems (Murgia et al., 1999; McCormick and Kelleher, 2012; Roh et al., 2012; Bostanci et al., 2014). Another pathway, a “maturation” of Zn2+-rich secretory granules into the lysosomes has been shown in the mammary gland (Lopez and Kelleher, 2009; Seo et al., 2011; McCormick et al., 2014); the presence of such a process in other tissues remains to be an interesting question. Whereas the Zn2+ entry into the lysosomes via endocytosis and autophagy appears to be a normal consequence of the lysosomal digestive function, its uptake from the cytoplasm into lysosomes is an emerging role for these organelles. Based on this evidence, we proposed a concept of the lysosomal Zn2+ sink, a process of absorption of Zn2+ from the cytoplasm into the lysosomes. This process could be especially important during abrupt changes in the cytoplasmic concentration of Zn2+, as it would give the cells time to update their Zn2+ chelation and extraction capacities (Kukic et al., 2014) (Fig. 1).
Fig. 1. Cellular zinc status.
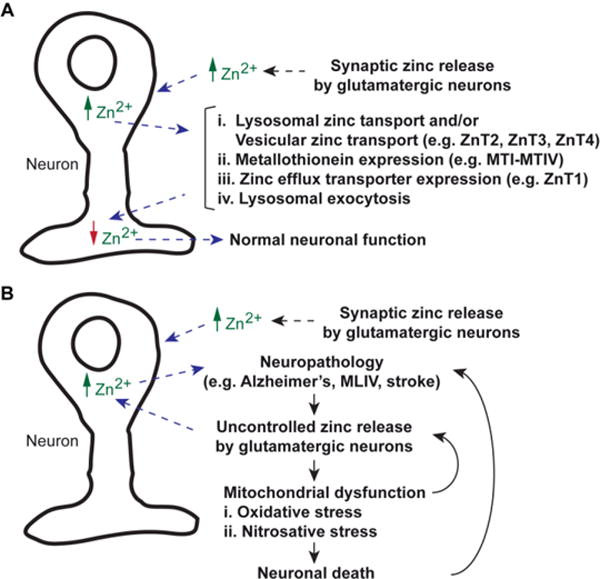
A) Normal zinc release by glutamatergic neurons results in extracellular increase of Zn2+. The ions are taken up by cells, which elevate intracellular Zn2+ levels. The ions are taken up by lysosomes or vesicular compartments until increases in the expression levels of Metallothionein and efflux zinc transporter have occurred. Lysosomal exocytosis is also a mechanism to reduce excess intracellular Zn2+. B) In pathological conditions caused by stroke or neurodegenerative diseases, glutamatergic neurons that release Zn2+ becomes a vicious cycle. Extracellular Zn2+ elevation perpetuates intracellular Zn2+ accumulation. The flood of Zn2+ results in oxidative and nitrosative stresses in mitochondria. Failure of lysosomes to buffer Zn2+ increase contributes to cellular stress, which subsequently results in cell death.
An extremely attractive aspect of the lysosomal biology that sets it apart from the Golgi as a Zn2+ sink is its dynamic regulation by the recently discovered lysosomal gene network. Such a network regulated by transcription factor EB (TFEB), and its relatives TFE3 (transcription factor binding IGHM enhancer 3) and MITF2 (microphthalmia-associated transcription factor 2) was shows to relay the information from mechanistic target-of-rapamycin (mTORC1) to transcriptional activity of a number of genes coding for the lysosomal and autophagic proteins (Martina et al., 2014). Since mTORC1 is a signaling nexus responsible or gauging the cellular energy and the functional status of the lysosomes (Settembre et al., 2011; Martina et al., 2012), a possible role of the lysosomes as a cellular Zn2+ clearance pathway casts a new light on these organelles as key players. Indeed, if the lysosomes are a powerful transition metal buffering and extraction pathway, as was recently proposed, then perhaps the definition of the lysosomes and the lysosomal gene network might be amended to include the oxidative stress response element. The recent evidence that the lysosomal biogenesis is stimulated by the transition metal exposure supports this idea (Peña and Kiselyov, 2015).
The growing knowledge of the lysosomal role in Zn2+ handling coincides with the discovery of the lysosomal exocytosis and lysosomal biogenesis as driving factors in cellular detoxification. Upregulation of the lysosomal biogenesis stimulated by the TFEB overexpression has been linked to improving cellular phenotypes in in vitro models of several diseases including Pompe, Alzheimer’s, Parkinson’s, Huntington’s, and hepatic SERPINA1 deficiency (La Spada, 2012; Decressac et al., 2013; Feeney et al., 2013; Spampanato et al., 2013; Polito et al., 2014). Such an improvement has been linked to increased removal of the storage bodies and misfolded protein aggregates. Lysosomal exocytosis has been proposed as a driving force behind such an improvement (Medina et al., 2011; Feeney et al., 2013). It is thus telling that TFEB overexpression and increased lysosomal exocytosis have been linked to improved removal of copper ions (Cu2+) and Zn2+, and suppressed oxidative stress in cells exposed to these metals (Peña et al., 2015; Peña and Kiselyov, 2015). On the contrary, suppression of the lysosomal exocytosis has been linked to increased oxidative stress in cells treated with Cu2+ or Zn2+. It is important to note that beyond the “lysosomal” genes, the TFEB-responsive network (Settembre and Medina, 2015) incorporates genes involved in regulation of oxidative stress such as heme oxygenase 1 (HMOX1). Indeed, the TFEB-dependent upregulation of HMOX1 expression has been shown in response to Cu2+ exposure of cultured cells (Peña and Kiselyov, 2015). To further the argument for the interaction of the lysosomal biogenesis and oxidative stress responses, regulation of the lysosomal biogenesis by FXR-CREB and PPARα transcription factors previously implicated in the oxidative stress responses has been shown (La Spada, 2012; Lee et al., 2014; Seok et al., 2014; Ghosh et al., 2015).
Taken together, this evidence strongly suggests that the lysosomes play an important role in the cellular defense against oxidative stress not only by destroying damaged organelles, but also by taking up and removing toxic metal from the cytoplasm. Below we summarize the available data and the current concepts involving these processes, as well as highlight recent discoveries on intracellular Zn2+-buffering mediated by Zn2+ transport proteins associated with the lysosomes.
The Lysosomal Zn2+ Sink
Zn2+ accumulation into the lysosomes via the endocytic/autophagic pathway is a logical and predictable consequence of the endocytic and autophagic activities, albeit has not been extensively pursued until recently. Lysosomal degradation of Zn2+-rich organelles and Zn2+-bound proteins in the acidic environment releases Zn2+. Indeed, elevated free vesicular (lysosomal) Zn2+ has been shown shortly after the first Zn2+-sensitive fluorescent dyes had become available (Gee et al., 2002).
A buildup of Zn2+ in the lysosomes of cells exposed to oxidative stress or high Zn2+ levels to induce Zn2+ uptake across the plasma membrane has been shown in various cell culture models using the high affinity dye, Fluozin-3 (Hwang et al., 2008; Chung et al., 2009; Lee et al., 2009; Eichelsdoerfer et al., 2010; Kukic et al., 2013; Cuajungco et al., 2014). Such buildup has led to lysosomal permeabilization followed by the release of the lysosomal digestive enzymes and cell death by the autophagic scenario. Due to the previous evidence of Zn2+ buildup in brains affected by stroke and other neurodegenerative diseases (Koh et al., 1996; Lees et al., 1998; Suh et al., 2000), these findings were interpreted as an explanation for cell death in these diseases. According to this model, an excess cytoplasmic Zn2+ entering the cells at increased rate through the plasma membrane (Sensi et al., 1997) or due to liberation of Zn2+ from the cytoplasmic Zn2+-binding proteins (Cuajungco and Lees, 1998; Frederickson et al., 2002; Lee et al., 2003), which floods the cytosol and subsequently, the lysosomes and mitochondria (Sensi et al., 2002; Hwang et al., 2008) (Fig. 1). Such a Zn2+ overload then triggers cell death (Cuajungco and Lees, 1997; Choi and Koh, 1998; Frederickson et al., 2004). Interestingly, a significant increase in cerebral Zn2+ levels of Mucolipin-1 knockout (Mcoln1−/−) mice, a model for Mucolipidosis type IV (MLIV), has been reported (Eichelsdoerfer et al., 2010; Kukic et al., 2013; Cuajungco et al., 2014). It was surmised that lysosomal Zn2+ overload could potentially contribute to MLIV pathology, as well as cause progressive neuronal and retinal cell degeneration (Eichelsdoerfer et al., 2010). MLIV is a human lysosomal storage disease caused by a loss-of-function mutation or deletion in the Mucolipin-1 (TRPML1) ion channel (Bargal et al., 2000; Bassi et al., 2000; Sun et al., 2000). TRPML1 confers non-selective permeability to calcium (Ca2+), Zn2+, ferrous iron (Fe2+), and manganese ions (Mn2+) (Grimm et al., 2007; Dong et al., 2008; Dong et al., 2009; Dong et al., 2010), suggesting that TRPML1 may function in metal homeostasis. Indeed, Zn2+ mishandling by the lysosomes in MLIV-affected cells has been revealed consistently (Eichelsdoerfer et al., 2010; Kukic et al., 2013; Cuajungco et al., 2014), in addition to a previous report that Fe2+ overload may also be a contributing factor in disease etiology (Dong et al., 2008). It is interesting to note that lysosomal permeabilization and the release of Cathepsin B have been shown in an in vitro model of MLIV (Colletti et al., 2012), which potentially correlates with lysosomal Zn2+ accumulation in MLIV cells as a pathological trigger. Meanwhile, abnormal iron homeostasis has been implicated in MLIV disease because many MLIV patients suffer from anemia (Altarescu et al., 2002); however, it was reported recently that a decrease in cerebral ferric iron (Fe3+) load but not total iron levels is a common feature of ten-day old Mcoln1−/− knockout mice (Grishchuk et al., 2015). The change in Fe3+ levels was correlated with abnormal myelination of the brains of Mcoln1−/− knockout mice relative to wild type controls (Grishchuk et al., 2015). Notwithstanding, anemia in MLIV patients is likely due to a decrease in iron uptake associated with gastrointestinal problems manifested by the disease.
Neuronal and retinal abnormalities are a hallmark of lysosomal storage diseases in which a very large fraction is associated with degenerative phenotypes and developmental delays. Both tissues are known for high Zn2+ requirements. Zn2+ is co-released with glutamate to modulate neuronal transduction (Howell et al., 1984) and very large changes in retinal Zn2+ content have been shown to accompany the light-dark cycle (Redenti and Chappell, 2005; Lengyel et al., 2007; Redenti et al., 2007). Owing to its importance, several reports have shown that depletion of Zn2+ induces degeneration of retinal pigment epithelium (Hyun et al., 2000; Hyun et al., 2001), while oxidant-induced Zn2+ overload may also contribute to retinal cell death (Yoon et al., 2000; Yoo et al., 2004; Chung et al., 2009). Despite these observations, the role of the lysosomes in retinal Zn2+ handling has not been pursued, and in general, there is a dearth of knowledge on the lysosomal function in the retina with respect to lysosomal biogenesis and exocytosis.
The first evidence of Zn2+ transporters localized to the lysosomal membrane came from mammary gland in which involution at the end of lactation appears to be associated with translocation of the transporter ZnT2 to the lysosomal membrane and a buildup of Zn2+ in the lysosomes. This is followed by the destabilization of the lysosomal membrane and cell death. In contrast to the neuronal tissue, lysosomal Zn2+ buildup in the mammary gland appears to be dynamically regulated component of a developmental program. For a complete account of Zn2+ handling and transporters in the involuting mammary gland, we refer to a recent comprehensive review on this topic by Kelleher and colleagues (McCormick et al., 2014). A confocal analysis of recombinant protein and short interfering RNA (siRNA)-mediated analysis showed that while recombinant ZnT2 and ZnT4 are routed to the lysosomes in HeLa cells, the native ZnT2 does not contribute to the lysosomal Zn2+ uptake in these cells. Instead, this seems to be a function of ZnT4 in HeLa cells. This is consistent with the previously shown exclusive localization of ZnT2 in the mammary gland tissue and a wider expression of ZnT4 (McCormick and Kelleher, 2012). The lysosomal localization of ZnT4 has been confirmed by the recently published lysosomal proteomic analysis (Chapel et al., 2013).
While the uptake of Zn2+ from the cytoplasm into the lysosomes and the resulting buildup and toxicity fits well into the idea of Zn2+-mediated cell death under degenerative conditions and during the mammary gland involution, such mechanisms may play a cytoprotective role in other tissues. However, this protective process requires elimination of the lysosomal Zn2+ at the end of the Zn2+ spike. Lysosomal exocytosis seems to have emerged as such a defense mechanism (Kukic et al., 2014). Originally proposed as a means of plasma membrane repair (Reddy et al., 2001), lysosomal exocytosis is now being recognized as a key cellular detoxification and stress repair pathway. Lysosomal exocytosis is driven by the lysosomal fusion with the plasma membrane, mediated by VAMP7 and synaptotagmin 7, and by the cytoplasmic calcium ion (Ca2+) spike (Logan et al., 2006). The latter was posited to depend on the endolysosomal Ca2+ release via TRPML1 and TRPML3 ion channels (Kiselyov et al., 2012; Samie et al., 2013; Miao et al., 2015), although its dependence on Ca2+ entry across the plasma membrane has been shown as well (Peña et al., 2015). As previously mentioned, lysosomal exocytosis of storage bodies and unfolded proteins was suggested to underlie the improvement of cellular phenotypes of several diseases (Medina et al., 2011). In these experiments, the in vitro disease models were transiently transfected with TFEB cDNA to cause overexpression of this transcription factor and stimulation of the lysosomal exocytosis. The resulting improvement was attributed to the increased lysosomal exocytosis and increased extraction of toxic material. The role of lysosomal exocytosis in the removal of excess (lysosomal) Zn2+ from cells has been recently shown using siRNA-mediated knockdown of VAMP7 and synaptotagmin 7, which resulted in the buildup of Zn2+ and oxidative stress in Zn2+-treated cells (Kukic et al., 2014).
Based on the data summarized in this chapter, a concept of the lysosomal Zn2+ sink was proposed. We postulated that Zn2+ uptake into the lysosomes via the lysosomal Zn2+ transporters quickly lowers the cytoplasmic Zn2+ levels and gives the cells time to induce expression, modify, and target ionic buffering through various efflux transporters and chelating proteins (Fig. 1). Such high capacity dynamic system may serve as a first line of defense mechanism against the cytoplasmic Zn2+ spike – lysosomes occupy 3% to 5% of the cellular volume according to some estimates (Draye et al., 1988). Lysosomes are thus able to respond to a wide range of signals and are dynamically regulated by the signaling loop of circuits concerning cell stress (e.g. oxidative stress, lysosomal damage, and starvation). An increase in the cytoplasmic Zn2+ clearance may serve a cytoprotective role, while such clearance may be decreased when cell death is a desirable outcome. As shown in a recent publication (Kukic et al., 2014) by one of us (KK), the rate of lysosomal exocytosis is a factor defining its cytoprotective role. While some molecules involved in the lysosomal exocytosis of Zn2+ are fairly well understood, some are still poorly understood or just beginning to shed light on their new roles. One of such molecules is discussed in the next chapter.
TMEM163 and zinc accumulation
Transmembrane-163 (TMEM163) protein, also known as synaptic vesicle 31 (SV31), was first identified in rat brain synaptosomes using proteomics (Burre et al., 2007). The TMEM163 gene is conserved across many vertebrate species (chimpanzee, Rhesus monkey, cow, dog, chicken, mouse, rat, zebrafish, and frog) and has over 100 orthologues. TMEM163 protein expression is detected in certain glutamatergic and γ-aminobutyric acid (GABA)-ergic neuronal populations (Burre et al., 2007; Barth et al., 2011). Its presence in synaptic-like micro-vesicles, large dense core vesicles, endosomes and lysosomes (Burre et al., 2007; Barth et al., 2011) overlaps with the enrichment of zinc in pre-synaptic vesicles of these neuronal populations (Frederickson, 1989). Furthermore, subcellular fractionation of PC12 cell lysates stably expressing the rat Tmem163 also showed that this protein is detected in the plasma membrane, endoplasmic reticulum (ER), Golgi, mitochondria, and peroxisomes (Barth et al., 2011).
An interaction between TMEM163 and TRPML1 was recently shown using genetic (yeast two-hybrid) and biochemical (co-immunoprecipitation) assays (Cuajungco et al., 2014). The interaction between the two proteins appears to influence intracellular zinc homeostasis, at least in a heterologous expression system using cultured cells (Cuajungco et al., 2014; Silva and Cuajungco, 2015). Moreover, the expression level of TMEM163 is down-regulated in MLIV patient fibroblasts (Cuajungco et al., 2014). It is not clear, however, how such a reduction in TMEM163 protein potentially contributes to the disease phenotype. Nevertheless, the tissue mRNA expression pattern of TMEM163 gene coincides well with that of MCOLN1 (TRPML1) gene (Grimm et al., 2010; Cuajungco et al., 2014). Specifically, higher relative TMEM163 transcripts are observed in the brain, lung, and testis, but notable levels are seen also in the pancreas, kidney, thymus, ovary, and intestines (Fig. 2). Confocal microscopy of heterologously expressed human TMEM163 shows plasma membrane (PM) and lysosomal localization (Fig. 3). A partial co-localization with TRPML1 is observed, suggesting that both proteins may have specific cellular function that is independent of each other (Fig. 3).
Fig. 2. Analyses of TMEM163 gene expression in human tissues.
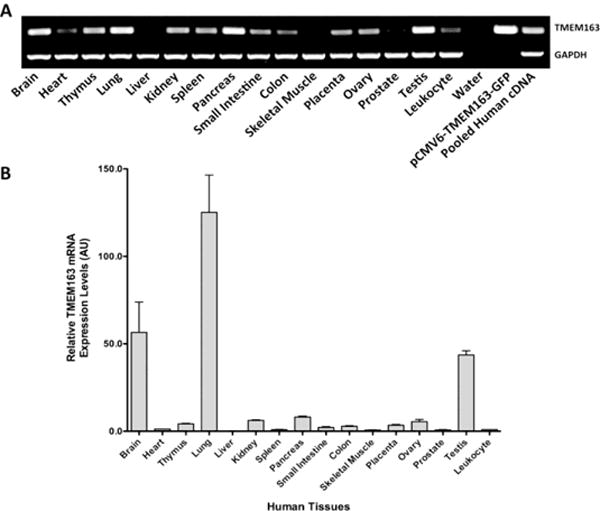
A) Standard PCR analysis of human TMEM163 transcripts using normalized multiple tissue cDNA (MTC) panel commercially purchased from Clontech. No template control (H2O) represented the negative control, while pCMV6-GFP-TMEM163 and non-normalized pooled cDNA were used as positive controls. The housekeeping gene, GAPDH, was used as an internal loading control. B) Real-time quantitative reverse-transcription polymerase reaction (RT-PCR) analysis of TMEM163 using the same MTC panel used in A. The samples were analyzed using the Livak method (ΔΔCq). The housekeeping gene, 18s rRNA, was used as a reference (normalizer). The leukocyte sample was used as the calibrator (value = 1), which makes the tissue mRNA levels all relative to leukocyte. Data are represented as mean ± SEM (n = 3). AU, arbitrary units; bp, basepair. Reprinted with permission from Cuajungco et al. (2014), Traffic, 15, 1247-1265. Copyright 2014 Wiley.
Fig. 3. Subcellular distribution of heterologously co-expressed TRPML1 and TMEM163 proteins.
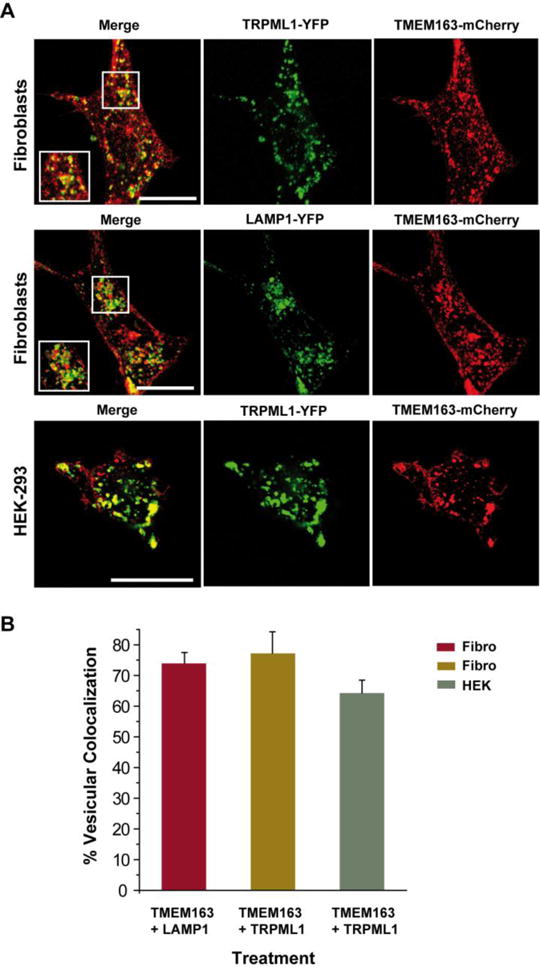
A) Representative laser scanning micrographs showing subcellular co-localization of TRPML1-YFP and TMEM163-mCherry upon heterologous expression in human primary fibroblast cells (top panel) and HEK-293 cells (bottom panel). TMEM163-mCherry partially co-localized with TRPML1-YFP and LAMP1-YFP (a marker for late endosomes and lysosomes). TMEM163 localized on the plasma membrane, but also exhibited a punctate distribution pattern with either TRPML1 or LAMP1. In HEK-293 cells, co-expression of TMEM163-mCherry with the TRPML1-YFP showed similar a subcellular distribution pattern to the fibroblast cells. Scale bar = 20 μm. B) Cell count showing the percentage of vesicular co-localization pattern between co-expressed TMEM163 plus LAMP1, and TMEM163 plus TRPML1 (for both human fibroblast and HEK-293 cells). The data showed that 70–80% of TMEM163 co-localized with LAMP1 and TRPML1 in late endosomes and lysosomes of fibroblast cells, while 60–70% of TMEM163 co-localized with TRPML1 in late endosomes and lysosomes of HEK-293 cells (n = 50 cells). Reprinted with permission from Cuajungco et al. (2014), Traffic, 15, 1247–1265. Copyright 2014 Wiley.
TRPML1 has been reported to have a di-Leucine motif [D/E]XXXL[L/I] or lysosomal targeting sequence (LTS) at the N-terminus, while another di-leucine motif situated at the C-terminus serves as an internalization signal for adaptor protein 2 (Vergarajauregui and Puertollano, 2006). Human TMEM163 (or rodent Tmem163) has a putative di-Leucine motif [D/E]XXXL[L/I] or lysosomal targeting sequence (LTS) with amino acid residues EDRGLL at its N-terminus position 65–70 (Fig. 4) (Cuajungco et al., 2014). The LTS motif is also present on certain Zrt- and Irt-like proteins (ZIP, also known as SLC39A) and ZnT proteins. For example, ETRALL is found on ZIP1 (144–149), DDDSLL (72–77) on ZnT4, and DAAHLL on ZnT2 (103–108) or ZnT3 (105–110). In general, proteins containing a di-Leucine motif (Kozik et al., 2010) are targeted by adaptor proteins for membrane trafficking to and from the plasma membrane, tubular endosomes or endosomal-lysosomal compartment, or Golgi network (Hirst et al., 2011).
Fig. 4. Amino acid sequence map of TMEM163 protein.
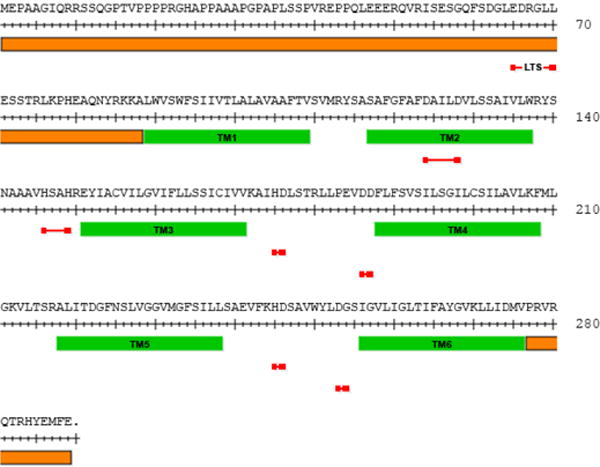
The map indicates H and D amino acid residues that could potentially bind zinc (red line). The predicted TM domains are shown as solid green bar, while both N- and C-termini are demarcated by a solid orange bar. The putative lysosomal targeting sequence (LTS) is indicated by a red line. The LTS contains a consensus sequence motif of [D/E]XXXL[L/I] residues where X is any amino acid.
The rodent Tmem163 protein is predicted to have six transmembrane domains with long N-terminus and short C-terminus regions predicted to be cytoplasmic (Burre et al., 2007; Barth et al., 2011). A closer inspection of human TMEM163’s protein sequence (Fig. 4) shows an apparent topological similarity with the ZnT proteins, whereby TMEM163 has a predicted long N-terminus but short C-terminus, while the ZnT proteins have a predicted short N-terminus but long C-terminus region. This may be, of course, a mere coincidence; however, we cannot rule out the possibility that TMEM163 may belong to the SLC39A or SLC30A family of influx or efflux transporter proteins, respectively. Further, the tissue specific expression pattern of TMEM163 coincides well with other ZnTs (Chimienti et al., 2006; Jackson et al., 2007; Bosomworth et al., 2012), and thus contributes to transporter redundancy in many cell types (Kambe et al., 2015).
The amino acid sequence alignment of rodent Tmem163 with mouse ZnT3, E. coli zinc transporter (YiiP), and R. metallidurans cobalt–zinc–cadmium resistance protein (CzcD) shows around 20% sequence identity (Barth et al., 2011). From the same alignment, two aspartate (D) residues on the predicted second transmembrane (TM) domain were conjectured to potentially bind Zn2+. It is interesting to note that Histidine (H) and/or D residues such as the HXXXD motif (where X is a non-polar amino acid) located within TM2 and TM5 helices of ZnT proteins, and HXXXH motif in TM4 and TM5 helices of ZIP proteins have been suggested to facilitate tetrahedral zinc coordination (Ohana et al., 2009; Kambe et al., 2015). Collectively, these amino acid residues have been designated as HD-DD motifs present in zinc transport proteins (Kambe et al., 2015), and site-directed mutagenesis of native HD residues within TM4 and TM5 helices of the ZnT5 protein has been shown to disrupt zinc binding and transport activity (Ohana et al., 2009). Indeed, metal-binding assays on cells heterologously expressing rodent Tmem163 showed a strong binding preference to Zn2+ or nickel (Ni2+), but weakly binds copper (Cu2+) (Barth et al., 2011). Future research needs to show if any of these H and/or D residues on human TMEM163 do bind Zn2+, Ni2+, or Cu2+. In addition, it would be interesting to know if other parts of TMEM163 that have the H-D motif such as those located between TM3 and TM4 domains, or TM5 and TM6 domains (Fig. 4) are potential binding site for Zn2+, Ni2+, or Cu2+.
The main function of Metallothionein (MT) proteins is to act as a zinc reservoir, and to control the distribution of Zn2+ to other zinc-binding proteins (Maret and Vallee, 1998). However, MT is not a long-term storage for Zn2+ due to its short biological half-life (Krezoski et al., 1988), which necessitates vesicular or compartmental storage of zinc. Specifically, Zn2+ may be stored in the neuronal synaptic vesicles, endosomes or lysosomes, endoplasmic reticulum [ER]) and mitochondria. Transport of Zn2+ into or out of these compartments is mediated by ZnTs, ZIPs, and divalent cation transporter protein families (Eide, 2006; Kambe et al., 2015). To further investigate the function of Tmem163, rat PC12 cells stably expressing the protein and subsequently exposed to Zn2+ showed accumulation within vesicular structures and cytoplasmic compartments as evidenced by the zinc-specific dye, Fluozin-3 (Barth et al., 2011). It was then suggested that rodent Tmem163 protein could be a Zn2+ efflux transporter. One of us (MPC) has found that cultured SH-SY5Y human neuroblastoma cells heterologously expressing TMEM163 markedly accumulate intracellular zinc following a brief exogenous zinc chloride (ZnCl2, 100 μM, 1 hour exposure). A concomitant increase in the relative MT1A transcript expression levels is observed in TMEM163-overexpressing SH-SY5Y neuroblastoma cells compared with controls (Fig. 5). This observation suggests that TMEM163 is a novel transporter of Zn2+, and is thus critical to Zn2+ homeostasis in specific tissues or organs. Interestingly, it was also observed that heterologously co-expressed TMEM163 and ZnT4 produces intracellular zinc elevation in cultured cells upon exogenous ZnCl2 (100 μM) exposure as evidenced by Fluozin-3 fluorescence (Cantrell et al., 2016). This finding suggests that TMEM163 and ZnT4 physically interact with each other in distinct cells that express both proteins. In line with this possibility, several ZnT proteins have been reported to interact and form heterodimers with each other (Fukunaka et al., 2009; Lasry et al., 2014; Golan et al., 2015; Zhao et al., 2016). It is worth noting that ZnT2 and ZnT4 heterodimers localize to the plasma membrane, whereas ZnT2 or ZnT4 homodimer each localize to their respective vesicular compartment (Golan et al., 2015). It is thus possible that TMEM163 and ZnT4 heterodimers confer distinct function when compared to their respective homodimers. Further work needs to be done in order to prove if TMEM163 is an influx or efflux transporter, and whether it interacts with ZnT4 or other ZnTs, as well as ZIP transporters.
Fig. 5. Cultured SH-SY5Y neuroblastoma cells heterologously expressing human TMEM163 increases Metallothionein-1A expression levels upon exogenous zinc exposure.
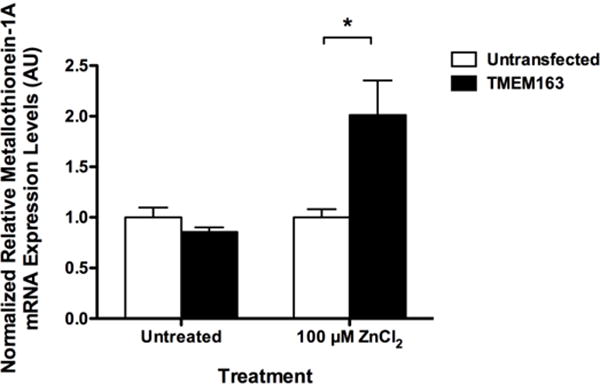
Real-time quantitative RT-PCR of Metallothionein-1A (MT1A) transcripts at 24 hours following transient ZnCl2 exposure (100 μM, 1 h) of TMEM163-expressing SH-SY5Y neuroblastoma and untransfected control cells. Significant up-regulation of MT1A transcripts is evident in the TMEM163-expressing cells exposed to zinc compared to untreated cells. This result suggests that TMEM163 mediates intracellular zinc flux upon exogenous zinc exposure. Data are represented as mean ± SEM (n = 3, Student’s t-test, paired, two-tailed, *p < 0.05). AU, arbitrary units.
In summary, the lysosomal Zn2+ handling appears to be a critical period that could mean survival or death upon cytoplasmic Zn2+ overload in many cell types, especially in neurons. The TRPML1, ZnT4, and TMEM163 proteins may be central to Zn2+ handling that involves the lysosomes and other membrane-bound compartments (Fig. 6). Future research to define the contributions of these proteins in cellular zinc homeostasis. There are still many gaps in our knowledge regarding the function of TMEM163, and the relevance of its interaction with TRPML1 or its putative interaction with other zinc transporters. For example, does TRPML1 mediate the subcellular trafficking of TMEM163? Does TRPML1 function cooperatively with TMEM163 in terms of releasing cations from the lysosomes into the cytosol according to previous reports suggesting that TRPML1 is a “release” channel? Finally, does the functional loss of TRPML1, which creates hyperacidic and zinc-elevated lysosomes, confers concomitant inhibition of TMEM163 function? Does TMEM163 belong to a new class of ZnT- or ZIP-like proteins, which could explain its redundant expression pattern with ZnT or ZIP in various tissues? These are just a few questions that must be answered in order to advance the field and fully understand the role of TMEM163 in normal and pathological states.
Fig. 6. Proposed cellular function of TMEM163 protein in zinc-rich cells or neurons.
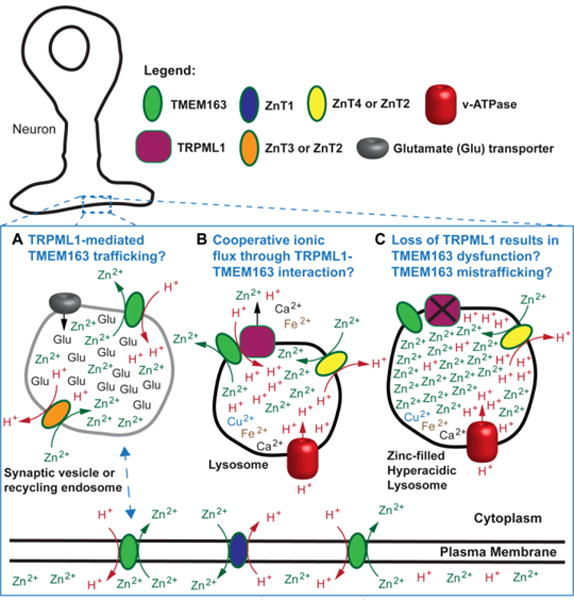
TMEM163 has been observed to localize in the plasma membrane, lysosomes, and vesicular compartments. A) The schematic model depicts that TMEM163 is a zinc transporter that is similar to the ZnT proteins in that it is a zinc (Zn2+)/proton (H+) exchanger. It is proposed that TRPML1 may be responsible to the subcellular trafficking of TMEM163 from the plasma membrane to endocytic compartments, synaptic vesicles, or lysosomes; and vice versa. B) The illustration shows that TRPML1 is a release channel that controls the flux of ions (H+, Ca2+, Zn2+, Fe2+, Mn2+) within the lysosomes. The physical interaction between TMEM163 and TRPML1 is hypothesized to result in cooperative release of Zn2+ and possibly other cations, in order to prevent pathological buildup. C) The loss of TRPML1 function produces hyperacidic lysosomes that is also filled with Zn2+ through the activity of ZnT4 proteins (ZnT2 in other cell types or ZnT3 in neurons). Consequently, the loss of TMEM163 and TRPML1 interaction prevents the cooperative release and exacerbates Zn2+ accumulation.
Acknowledgments
MPC was partly funded by grants from the NIH BD2K R25-MD01397-01, NIH AREA R15-NS070774-01, NIH MARC U*STAR Program T34-GM008612-20, National Science Foundation MCB-0920127, and Cal State Fullerton Intramural Grants program. KK is supported by the MLIV foundation.
Footnotes
The authors declare no conflicts of interest.
References
- Altarescu G, Sun M, Moore DF, Smith JA, Wiggs EA, Solomon BI, Patronas NJ, Frei KP, Gupta S, Kaneski CR, Quarrell OW, Slaugenhaupt SA, Goldin E, Schiffmann R. The neurogenetics of mucolipidosis type IV. Neurology. 2002;59:306–313. doi: 10.1212/wnl.59.3.306. [DOI] [PubMed] [Google Scholar]
- Balaji RV, Colvin RA. A proton-dependent zinc uptake in PC12 cells. Neurochemical research. 2005;30:171–176. doi: 10.1007/s11064-004-2438-6. [DOI] [PubMed] [Google Scholar]
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Barth J, Zimmermann H, Volknandt W. SV31 is a Zn2+-binding synaptic vesicle protein. J Neurochem. 2011;118:558–570. doi: 10.1111/j.1471-4159.2011.07344.x. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics : integrated biometal science. 2012;4:771–779. doi: 10.1039/c2mt20088k. [DOI] [PubMed] [Google Scholar]
- Bostanci Z, Alam S, Soybel DI, Kelleher SL. Prolactin receptor attenuation induces zinc pool redistribution through ZnT2 and decreases invasion in MDA-MB-453 breast cancer cells. Exp Cell Res. 2014;321:190–200. doi: 10.1016/j.yexcr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Burre J, Zimmermann H, Volknandt W. Identification and characterization of SV31, a novel synaptic vesicle membrane protein and potential transporter. J Neurochem. 2007;103:276–287. doi: 10.1111/j.1471-4159.2007.04758.x. [DOI] [PubMed] [Google Scholar]
- Cantrell QW, Silva J, Nguyen C, Hildebrand LD, Rivas T, Shoemaker R, Rojas A, Cuajungco MP. Transmembrane (TMEM)-163 protein is a novel zinc transporter. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30 Supplement. [Google Scholar]
- Chapel A, Kieffer-Jaquinod S, Sagne C, Verdon Q, Ivaldi C, Mellal M, Thirion J, Jadot M, Bruley C, Garin J, Gasnier B, Journet A. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Molecular & cellular proteomics : MCP. 2013;12:1572–1588. doi: 10.1074/mcp.M112.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Chung H, Yoon YH, Hwang JJ, Cho KS, Koh JY, Kim JG. Ethambutol-induced toxicity is mediated by zinc and lysosomal membrane permeabilization in cultured retinal cells. Toxicol Appl Pharmacol. 2009;235:163–170. doi: 10.1016/j.taap.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Colletti GA, Miedel MT, Quinn J, Andharia N, Weisz OA, Kiselyov K. Loss of lysosomal ion channel transient receptor potential channel mucolipin-1 (TRPML1) leads to cathepsin B-dependent apoptosis. J Biol Chem. 2012;287:8082–8091. doi: 10.1074/jbc.M111.285536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Davis N, Nipper RW, Carter PA. Zinc transport in the brain: routes of zinc influx and efflux in neurons. J Nutr. 2000;130:1484S–1487S. doi: 10.1093/jn/130.5.1484S. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Basilio LC, Silva J, Hart T, Tringali J, Chen CC, Biel M, Grimm C. Cellular Zinc Levels Are Modulated by TRPML1-TMEM163 Interaction. Traffic. 2014;15:1247–1265. doi: 10.1111/tra.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. Nitric oxide generators produce accumulation of chelatable zinc in hippocampal neuronal perikarya. Brain research. 1998;799:118–129. doi: 10.1016/s0006-8993(98)00463-6. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1817–1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nature communications. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Shen D, Chen S, Liu M, Wang Y, Mills E, Cheng X, Delling M, Xu H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J Biol Chem. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draye JP, Courtoy PJ, Quintart J, Baudhuin P. A quantitative model of traffic between plasma membrane and secondary lysosomes: evaluation of inflow, lateral diffusion, and degradation. J Cell Biol. 1988;107:2109–2115. doi: 10.1083/jcb.107.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, Cuajungco MP. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Falchuk KH, Montorzi M, Vallee BL. Zinc uptake and distribution in Xenopus laevis oocytes and embryos. Biochemistry. 1995;34:16524–16531. doi: 10.1021/bi00050a037. [DOI] [PubMed] [Google Scholar]
- Feeney EJ, Spampanato C, Puertollano R, Ballabio A, Parenti G, Raben N. What else is in store for autophagy? Exocytosis of autolysosomes as a mechanism of TFEB-mediated cellular clearance in Pompe disease. Autophagy. 2013;9:1117–1118. doi: 10.4161/auto.24920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Cuajungco MP, LaBuda CJ, Suh SW. Nitric oxide causes apparent release of zinc from presynaptic boutons. Neuroscience. 2002;115:471–474. doi: 10.1016/s0306-4522(02)00399-8. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Maret W, Cuajungco MP. Zinc and excitotoxic brain injury: a new model. Neuroscientist. 2004;10:18–25. doi: 10.1177/1073858403255840. [DOI] [PubMed] [Google Scholar]
- Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims KB, Berry-Kravis E, Pahan K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: implications for lysosomal storage disorders. The Journal of biological chemistry. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan Y, Berman B, Assaraf YG. Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation. J Biol Chem. 2015;290:9050–9063. doi: 10.1074/jbc.M114.617332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Small molecule activators of TRPML3. Chemistry & biology. 2010;17:135–148. doi: 10.1016/j.chembiol.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk Y, Peña KA, Coblentz J, King VE, Humphrey DM, Wang SL, Kiselyov KI, Slaugenhaupt SA. Impaired myelination and reduced brain ferric iron in the mouse model of mucolipidosis IV. Dis Model Mech. 2015 doi: 10.1242/dmm.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS. The fifth adaptor protein complex. PLoS Biol. 2011;9:e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun HJ, Sohn J, Ahn YH, Shin HC, Koh JY, Yoon YH. Depletion of intracellular zinc induces macromolecule synthesis- and caspase-dependent apoptosis of cultured retinal cells. Brain research. 2000;869:39–48. doi: 10.1016/s0006-8993(00)02340-4. [DOI] [PubMed] [Google Scholar]
- Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:460–465. [PubMed] [Google Scholar]
- Jackson KA, Helston RM, McKay JA, O’Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J Biol Chem. 2007;282:10423–10431. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov KK, Ahuja M, Rybalchenko V, Patel S, Muallem S. The intracellular Ca(2)(+) channels of membrane traffic. Channels (Austin) 2012;6:344–351. doi: 10.4161/chan.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kozik P, Francis RW, Seaman MN, Robinson MS. A screen for endocytic motifs. Traffic. 2010;11:843–855. doi: 10.1111/j.1600-0854.2010.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezoski SK, Villalobos J, Shaw CF, 3rd, Petering DH. Kinetic lability of zinc bound to metallothionein in Ehrlich cells. Biochem J. 1988;255:483–491. [PMC free article] [PubMed] [Google Scholar]
- Kukic I, Kelleher SL, Kiselyov K. Zn2+ efflux through lysosomal exocytosis prevents Zn2+-induced toxicity. J Cell Sci. 2014;127:3094–3103. doi: 10.1242/jcs.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J. 2013;451:155–163. doi: 10.1042/BJ20121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Spada AR. PPARGC1A/PGC-1alpha, TFEB and enhanced proteostasis in Huntington disease: defining regulatory linkages between energy production and protein-organelle quality control. Autophagy. 2012;8:1845–1847. doi: 10.4161/auto.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasry I, Golan Y, Berman B, Amram N, Glaser F, Assaraf YG. In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J Biol Chem. 2014;289:7275–7292. doi: 10.1074/jbc.M113.533786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD. Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516:112–115. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–347. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Cho KS, Koh JY. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia. 2009;57:1351–1361. doi: 10.1002/glia.20854. [DOI] [PubMed] [Google Scholar]
- Lees GJ, Cuajungco MP, Leong W. Effect of metal chelating agents on the direct and seizure-related neuronal death induced by zinc and kainic acid. Brain research. 1998;799:108–117. doi: 10.1016/s0006-8993(98)00483-1. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Flinn JM, Peto T, Linkous DH, Cano K, Bird AC, Lanzirotti A, Frederickson CJ, van Kuijk FJ. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007;84:772–780. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Logan MR, Lacy P, Odemuyiwa SO, Steward M, Davoine F, Kita H, Moqbel R. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy. 2006;61:777–784. doi: 10.1111/j.1398-9995.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Diab HI, Li H, Puertollano R. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci. 2014;71:2483–2497. doi: 10.1007/s00018-014-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick NH, Hennigar SR, Kiselyov K, Kelleher SL. The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution. Journal of mammary gland biology and neoplasia. 2014;19:59–71. doi: 10.1007/s10911-013-9314-4. [DOI] [PubMed] [Google Scholar]
- McCormick NH, Kelleher SL. ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells. Am J Physiol Cell Physiol. 2012;303:C291–297. doi: 10.1152/ajpcell.00443.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell. 2015;161:1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia C, Vespignani I, Cerase J, Nobili F, Perozzi G. Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. The American journal of physiology. 1999;277:G1231–1239. doi: 10.1152/ajpgi.1999.277.6.G1231. [DOI] [PubMed] [Google Scholar]
- Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña KA, Coblenz J, Kiselyov K. Brief exposure to copper activates lysosomal exocytosis. Cell Calcium. 2015;57:257–262. doi: 10.1016/j.ceca.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña KA, Kiselyov K. Transition metals activate TFEB in overpexpressing cells. Biochem J. 2015;470:65–76. doi: 10.1042/BJ20140645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, Sardiello M, Ballabio A, Zheng H. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO molecular medicine. 2014;6:1142–1160. doi: 10.15252/emmm.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Redenti S, Chappell RL. Neuroimaging of zinc released by depolarization of rat retinal cells. Vision research. 2005;45:3520–3525. doi: 10.1016/j.visres.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Redenti S, Ripps H, Chappell RL. Zinc release at the synaptic terminals of rod photoreceptors. Exp Eye Res. 2007;85:580–584. doi: 10.1016/j.exer.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K. Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab. 2012;15:88–99. doi: 10.1016/j.cmet.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Weiss JH. Mitochondrial sequestration and Ca(2+)-dependent release of cytosolic Zn(2+) loads in cortical neurons. Neurobiol Dis. 2002;10:100–108. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- Seo YA, Lopez V, Kelleher SL. A histidine-rich motif mediates mitochondrial localization of ZnT2 to modulate mitochondrial function. Am J Physiol Cell Physiol. 2011;300:C1479–1489. doi: 10.1152/ajpcell.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B, Kemper JK. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;516:108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Medina DL. TFEB and the CLEAR network. Methods Cell Biol. 2015;126:45–62. doi: 10.1016/bs.mcb.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Silva J, Cuajungco MP. Intracellular Zinc Dyshomeostasis Caused by a Disrupted TRPML1-TMEM163 Protein Interaction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29 Supplement. [Google Scholar]
- Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F, Zare H, Polishchuk R, Puertollano R, Parenti G, Ballabio A, Raben N. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO molecular medicine. 2013;5:691–706. doi: 10.1002/emmm.201202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain research. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Lee JY, Lee SE, Koh JY, Yoon YH. Protection by pyruvate of rat retinal cells against zinc toxicity in vitro, and pressure-induced ischemia in vivo. Invest Ophthalmol Vis Sci. 2004;45:1523–1530. doi: 10.1167/iovs.03-1315. [DOI] [PubMed] [Google Scholar]
- Yoon YH, Jung KH, Sadun AA, Shin HC, Koh JY. Ethambutol-induced vacuolar changes and neuronal loss in rat retinal cell culture: mediation by endogenous zinc. Toxicol Appl Pharmacol. 2000;162:107–114. doi: 10.1006/taap.1999.8846. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Feresin RG, Falcon-Perez JM, Salazar G. Differential Targeting of SLC30A10/ZnT10 Heterodimers to Endolysosomal Compartments Modulates EGF-Induced MEK/ERK1/2 Activity. Traffic. 2016;17:267–288. doi: 10.1111/tra.12371. [DOI] [PubMed] [Google Scholar]


