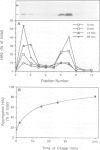
Abstract
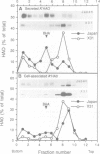
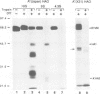
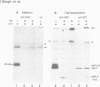
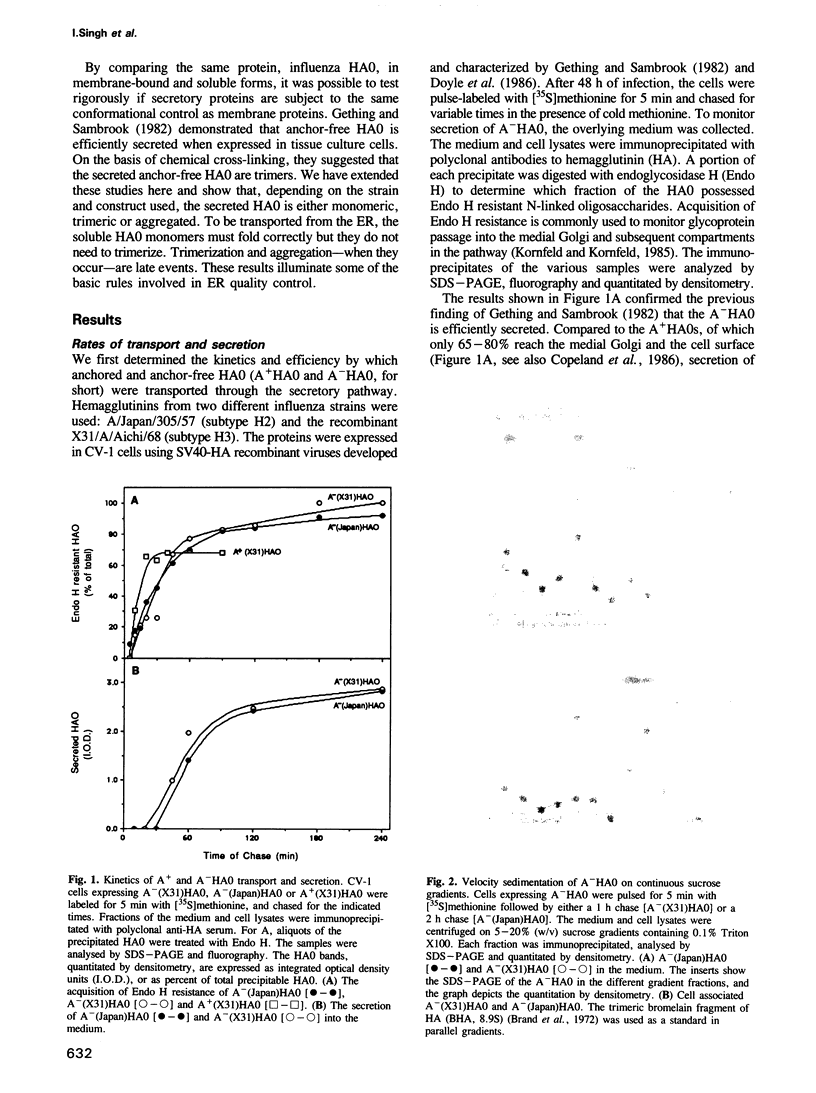
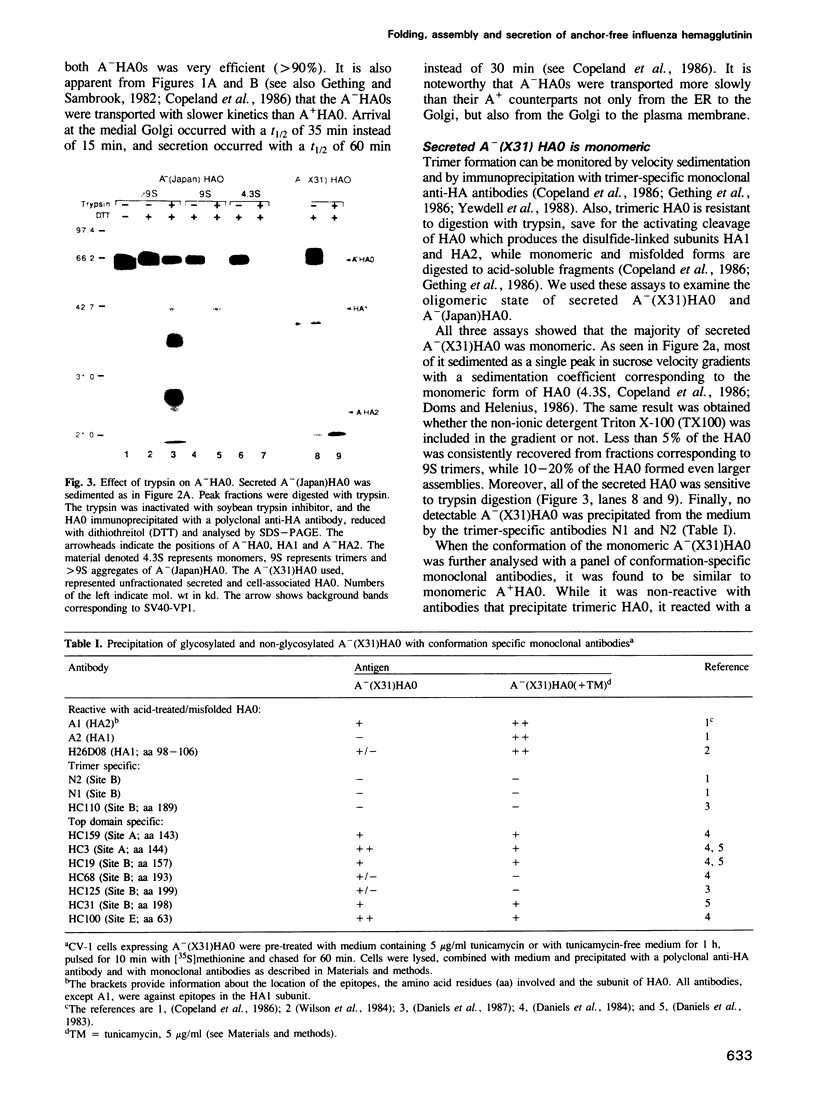
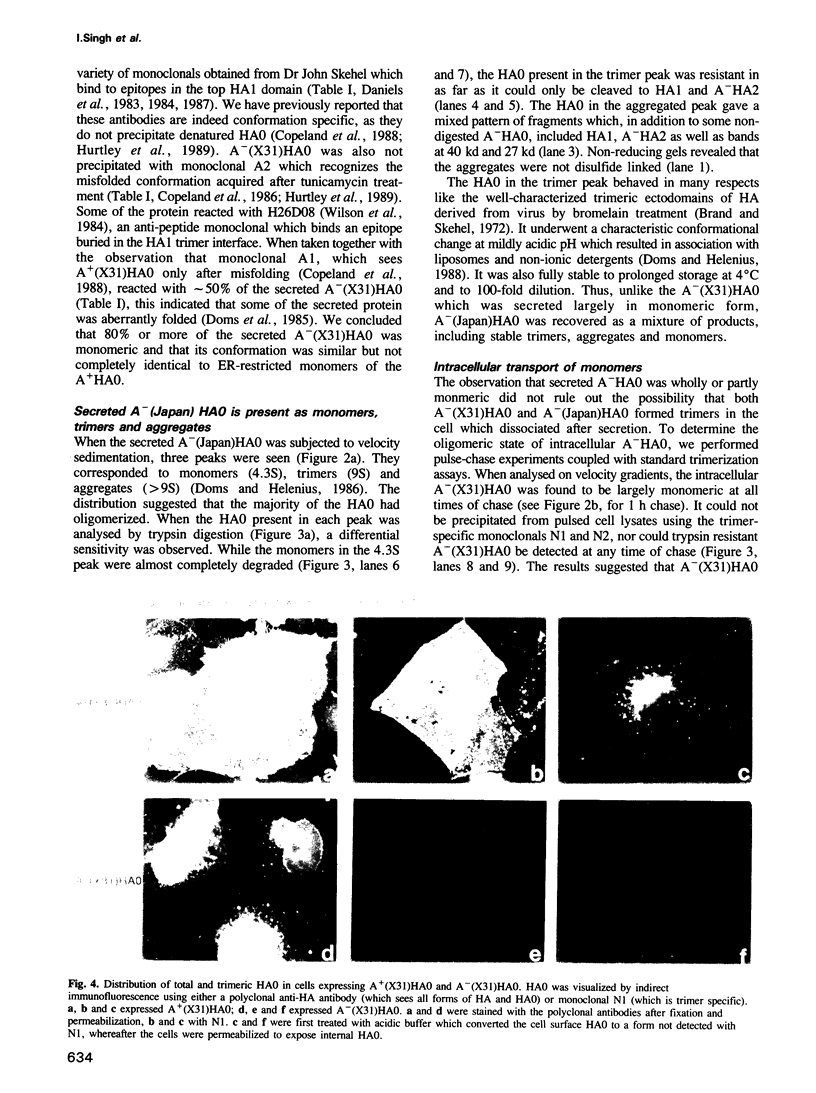
The influenza hemagglutinin precursor (HA0) and many other glycoproteins fold and oligomerize in the endoplasmic reticulum (ER). Only correctly folded oligomers are transported to the cell surface. To analyse the rules which determine this type of ER sorting, we have extended our analysis of hemagglutinin transport to two soluble, anchor-free recombinant HA0s derived from X31/A/Aichi/68 and A/Japan/305/57 influenza A. The results showed that individual monomers rapidly acquired a folded structure similar to that of monomeric membrane-anchored HA0. They were efficiently transported and secreted, but oligomerization was not required for secretion. Trimers or higher order complexes were either not formed (X31 HA0), or appeared during passage through the late compartments of the secretory pathway, with no effect on the rate of transport (Japan HA0). However, when initial folding was disturbed by inhibition of N-linked glycosylation, anchor-free X31 HA0 was misfolded and retained in the ER as disulfide-linked complexes associated with binding protein, BiP (GRP78). The complexes were similar to those seen for the nonglycosylated membrane-bound HA0, but instead of forming immediately after synthesis they appeared with a half-time of 6 min. Taken together, the data demonstrate that the structural criteria that makes the anchor-free HA0 transport competent are less stringent than those for the membrane form; they must fold correctly but do not need to oligomerize.
Full text
PDF



Images in this article
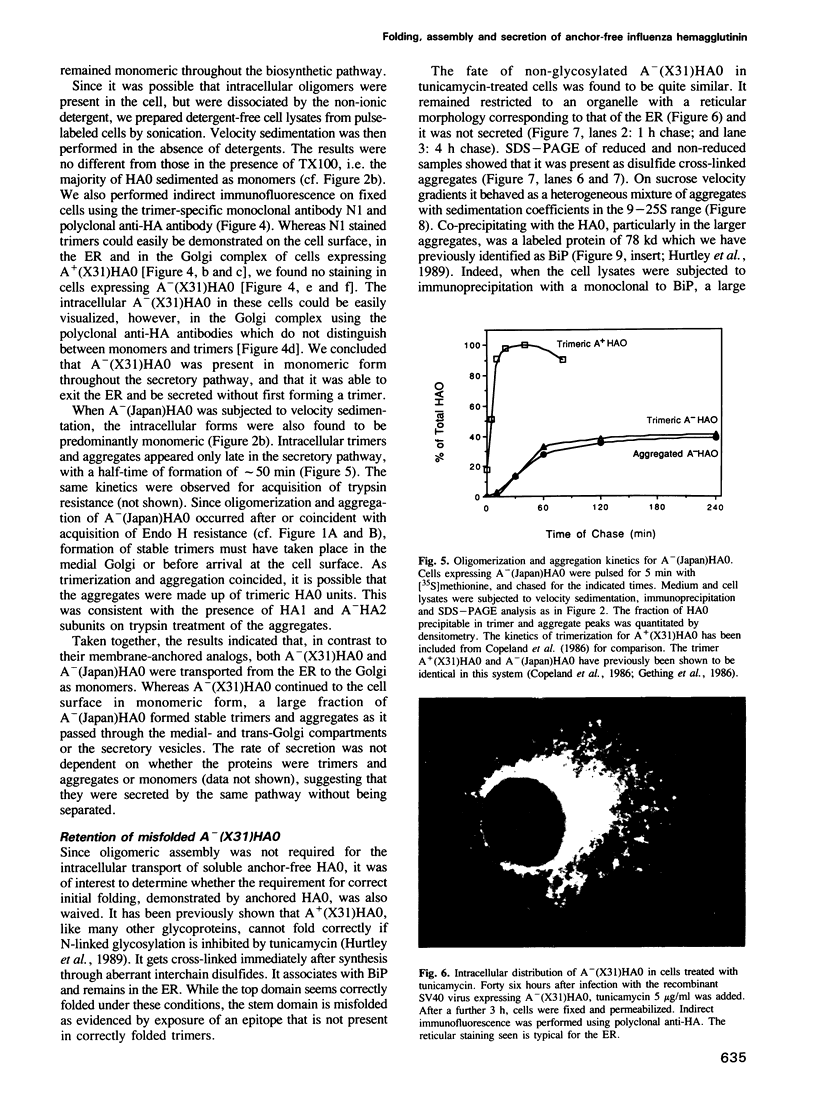
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Daniels P. S., Jeffries S., Yates P., Schild G. C., Rogers G. N., Paulson J. C., Wharton S. A., Douglas A. R., Skehel J. J., Wiley D. C. The receptor-binding and membrane-fusion properties of influenza virus variants selected using anti-haemagglutinin monoclonal antibodies. EMBO J. 1987 May;6(5):1459–1465. doi: 10.1002/j.1460-2075.1987.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983 Aug;64(Pt 8):1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C., Naeve C. W., Webster R. G., Rogers G. N., Paulson J. C. Antigenic analyses of influenza virus haemagglutinins with different receptor-binding specificities. Virology. 1984 Oct 15;138(1):174–177. doi: 10.1016/0042-6822(84)90158-2. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Gething M. J., Henneberry J., White J., Helenius A. Variant influenza virus hemagglutinin that induces fusion at elevated pH. J Virol. 1986 Feb;57(2):603–613. doi: 10.1128/jvi.57.2.603-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Doms R. W., Keller D. S., Helenius A., Balch W. E. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987 Nov;105(5):1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. J., Sambrook J., Helenius A., White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985 Jul;101(1):19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Sambrook J., Gething M. J. Analysis of progressive deletions of the transmembrane and cytoplasmic domains of influenza hemagglutinin. J Cell Biol. 1986 Oct;103(4):1193–1204. doi: 10.1083/jcb.103.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding and intracellular transport: studies on influenza virus haemagglutinin. Biochem Soc Symp. 1989;55:155–166. [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- PALADE G. E. Intracisternal granules in the exocrine cells of the pancreas. J Biophys Biochem Cytol. 1956 Jul 25;2(4):417–422. doi: 10.1083/jcb.2.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens J. E., Roth M. G., Matlin K. S. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989 Mar;108(3):821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tooze J., Kern H. F., Fuller S. D., Howell K. E. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989 Jul;109(1):35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Yellen A., Bächi T. Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell. 1988 Mar 25;52(6):843–852. doi: 10.1016/0092-8674(88)90426-6. [DOI] [PubMed] [Google Scholar]