Abstract
Azathioprine (AZA) and 6-mercaptopurine (6-MP) are the most widely used immunosuppressive therapies in inflammatory bowel disease. Pretreatment measurement of thiopurine methyltransferase (TPMT) activity is recommended and although conventional practice is to use a dose of 2 mg/kg AZA (1 mg/kg 6-MP), higher doses of 2.5 mg/kg AZA or more may be required in some patients, particularly if TPMT activity is high. Dose raising is limited by toxicity, and a robust monitoring system is mandatory. Patients with side effects to AZA may tolerate 6-MP but pancreatitis is a contraindication to switching. Metabolite monitoring is not widely available but may be useful, particularly if non-compliance is possible or where metabolite shunting to 6-methylmercaptopurine is suspected, on the basis of non-response or toxicity. It may allow dose optimisation before switching to alternative immunosuppressants. The drug appears safe in pregnancy and breast feeding. Long term duration of therapy is a balance between benefits in relation to the underlying disease extent, activity and aggressiveness, and the risk of neoplasia, particularly lymphoma.
Introduction
The explosion of genetic understanding of inflammatory bowel disease (IBD) has highlighted the fundamental role of defective innate immune mechanisms and abnormal immune regulation in pathogenesis but the mainstay of therapy remains anti-inflammatory and immunosuppressive treatments directed at the secondary immune response and tissue damage that results. Of these, the thiopurine drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) are the most widely used. They have a slow onset of action (2–4 months), and Cochrane meta-analysis1 2 gives a number needed to treat (NNT) of 5–6 for response to treatment, and 3 for steroid sparing. Meta-analysis of four controlled trials concluded that AZA/6-MP use was effective in preventing both clinical and endoscopic recurrence of postoperative Crohn's disease (CD).3 The threshold for using thiopurines in CD has gradually lowered and they are being used earlier and more widely4 for patients with extensive disease who require more than one course of corticosteroids or who have extensive or severe disease.5 In ulcerative colitis (UC), thiopurines have been less widely used. Meta-analysis shows clear benefit in maintenance of remission in UC in mesalazine treatment failure or intolerance, and in steroid dependence (NNT=5).6
Pharmacology and pharmacogenetics
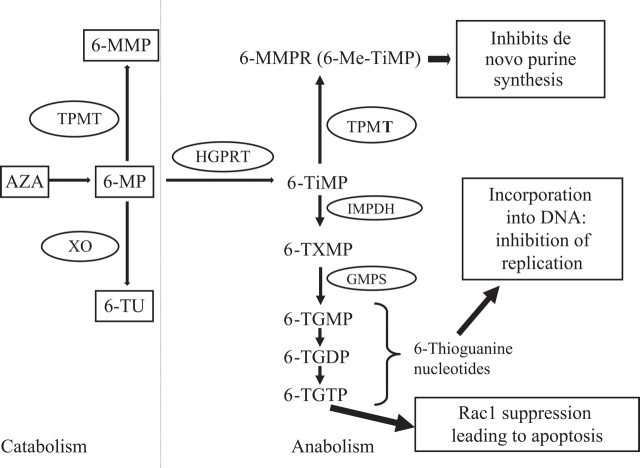
The purine analogue 6-MP was initially used in the 1950s in haematological malignancy. It undergoes extensive first pass metabolism by xanthine oxidase in intestinal mucosa and liver,7 and AZA was developed as a prodrug, by addition of an imidazole ring, to increase bioavailability. Absorption of AZA is 16–50% in health and reduced if intestinal transit time is rapid.8 Eighty-eight per cent of absorbed AZA is rapidly converted non-enzymatically in the liver to 6-MP. 6-MP is then metabolised by three enzymes, as shown in figure 1.9 The molecular weight of 6-MP is 55% of that of AZA, so 2.08 mg of AZA is equivalent to 1 mg of 6-MP, assuming 100% oral bioavailability.
Figure 1.
Pathway for thiopurine metabolism. 6-Me-TIMP, 6-methyl thioinosine monophosphate; 6-MMP, 6-methylmercaptopurine; 6-MMPR, 6-methylmercaptopurine ribonucleotides; 6-MP, 6-mercaptopurine; 6-TGDP, 6-thioguanosine diphosphate; 6-TGMP, 6-thioguanosine monophosphate; 6-TGTP, 6-thioguanosine triphosphate; 6-TIMP, 6-thioinosine monophosphate; 6-TU, 6-thiouracil; 6-TXMP, 6-thioxanthine monophosphate; AZA, azathioprine; GMPS, guanosine monophosphate synthetase; HGPRT, hypoxanthine guanine phosphoribosyl transferase; IMPDH, inosine monophosphate dehydrogenase; TPMT, thiopurine S-methyltransferase; XO, xanthine oxidase. 6-TGMP, 6-TGDP and 6-TGTP together are called 6-thioguanine nucleotides.
With a trimodal distribution of thiopurine methyltransferase (TPMT) activity, 89% of Caucasians are homozygous for a gene with high activity, 11% heterozygous with intermediate activity and 1 in 300 homozygous for low activity.10 TPMT*3A is the commonest mutant allele, seen predominantly in Caucasians. TPMT*3C is the most prevalent variant in African American and Asian populations. Clinical efficacy and marrow toxicity of AZA/6-MP are in part determined by TPMT activity. Low activity is associated with elevated 6-thioguanine nucleotide (6-TGN) and, consequently, risk of myelosuppression.11 Conversely, high TPMT activity results in low erythrocyte 6-TGN levels, and these patients may need a higher dose of AZA/6-MP to achieve a therapeutic response.12
Routine TPMT testing?
Checking TPMT prior to starting AZA/6-MP has become routine in most hospitals. This can be justified by the avoidance of potentially fatal myelotoxicity in individuals homozygous or compound heterozygous for alleles with low TPMT activity. Patients with intermediate activity are predicted to need a 50% reduction in standard doses (table 1). Conversely, prior knowledge of high TPMT activity should encourage use of higher doses and increasing the dose at an early stage to maximise clinical response.13 In view of the different mutations associated with TPMT deficiency, measurement of erythrocyte TPMT activity is preferred to genotyping.14 Blood transfusion in the previous 3 months will affect levels because of TPMT activity in donor blood and can therefore mask patients with very low activity. Other factors will also affect levels, including food, drug therapy and uraemia.
Table 1.
Strategies to optimise thiopurine therapy
| Strategy | Comment | References |
|---|---|---|
| TPMT measurement (levels as pmol/h/mg Hb) | Low TPMT (<10) – contraindicated | Travis,5 Cuffari,20 Dubinsky,21 Barbe22 |
| Intermediate TPMT (10–24) – AZA dose range 1 mg/kg daily | ||
| Normal TPMT (25–50) – AZA dose range 2–2.5 mg/kg daily | ||
| Metabolite monitoring (measurement of 6-TGN and 6-MMP levels) | Consider measurement of metabolites if poor response to therapy or abnormal LFTs | |
| Absent 6-TGN levels suggest non-adherence | ||
| Low 6-TGN (below 230–260 pmol/8 × 108 RBCs) with good adherence suggests dose increase is needed | Osterman64 | |
| High 6-MMP suggests preferential methylation. Options include: | Dubinsky,21 65 | |
| splitting doses to twice daily, which has been reported to reduce methylation while maintaining 6-TGN levels and efficacy; | Shih66 | |
| reduce AZA dose (25–50% of usual dose) and add low dose allopurinol. May normalise raised LFTs. Only recommended in specialist centres with relevant expertise | Sparrow67 68 |
6-MP dose is half that of AZA.
6-MMP, 6-methylmercaptopurine; 6-MP, 6-mercaptopurine; 6-TGN, 6-thioguanine nucleotides; AZA, azathioprine; LFTs, liver function tests; RBC, red blood cells; TPMT, thiopurine methyltransferase.
Using decision analysis, measurement of TPMT has been shown to be cost effective.13 15 The assay costs £30 in the UK, and it costs £9000 to prevent one potential fatal toxicity and avert a proportion of toxicity in 30 heterozygous individuals.
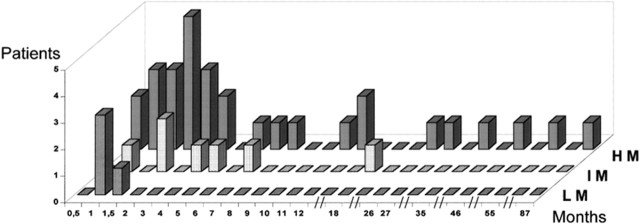
Myelosuppression during thiopurine treatment may not be solely attributed to low TPMT activity. Among 41 patients with CD who developed either leucopenia or thrombocytopenia after thiopurine therapy, only 27% had one or two mutant alleles.16 In the same study, as shown in figure 2, Colombel et al showed that early myelotoxicity occurred in those homozygous for an inactivating mutation, from 1 to 18 months in heterozygotes, but at any time in patients with no mutation. Delayed neutropenia is likely due to other factors (eg, concomitant medications, varicella and parvovirus B19 infection).17 18 TPMT testing alone is no substitute for regular blood monitoring.
Figure 2.
Time to bone marrow toxicity. Delay (months) between the first administration of azathioprine/6-mercaptopurine and the occurrence of bone marrow toxicity. Patients were classified as low methylators (LM; n=4), intermediate methylators (IM; n=7) and high methylators (HM; n=20) (courtesy of Colombel et al16).
Dosage and duration
Approximately 40% of patients will not respond to 2 mg/kg/day dose of AZA or equivalent 6-MP dose.19 Non-responders to low doses will benefit from dose escalation to AZA 2–2.5 mg/kg/day20–22 but benefit of dose escalation above this level is less.23 Target doses should be adjusted using TPMT levels, as shown in table 1.
Mean time to response in CD patients treated with 6-MP was reported as 3.1 months24 although later studies suggest that this could be as early as 4–8 weeks.25 Overall duration of thiopurines, after remission is reached, remains contentious. A randomised study26 from Lemann et al showed that continuation of AZA beyond 42 months, for a further 18 months, was beneficial compared with cessation. Reassuringly, many patients who did relapse after cessation responded well to reintroduction. Other studies have recommended indefinite thiopurine use once remission is achieved.27
Haematological monitoring
Bone marrow suppression can occur at any time during treatment with AZA/6-MP, and can occur suddenly after months or years.16 28 Overall reported frequency varies from 5% to 13%.29 30 Regular blood count monitoring becomes important but the optimal monitoring schedule is unclear. The British National Formulary recommends weekly full blood counts for 4 weeks.31 European guidelines recommend less frequent monitoring,5 at 2–3 month intervals but checking shortly (2 weeks) after any dose increase. Dose adjustment should be based on total white cell count (WCC) with reduction if this falls below 3.5 × 109/ml and cessation if below 3.0. Adjusting the dose based on neutrophil or lymphocyte depletion appears to offer no great advantage.
Fraser et al29 showed that the WCC and neutrophil counts were both good predictors of achieving and maintaining remission but lymphocyte count had no value for predicting remission. Colonna and Korelitz found a strong positive correlation between the extent of 6-MP induced leucopenia and clinical outcome in refractory CD patients.32 Campbell and Ghosh in a cohort of 173 patients however showed no relation between lowest neutrophil count and relapse rate. AZA dose titration to achieve neutropenia is not necessary.33
Non-haematological adverse drug reactions
These can be divided into dose dependent pharmacologically explainable events (type A) and dose independent hypersensitivity reactions (type B). Type A adverse events are associated with formation of toxic metabolites and usually occur over the first 4–8 weeks of treatment. They include general malaise, nausea and vomiting, and hepatotoxicity. In general, stopping AZA will improve symptoms and reintroducing this successfully at a later stage is possible. Type B reactions often occur within 2–4 weeks after the start of treatment, and result in immune mediated symptoms such as fever, rash and arthralgia but pancreatitis and hepatitis may also be idiosyncratic. Reintroduction of AZA after stoppage will cause the same reactions. Reported frequency of toxicity varies widely: nausea from 5% to 11%, hepatotoxicity 2% to 9% and pancreatitis 2% to 5%, according to definitions and dosage.29 30 34
Switching to 6-MP can be worthwhile in AZA intolerance. Seven case series have shown that 6-MP was tolerated in 48–77% of AZA intolerant patients. 6-MP is likely to be tolerated in patients failing AZA because of nausea and vomiting or a flu-like illness, which are associated with the AZA imidazole ring. Patients failing AZA because of hepatotoxicity fared less well, and all patients with pancreatitis failed a trial of 6-MP in the case series by Lees et al.35 However, Hindorf et al in their series of 135 patients reported that 12/17 (71%) patients who had hepatotoxicity with AZA tolerated 6-MP.36 Therefore, a cautious trial of 6-MP could be considered in AZA intolerance, with the exception of those with AZA induced pancreatitis. Metabolite monitoring makes it possible to select preferential methylators (high 6-methylmercaptopurine (6-MMP) levels) who are more likely to benefit from split dosing or dose reduction and addition of allopurinol (specialist centres only).
The antimetabolite thioguanine (TG), a direct precursor of 6-TGNs, has been used to avoid accumulation of the potentially hepatotoxic metabolite 6-MMP ribonucleotides. Although early studies showed good efficacy and short term safety data, Dubinsky et al in their study of 111 patients on TG reported that 29 (26%) patients had abnormal liver enzymes or thrombocytopenia, and of these who had liver biopsy, nodular regenerative hyperplasia (NRH) was diagnosed in 76%.37 Some of these patients may already have had abnormal liver function tests from preferential methylation. NRH causes non-cirrhotic portal hypertension38 so TG therapy remains controversial. It should only be used in specialist centres with experience of metabolite monitoring and in very limited circumstances (eg, AZA induced pancreatitis, in patients where alternatives such as methotrexate (MTX), mycophenolate mofetil or surgical therapy is not practicable). Vernier-Massouille et al reported that NRH does not only occur in patients receiving TG. Thirty-seven patients were identified in this GETAID survey of 36 hospitals and all cases had been treated with AZA in standard doses39 (6-MP was used little in the centres studied). The risk was estimated at 0.5% at 5 years and 1.25% at 10 years therapy although CIs were large. There was an association with male sex and stricturing disease behaviour.
Infection
In the absence of undiagnosed leucopenia, infectious complications are uncommon. There is an increased risk of viral infections, particularly herpes zoster, cytomegalovirus and viral warts. More serious infections are rare, and more often related to concomitant corticosteroid therapy. The use of AZA/6-MP alone or used in combination with corticosteroids in patients undergoing elective bowel surgery in IBD does not significantly increase the risk of postoperative infectious complications.40 Live vaccination is contraindicated on thiopurine therapy. Newly diagnosed IBD patients (particularly those with CD, extensive UC or where early immunosuppressive therapy is likely) should have assessment of risk factors for infection, full immunisation history and serology assessment. Protocols should reflect local prevalence. If serology is negative, varicella zoster and hepatitis B vaccination should be given, and human papilloma virus (if not already given). Boosters for influenza and pneumococcal vaccine should be given to those receiving ongoing therapy.41
Risk of lymphoma and cancers
Epidemiological evidence of AZA use in transplant patients is sufficient for the International Agency for Research on Cancer to classify AZA as a human carcinogen.42 Most of these transplant associated cancers (non-Hodgkin's lymphoma, hepatobiliary carcinoma and renal mesenchymal tumours) can be accounted for by activation of oncogenic viruses, and there are similarities in the pattern of malignancy to those of HIV infected patients. In skin squamous cell carcinoma however (which is the most common treatment related transplant malignancy), the link to a viral aetiology has not been shown. Other putative mechanisms include impaired mismatch repair and photoreactivity of DNA 6-TG in the skin.43 Any increase in colorectal cancer in thiopurine treated IBD has generally been attributed to the underlying disease, and indeed a report from the CESAME cohort showed that IBD patients with longstanding extensive colitis receiving thiopurines had a 3.5 fold reduction in advanced colorectal neoplasia.44 Connell et al in their study45 also reported a numerically significant risk of cervical cancer but it was not statistically significant and it remains controversial whether thiopurine treated patients should have enhanced cervical screening. In contrast with individual studies showing increased risk of various cancers, a recent meta-analysis of nine cohort studies showed no overall increase in cancer risk.46
There is an increased risk of lymphoma in IBD.47 Whether treatment with thiopurines increases this has been a matter of debate. Askling et al in their cohort of 47 679 Swedish patients with IBD reported no increased risk of lymphoma in UC (standardised incidence ratio (SIR) 1.0 (95% CI 0.8 to 1.3)) but increased myeloid leukaemia (SIR 1.8 (95% CI 1.2 to 2.6)) in UC. In CD there was a borderline increase in lymphoma risk (SIR 1.3 (95% CI 1.0 to 1.6)) but confined to the first few years of follow-up, and little to indicate a long term increase in risk.48 In an analysis of the French CESAME cohort of 19 486 IBD patients (44.5% of whom had received thiopurines at some time), Beaugerie et al reported a significant increased risk of lymphoproliferative diseases (hazard ratio 5.3 (95% CI 2.0 to 13.9)) in thiopurine users compared with those who had never received the drugs. There was an association with Epstein–Barr infections, and the risk appeared to reduce to background rates once thiopurines were stopped.49 The aggressive and generally fatal hepatosplenic T cell lymphomas that occur in young (often male) patients have been reported with thiopurines alone, and in combination with infliximab.50–52
A decision analysis using ‘Markov’ modelling by Lewis et al53 showed that the benefits of AZA treatment in IBD outweigh the risk of developing lymphoma. With a maximum of 4 years of therapy, AZA results in a marginal increase in quality adjusted life years. This benefit decreased with increasing patient age (where baseline lymphoma risk is higher), and therefore young patients (who have the lowest baseline lymphoma risk and greatest life expectancy in the absence of a CD related death) have the greatest benefit. Kandiel's meta-analysis54 showed that the NNT to cause one additional lymphoma per year ranged from approximately 4357 persons aged 20–29 years to 355 persons aged 70–79 years.
Drug interactions
The commonest drug interaction of clinical relevance is with infliximab. In a study of 32 patients taking thiopurines for CD, 6-TGN levels rose, with a fall in WCC within 3 weeks after starting infliximab.55 Thiopurine dose reduction is more likely in those with low TPMT levels who start infliximab. Mesalazine inhibits TPMT in vitro but this is rarely clinically relevant.56 57 MTX inhibits xanthine oxidase, and co-administration with thiopurines can lead to severe myelotoxicity with no increase in clinical benefit.58 Although the t1⁄2β of 6-MP and AZA are very short in plasma, ranging from 1 to 2 h, 6-TGN accumulates in erythrocytes and has a t1/2β between 3 and 13 days, reflecting systemic exposure to cumulative doses of thiopurines.9 On this basis, an interval of at least 2 and preferably 4 weeks should occur before switching from thiopurines to MTX.
Pregnancy and lactation
Recent data from a Danish cohort study59 add to the growing body of evidence that thiopurines are safe in pregnancy. The adverse pregnancy outcomes reported in some studies are most likely related to the underlying disease.60 61 Having previously been considered unsafe during lactation, maternal use is now thought to be safe, based on at least 35 case reports,62 low MP levels in breast milk of mothers taking thiopurines and undetectable thiopurine metabolite levels in breast fed infants.63
Conclusion
In spite of more than 50 years of use, the understanding of how these drugs work is still growing. Measurement of TPMT status, and metabolite monitoring, will allow more rational dosing and ensure optimal use of these drugs before switching to alternatives. Well organised monitoring of blood counts is vital for safety. Once in remission, it is best to individualise decisions on duration of therapy. On the one hand, it is important to emphasise to the patient the benefits (undoubtedly measurable even after many years of therapy but with a diminishing underlying risk of flare with each successive year of prolonged remission). Patients should however be aware of the hazards—including the risk of bone marrow suppression (particularly if compliance with blood monitoring becomes haphazard)—and the potential for lymphoma.
Footnotes
Competing interest: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Prefontaine E, MacDonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2000;3:CD000545. [DOI] [PubMed] [Google Scholar]
- 2.Prefontaine E, Sutherland LR, Macdonald JK, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2009;1:CD000067. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Deltenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn's disease: a meta-analysis. Am J Gastroenterol 2009;104:2089–96. [DOI] [PubMed] [Google Scholar]
- 4.Ramadas AV GS, Thomas G, Williams GT, et al. Reduced Crohn's resections with increasing immunosuppressant use in a population-based Crohn's disease cohort in Cardiff (1986-2005). Gut 2009;58(Suppl II):A26. [Google Scholar]
- 5.Travis SP, Stange EF, Lémann M, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut 2006;55(Suppl 1):i16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmer A, McDonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2007;1:CD000478. [DOI] [PubMed] [Google Scholar]
- 7.Schwab M, Klotz U. Pharmacokinetic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet 2001;40:723–51. [DOI] [PubMed] [Google Scholar]
- 8.Tremaine WJ. Refractory IBD: medical management. Neth J Med 1997;50:S12–14. [DOI] [PubMed] [Google Scholar]
- 9.Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol 1992;43:329–39. [DOI] [PubMed] [Google Scholar]
- 10.Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine methyltransferase. Drug Metab Dispos 2001;29:601–5. [PubMed] [Google Scholar]
- 11.Gardiner SJ, Gearry RB, Begg EJ, et al. Thiopurine dose in intermediate and normal metabolizers of thiopurine methyltransferase may differ three-fold. Clin Gastroenterol Hepatol 2008;6:654–60. [DOI] [PubMed] [Google Scholar]
- 12.Ansari A, Arenas M, Greenfield SM, et al. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2008;28:973–83. [DOI] [PubMed] [Google Scholar]
- 13.Dubinsky MC, Reyes E, Ofman J, et al. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol 2005;100:2239–47. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson J, Ansari A, Marinaki T, et al. Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem 2004;41:294–302. [DOI] [PubMed] [Google Scholar]
- 15.Winter J, Walker A, Shapiro D, et al. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2004;20:593–9. [DOI] [PubMed] [Google Scholar]
- 16.Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000;118:1025–30. [DOI] [PubMed] [Google Scholar]
- 17.Kamper AM, Malbrain M, Zachee P, et al. Parvovirus infection causing red cell aplasia and leukopenia in rheumatoid arthritis. Clin Rheumatol 1994;13:129–31. [DOI] [PubMed] [Google Scholar]
- 18.Veraldi S, Rizzitelli G, Lunghi G, et al. Primary infection by human parvovirus B19. Dermatology (Basel) 1993;186:72–4. [DOI] [PubMed] [Google Scholar]
- 19.Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med 1995;123:132–42. [DOI] [PubMed] [Google Scholar]
- 20.Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut 2001;48:642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubinsky MC, Yang H, Hassard PV, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 2002;122:904–15. [DOI] [PubMed] [Google Scholar]
- 22.Barbe L, Marteau P, Lémann M, et al. Dose raising of azathioprine beyond 2.5 mg/kg/day in Crohn's disease patients who fail to improve with a standard dose. Gastroenterology 1998;114(Suppl 1):A925. [Google Scholar]
- 23.Rayner CK, Hart AL, Hayward CM, et al. Azathioprine dose escalation in inflammatory bowel disease. Aliment Pharmacol Ther 2004;20:65–71. [DOI] [PubMed] [Google Scholar]
- 24.Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980;302:981–7. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterology 1999;117:527–35. [DOI] [PubMed] [Google Scholar]
- 26.Lémann M, Mary JY, Colombel JF, et al. A randomized, double-blind, controlled withdrawal trial in Crohn's disease patients in long-term remission on azathioprine. Gastroenterology 2005;128:1812–18. [DOI] [PubMed] [Google Scholar]
- 27.Kim PS, Zlatanic J, Korelitz BI, et al. Optimum duration of treatment with 6-mercaptopurine for Crohn's disease. Am J Gastroenterol 1999;94:3254–7. [DOI] [PubMed] [Google Scholar]
- 28.Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002;50:485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein ES, Marion JF, Wheeler S, et al. Toxicities and infections associated with chronic 6-mercaptopurine (6-MP) use in Crohn's disease (CD): do we need to discontinue treatment? Gastroenterology 1998;114(Suppl 1):A986–86. [Google Scholar]
- 31.British Medical Association and Royal Pharmaceutical Society of Great Britain. British national formulary. 57th edn London: Pharmaceutical Press, 2009. [Google Scholar]
- 32.Colonna T, Korelitz BI. The role of leukopenia in the 6-mercaptopurine-induced remission of refractory Crohn's disease. Am J Gastroenterol 1994;89:362–6. [PubMed] [Google Scholar]
- 33.Campbell S, Ghosh S. Is neutropenia required for effective maintenance of remission during azathioprine therapy in inflammatory bowel disease? Eur J Gastroenterol Hepatol 2001;13:1073–6. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein GR, Abreu MT, Cohen R, et al. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130:940–87. [DOI] [PubMed] [Google Scholar]
- 35.Lees CW, Maan AK, Hansoti B, et al. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther 2008;27:220–7. [DOI] [PubMed] [Google Scholar]
- 36.Hindorf U, Johansson M, Eriksson A, et al. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009;29:654–61. [DOI] [PubMed] [Google Scholar]
- 37.Dubinsky MC, Vasiliauskas EA, Singh H, et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology 2003;125:298–303. [DOI] [PubMed] [Google Scholar]
- 38.Ferlitsch A, Teml A, Reinisch W, et al. 6-thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol 2007;102:2495–503. [DOI] [PubMed] [Google Scholar]
- 39.Vernier-Massouille G, Cosnes J, Lemann M, et al. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut 2007;56:1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aberra FN, Lewis JD, Hass D, et al. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003;125:320–7. [DOI] [PubMed] [Google Scholar]
- 41.Rahier JF, Yazdanpanah Y, Colombel JF, et al. The European (ECCO) Consensus on infection in IBD: what does it change for the clinician? Gut 2009;58:1313–15. [DOI] [PubMed] [Google Scholar]
- 42.International Agency for Research on Cancer (IARC) – Summaries and evaluations. Azathioprine. IARC monographs 1987;26(Suppl 7):119. [Google Scholar]
- 43.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 2008;8:24–36. [DOI] [PubMed] [Google Scholar]
- 44.Beaugerie L, Seksik P, Bouvier A-M, et al. Thiopurine therapy is associated with a three-fold decrease in the incidence of advanced colorectal neoplasia in IBD patients with longstanding extensive colitis: results from the CESAME cohort. Gastroenterology 2009;136(Suppl 1):A54. [Google Scholar]
- 45.Connell WR, Kamm MA, Dickson M, et al. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet 1994;343:1249–52. [DOI] [PubMed] [Google Scholar]
- 46.Masunaga Y, Ohno K, Ogawa R, et al. Meta-analysis of risk of malignancy with immunosuppressive drugs in inflammatory bowel disease. Ann Pharmacother 2007;41:21–8. [DOI] [PubMed] [Google Scholar]
- 47.Aithal GP, Mansfield JC. Review article: the risk of lymphoma associated with inflammatory bowel disease and immunosuppressive treatment. Aliment Pharmacol Ther 2001;15:1101–8. [DOI] [PubMed] [Google Scholar]
- 48.Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut 2005;54:617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–25. [DOI] [PubMed] [Google Scholar]
- 50.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol 2006;4:621–30. [DOI] [PubMed] [Google Scholar]
- 51.Rosh JR, Gross T, Mamula P, et al. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn's disease: a cautionary tale? Inflamm Bowel Dis 2007;13:1024–30. [DOI] [PubMed] [Google Scholar]
- 52.Siegel CA, Hur C, Korzenik JR, et al. Risks and benefits of infliximab for the treatment of Crohn's disease. Clin Gastroenterol Hepatol 2006;4:1017–24. [DOI] [PubMed] [Google Scholar]
- 53.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma. Gastroenterology 2000;118:1018–24. [DOI] [PubMed] [Google Scholar]
- 54.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54:1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roblin X, Serre-Debeauvais F, Phelip JM, et al. Drug interaction between infliximab and azathioprine in patients with Crohn's disease. Aliment Pharmacol Ther 2003;18:917–25. [DOI] [PubMed] [Google Scholar]
- 56.Dewit O, Vanheuverzwyn R, Desager JP, et al. Interaction between azathioprine and aminosalicylates: an in vivo study in patients with Crohn's disease. Aliment Pharmacol Ther 2002;16:79–85. [DOI] [PubMed] [Google Scholar]
- 57.Hande S, Wilson-Rich N, Bousvaros A, et al. 5-aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm Bowel Dis 2006;12:251–7. [DOI] [PubMed] [Google Scholar]
- 58.Giverhaug T, Loennechen T, Aarbakke J. The interaction of 6-mercaptopurine (6-MP) and methotrexate (MTX). Gen Pharmacol 1999;33:341–6. [DOI] [PubMed] [Google Scholar]
- 59.Langagergaard V, Pedersen L, Gislum M, et al. Birth outcome in women treated with azathioprine or mercaptopurine during pregnancy: a Danish nationwide cohort study. Aliment Pharmacol Ther 2007;25:73–81. [DOI] [PubMed] [Google Scholar]
- 60.Dejaco C, Angelberger S, Waldhoer T, et al. Pregnancy and birth outcome under thiopurine therapy for inflammatory bowel disease. Gastroenterology 2005;128(Suppl 2):A12. [Google Scholar]
- 61.Francella A, Dyan A, Bodian C, et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology 2003;124:9–17. [DOI] [PubMed] [Google Scholar]
- 62.Gardiner SJ, Begg EJ, Sau A, et al. Thiopurine treatment in inflammatory bowel disease. Clin Pharmacokinet 2007;46:803–4. [DOI] [PubMed] [Google Scholar]
- 63.Christensen LA, Dahlerup JF, Nielsen MJ, et al. Azathioprine treatment during lactation. Aliment Pharmacol Ther 2008;28:1209–13. [DOI] [PubMed] [Google Scholar]
- 64.Osterman MT, Kundu R, Lichtenstein GR, et al. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006;130:1047–53. [DOI] [PubMed] [Google Scholar]
- 65.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000;118:705–13. [DOI] [PubMed] [Google Scholar]
- 66.Shih DQ, Nguyen M, Ibañez P, et al. W1206 split-dose administration of 6-mercaptopurine/azathioprine: a effective novel strategy for ibd patients with preferential 6mmp metabolism. Gastroenterology 2009;136(Suppl 1):A–677–78. [Google Scholar]
- 67.Sparrow MP, Hande SA, Friedman S, et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther 2005;22:441–6. [DOI] [PubMed] [Google Scholar]
- 68.Sparrow MP, Hande SA, Friedman S, et al. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol 2007;5:209–14. [DOI] [PubMed] [Google Scholar]