Abstract
Safe cost effective nutritional support is provided by a multidisciplinary team whose activity is overseen by a Nutrition Steering Group that reports directly to the Hospital Trust Board. When a nutrition support team (NST) is first formed, a nutrition nurse specialist enables parenteral nutrition to be given safely. An NST needs to have a clearly agreed scope of practice and needs to be able to justify its presence in terms of quality and cost savings.
Introduction
Patients who are malnourished or who are at risk of becoming malnourished are often poorly managed due to inadequate nutritional assessment1–3 and poor medical and nursing knowledge about artificial nutritional support (ANS).4–6 As a result, nutritional support may be given inappropriately with a high incidence of complications. ANS involves giving a liquid feed into the gut (enteral nutrition (EN)) or into a vein and so bypassing the gut (parenteral nutrition (PN)). EN is given to patients who cannot safely take food (oral failure). PN is given to patients whose gut cannot absorb adequate nutrients because their gut has been removed, does not function, is blocked or is perforated (intestinal failure). ANS may be needed by all specialities although EN is commonly needed for patients who have neurological problems (eg, stroke, motor neuron disease, multiple sclerosis, cerebral palsy or rarely dementia), head and neck cancer and trauma. PN is most often given perioperatively, to patients with a short bowel, enterocutaneous fistula or obstruction, patients undergoing chemotherapy or those who have been unable to improve their nutritional status with EN. There are many more patients needing EN than PN both in hospital and in the community. In 2007, in the UK, there were 365/million population EN and 13/million population PN patients at home.7 If healthcare staff are not skilled and experienced in giving ANS, serious life threatening complications can occur; with EN aspiration pneumonia and local gastrostomy problems8 9; with PN catheter related sepsis (CRS) and central vein thrombosis.
Many disciplines with different, but essential skills (summarised in table 1), are involved in the safe administration of ANS and these staff need to regularly communicate with each other to give coordinated planned care. This is most commonly and effectively done by combining the specialist skills of different disciplines into one nutrition support team (NST) in which the members all work closely together. The composition of an NST is variable in different hospitals but likely to consist of at least a clinician, nutrition nurse specialist (NNS) and dietitian for ward rounds. Gastroenterologists have largely taken on the role of leading an NST because they can achieve access to the gut via endoscopic techniques and they manage patients with intestinal problems. However, some teams are run effectively by a non-gastroenterologist such as a general surgeon, chemical pathologist, anaesthetist or endocrinologist. The core member of an NST that allows safe PN is an NNS and this was clearly demonstrated in the study from the Central Middlesex Hospital10 (table 2) and confirmed in other studies.11 The NNS achieves the administration of safe PN with a low rate of CRS partly by personally caring for the central feeding catheters (particularly when the team is first formed) and later by teaching others to put up and take down a PN bag (certainly necessary when there are many patients having PN and at weekends). The techniques used to do this involve strict asepsis and are different to the clean techniques used by other disciplines to care for Hickman-type lines. Once PN is performed safely, attention can be directed more to the nutritional/fluid requirements with the dietetic input and to the composition/compounding of parenteral feeding bags with a pharmacist. Thus the traditional NST core membership consists of a clinician, NNS, dietitian and pharmacist12 13 although other specialists may be involved (eg, chemical pathologist, microbiologist, radiologist) (table 1). A NST improves the quality of patient care largely though education that improves nutritional assessment, appropriate nutrient delivery and reduces mechanical, infective and metabolic complications.14–25 The rate of CRS should fall to less than 1/100 catheter days after an NST had been formed.11 26
Table 1.
Members of a nutrition support team36
| Essential member | Roles |
|---|---|
| Clinician | Overall responsibility, coordinated care, may insert enteral and intravenous feeding tubes. Liaises with the patient's primary team. Understands the underlying disease(s) and prognosis. Prescribes the parenteral feeding solution. |
| Nutrition nurse specialist | Teaches and supervises care of tubes and catheters and recognises and manages complications. Places or assists in placement of enteral and parenteral feeding catheters. Acts as the patient's advocate, who also trains patients/carers to manage at home. |
| Dietitian | Nutritional assessment, calculates requirements, designs feeding regimen and monitors nutritional and fluid status. |
| Pharmacist | Responsible for providing enteral feeds and sterile parenteral nutrition solutions (may include compounding). Optimises composition and advises on compatibility/stability issues and drug/nutrient interactions. |
| Other staff in the extended NST and certainly needed for HIFNET sector 3 or 4 centres29 37 38 | |
| Stoma care/tissue viability nurses for stoma/wound management | |
| Surgeon in cases of intestinal failure | |
| Social worker | |
| Physiotherapist | |
| Occupational therapist | |
| Psychiatrist/psychologist | |
| Interventional radiologist to insert some enteral feeding tubes and to insert difficult lines for parenteral nutrition. | |
| Microbiologist to advise on the treatment of and abdominal sepsis in those with intestinal failure. | |
| Speech and language therapist for patients with difficulty swallowing | |
| Other specialists as necessary (eg, haematologist, urologist, gynaecologist, etc) | |
| Community workers (eg, dieticians and nurses) | |
The roles may overlap and all are involved in monitoring progress.
All core personnel need cover for all roles in case time away (courses, holiday sickness etc).
HIFNET, home parenteral nutrition and intestinal failure network; NST, nutrition support team.
Table 2.
Catheter related sepsis and the effect of a nutrition nurse specialist who was employed in the middle of a study looking at sepsis and tunnelled versus untunnelled lines8
| Before nurse | After nurse | |||
|---|---|---|---|---|
| N | CRS (%) | N | CRS (%) | |
| Tunnelled | 26 | 6 (23) | 26 | 0 (0) |
| Untunnelled | 25 | 11 (44) | 22 | 2 (10) |
The dramatic improvement in CRS is obvious after her training and policies were implemented.
CRS, catheter related sepsis.
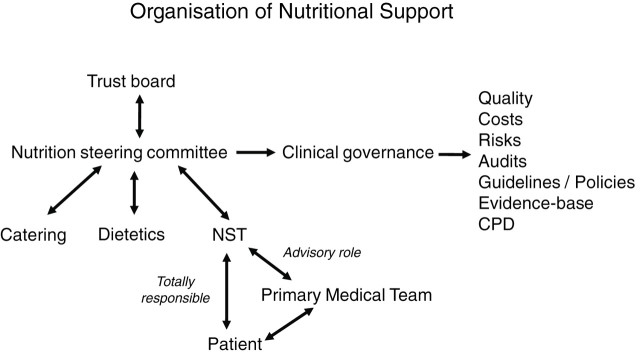
The UK National Institute for Health and Clinical Excellence (NICE) guidelines recommend that nutritional support is provided in all hospitals by a multidisciplinary NST.27 It also recommends that all acute hospital Trusts employ at least one NNS.28 Although this is recommended only about 60% of hospitals in the UK have an NST. The UK NICE guidelines also recommend that all hospital Trusts have a nutrition steering committee (NSC) to coordinate the nutritional care within a hospital and in addition to the NST this includes catering and dietetic services (figure 1). The difficulty at local level lies in justifying the expense of having a team in terms of cost and quality benefits. This paper discusses a well functioning multidisciplinary coordinated NST and shows how it can be set up, run and its continuation justified. The information is directed mainly at an adult NST but the same principles apply for one in paediatrics.29–31
Figure 1.
Organisation of nutrition support. NST, nutrition support team.
For malnutrition, unlike obesity, in England there is no National Service Framework, it is not currently a National Quality Board key priority, it is not a specific core standard inspected by the Care Quality Commission as part of its annual health check and nutritional screening is not part of the General Practice Quality Outcome Framework. However, the profile of malnutrition as common and needing treatment is increasing. There is pressure on a Trust to show that it is implementing the NICE recommendations. ‘Meeting nutritional needs’ with ‘sufficient food and drink and a choice of food and drink to meet your diverse needs’ is one of the 16 principle essential standards of quality and safety that are being implemented from the Health and Social Care Act 2008 and Trusts are expected to adhere to these. Another source of pressure comes from the annual Patient Environment Action Teams assessment which in addition to questions about food and drink includes questions about nutritional screening and asks about the presence of a Trust Nutritional Steering Group.32
Setting up an NST
If a Trust does not have an NST, it may still be necessary to justify the benefits of having one both in terms of quality improvement and cost savings. While quality of care should be the main reason for setting up an NST, cost savings are more influential. The process of setting up a team is slow and involves changing the culture of an organisation to one that realises the importance of such a team and questions why it does not have one. This process involves making many presentations to management and healthcare workers. This can be done simply using three questions; where are we now, where do we want to be and how do we get there? In addition a strengths, weaknesses, opportunities and threats analysis (SWOT analysis) may be used. In making a business case, alternatives need to be proposed and these include doing nothing, having a fully functioning salaried team with backup or inbetween situations (eg, employing a nutrition support nurse only).
There are two types of financial cost savings to calculate: tangible and intangible. Tangible cost savings include all equipment, investigations and medication costs but exclude nursing, medical and laboratory time and bed occupancy costs. Intangible costs include staff time and bed occupancy costs. Some hospital Trusts will say that intangible costs will be incurred anyway as a bed is occupied and so should not be taken into account.11 Whatever the view of the management, it is wise to present both the tangible and intangible savings. In the USA, retrospective or prospective observational studies have shown an NST to reduce intangible costs.24 33–35 One UK study has shown a saving in both tangible and intangible costs. The intangible costs were mainly from avoided PN episodes and reduced CRS such but they were still great enough to finance a full time NNS and part time dietitian.11 Additional cost savings can be made by an NST, reducing the number of percutaneous endoscopic gastrostomy (PEG) tubes that are inserted.36
Where do we want to be? A fully functioning NST able to perform as a sector 1 centre
The overall aim of a multidisciplinary NST is to provide safe up to date appropriate nutritional support to an individual patient, who is malnourished or at risk of malnutrition, in a coordinated fashion. In many of the patients the care also will involve treating over hydration (usually due to excess intravenous saline) and dehydration (often due to a high output stoma or fistula). The practicalities of an NST, day to day working and key operational issues for a successful NST are outlined below. Most centres in England will be expected to fall into the sector 1 category as specified by the home PN and intestinal failure network so are able to provide safe PN in hospital. Some will also manage patients on home PN (sector 2). Selected well established centres may be able to manage patients with more complex intestinal failure (sectors 3 and 4).37 38
Staff and allocated time
A multidisciplinary NST is led by a clinician with a special interest in nutritional support (often a gastroenterologist)12 39 40 and having at least two sessions dedicated to nutritional support. There must be a full time NNS to ensure safe PN with a very low rate of CRS. A senior dietitian and pharmacist complete the traditional core NST. The dietitian needs to be employed full time or occasionally when the team is first formed as part time. There needs to be a second NNS identified to cover away time (eg, study leave, holiday and sickness).
Operation
The team needs to be clear about how it will operate,15 including the routes by which patients are referred to the team. The NST can be ‘totally responsible (complete autonomy)’ in which the team assesses the nutritional and fluid requirements of the patient, established the access for feeding, writes the prescription, monitors progress and manages any complications. It is easier if the team is also responsible for the medical management of the patient but this is not always practical. An alternative way of working is to be ‘consultative (or supervisory)’ and to see patients and advise on their management. This is more difficult and less efficient as it relies on good communication with the primary medical team who need to act upon the advice offered. Often a team will work in a totally responsible way on some wards (eg, medical gastrointestinal and surgical wards) and in a consultative capacity on others (eg, intensive therapy unit, haematology and oncology). It would be impractical for an NST to be totally responsible for the medical management of all patients needing ANS and the team needs to be clear for which patients it wants to be totally responsible (eg, just those requiring PN).
Types of patient—scope
The team needs to be clear about which type of patients it will see. Initially this is likely to be all patients being considered for PN. After it has been functioning for several months it may take on some EN—for example, patients considered for postpyloric feeding (nasojejunal (NJ) tubes). Later it may see all patients considered for PEGs and indeed those considered for any form of enteral feeding (nasogastric feeding, radiologically inserted gastrostomy/jejunostomy and patients needing or with a needle jejunostomy, etc). The team needs to be careful about committing to managing patients needing all forms of ANS as the number of patients can rapidly become large, and the ethics of PEG placement can be very difficult and time consuming. The team needs initially to be seen as successful in a defined area and this may not be the case if overstretched. Most teams deal with adults or children and it is uncommon to manage both.
Ward rounds
The team needs to consider how often it will do ward rounds and who will be present on these. During the working week this may be twice a week with the consultant and on other days by the NNS, dietitian and ideally a pharmacist. At least one member of an NST must see all patients needing PN daily.
Wards visited
The team must consider which wards it will routinely visit. A presence on a ward is vital and makes staff aware of its presence and will encourage referrals. The route of the ward round may include starting on general surgical wards going to the intensive therapy unit, oncology, haematology and other selected wards depending on the hospital's case mix. It may finish on the home gastroenterology ward. Consideration of where patients are managed, especially for PN, needs to be made. It is easiest and safest to manage patients needing PN on one dedicated ward (often the home gastroenterology ward) although this can be difficult as patients move across the directorates and cross charging may be necessary.
Procedures
The team must decide which procedures it will perform itself. Some teams insert all central parenteral lines themselves with or without ultrasound and/or x-ray guidance. Others may insert the peripherally inserted central catheters themselves and ask radiologists, surgeons or anaesthetists to do other central lines. There is no evidence that the place a central feeding line is inserted relates to the subsequent occurrence of CRS, providing the insertion is performed using an aseptic technique. Most enteral tubes will be inserted by the gastroenterologists (NJ, PEG, PEG with a jejunal extension or percutaneous endoscopic jejunostomy) who may need to be able to move equipment to the bedside (eg, for an NJ tube on the intensive therapy unit). The profile of an NST will increase if it can rapidly (within 24 h) insert an enteral tube or parenteral feeding catheter.
Budget
The team should have a budget for lines and enteral feeding tubes (usually from the medical directorate). It may need an arrangement for cross charging other directorates if it supplies the catheters/tubes to patients from other directorates.
Office and clinical space
It helps communication if the team members do their administration work in the same place. This includes patient phone calls for those having ANS at home and checking blood and microbiology results. The team will need secretarial support and computer facilities.
A clinical room which has the facilities for aseptic procedures to be performed is needed. In this room some parenteral lines (eg, peripherally inserted central catheters) can be inserted and patients from home can be seen if they have problems with their enteral or parenteral feeding catheters. In addition, some drugs may be given there (eg, biphosphonate, iron or vitamin infusions or injections). The room needs facilities for hand washing.
Clinics
A weekly clinic in which to see patients needing ANS is needed and ideally the patients should see the multidisciplinary NST altogether. Initially it may be at the end or beginning of an established clinic until the number of patients justifies a complete dedicated clinic.
Coordination of care between hospital and community
The team needs to establish good links with the local community staff who will care for patients needing enteral feeding. There needs to be clear information/guidelines about the care and contact details if there are problems. When this works well patients will be discharged home more quickly and emergency admissions can be prevented. PN is more complex to set up at home and often a company will be contracted by a Primary Care Trust to set up and take down a PN bag and they may also teach the patient to do the aseptic procedures. While it is often a good idea to train the district nurses to do the procedures, they are only likely to have one patient needing PN and it can be difficult to train enough district nurses to be able to reliably cover every day.
Meetings and audit
The NST may meet weekly to discuss its operation, selected patients, data collection/audits and journals. The meetings may include other specialists (stoma care, tissue viability, radiologist, microbiologist, surgeon(s), occupational therapist, speech and language therapist, physiotherapist, etc). The continuation of the NST will at first will depend on it keeping good audit data. As a minimum, it must record details of all patients seen and data about every episode of CRS and every other complication of PN.41 It should present infection data per 100 catheter days (at home it is presented per 1000 days); percentage of infections is not such a good indicator as it gives no indication of the duration of the feeding before an infection occurred.
Participation in the NSC and other committees
Members of the NST will be members of and will report to the NSC which oversees their actions and reports directly to the Trust Board (see figure 1). Some members of the NST may become involved in hospital catering.
Education, policies and guidelines
The NST is likely to undertake a large educational role both teaching (undergraduates and postgraduates in medicine and nursing, all healthcare workers and many carers/patients) and writing guidelines/policies and setting standards. These are likely to be about nutritional assessment, and indications and management of EN and PN. The NNS may teach and establish link nurses on the relevant wards.
Accountability
Each member of the NST is accountable operationally to the consultant leading the NST and professionally to the head of their specialty.
Where are we now?
In a hospital with no NST, there is likely to be a problem of unrecognised malnutrition which may be poorly treated with high complication rates and high rates of feeding bags being wasted. There may be no or few policies/guidelines and care is likely to be fragmented and uncoordinated. To demonstrate the poor current situation background local data needs to be collected; this may include looking at the prevalence of inpatients who are undernourished or at risk of becoming undernourished and determining how many of these are unrecognised2 and the staff knowledge of undernutrition and its treatment.4 The rate of CRS, waiting time for venous access for PN, waiting time for NJ or PEG tube placement and the number of wasted parenteral feeding bags is relatively easy to obtain (table 3). The number of PEGs inserted and their appropriateness may also be audited.
Table 3.
Measurable cost benefits of a nutrition support team
| PN |
| Less inappropriate PN (avoided PN episodes)* |
| Less catheter related sepsis (and central vein thrombosis) |
| Less blood testing |
| Fewer PN bags wasted |
| EN |
| Less PEGs inserted30 |
| Faster rate of insertion and thus faster patient discharge |
But may in practice be cancelled out by more appropriate PN given for longer and more jejunal feeding tubes being inserted.
EN, enteral nutrition; PEGs, percutaneous endoscopic gastrostomy; PN, parenteral nutrition.
How do we get there?
The first step is forming a multidisciplinary nutritional support group, all of whom have a clear vision of what is needed in the future. It helps if these people have worked previously as part of an NST. If few people have worked or seen an NST working then selected individuals can be asked to visit a recognised unit with an NST to observe working practice. To carry weight this group needs to consist of senior members of the professions (eg, head of nursing and dietetics, the medical director, head of pharmacy and the potential lead clinician). They need to plan a strategy that will change the culture of the organisation. In the past this was slow but with nutritional support attracting a higher profile and having the backing of NICE guidelines it may be faster.
The key people in the Trust (chief executive and all stakeholders/users of the service) need to be made aware of the current situation and this may involve making presentations using members from each specialty. In general the presentations should demonstrate that there is a problem and a solution for the Trust. The presentations need to be relevant to the Trust and this is where local data are so helpful. For example, if the Trust specialises in oncology the benefit of nutritional support in the management of cancer can be stressed ideally with data about the size of the local problem and a description of what is currently happening. The steps to achieve the vision need to be clear with a timescale and measurable outcomes.
The presence of an NST in a neighbouring hospital can engender a positive competitive spirit, and it may be possible that having an NST will help a hospital achieve foundation status.
While efforts are being made to change the culture of the organisation and while these presentations are being made an NSC can be formed as recommended by NICE. The new NSC may consist of many of the people in the original multidisciplinary nutritional support group. The NSC can, as its first task, make a business case for the employment of an NNS (table 4). Then, if this is successful, move on to employing a dietitian, then a second NNS and a dedicated pharmacist. The team's reputation in the early days is crucial and a few episodes of bad practice or bad luck can severely damage its credibility. Planning how a team works and it standards and policies/guidelines can reduce the chance of bad luck. This is especially important for when one team member is away. Thus there must be plans for backup staff to cover holiday, study leave and sick leave.
Table 4.
Topics to include in making a business case for nutrition support team (especially nutrition nurse specialist)
| Quality benefits |
| Government/Trust strategy |
| Care Quality Commission—annual health check (complying with NICE) |
| Health and Social Care Act 2008 |
| National Confidential Enquiry into Patient Outcome and Death201028 |
| Patient Environment Action Teams assessment |
| Foundation status |
| Stakeholders (eg, surgery, oncology, haematology and critical care) |
| Options pros/cons, SWOT analysis. Do nothing—full team |
| Costings (tangible and intangible) |
| Time scale |
| Monitoring |
| Audits |
NICE, National Institute for Health and Clinical Excellence; SWOT, strengths, weaknesses, opportunities and threats.
Maintaining and continuation on an NST
The maintenance of an NST is done by having a presence such that the team is seen by all specialities as essential. It needs to see patients quickly and take responsibility for some procedures and for prescribing the feed and monitoring its outcome. Its role in education of healthcare workers, carers and patients will become vital. It will have a major contribution via the NSC into all of the Trust's policy on nutritional issues, especially nutritional support. The NST should always be able to produce data about the patients it has seen and the rates of CRS, avoided PEGs insertions and wasted PN bags. While hospitals are trying to reduce costs each NST must be able to demonstrate its quality and cost benefits at any time.
Summary
A multidisciplinary NST is an effective way of providing nutritional support to an individual patient. The two key steps in setting up an NST are to establish an NSC which reports directly to the Trust board and to appoint an NNS who can perform and teach the aseptic techniques to safely set up and take down a parenteral feeding bag. The NST needs to be seen to take responsibility for a patient's nutritional (and fluid) management. By keeping careful data it should be able to justify itself in terms of improved quality of care and cost savings. By providing high quality nutritional care the NST should quickly be regarded as essential to the Trust.
Footnotes
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308:945–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nightingale JM, Walsh N, Bullock ME, et al. Three simple methods of detecting malnutrition on medical wards. J R Soc Med 1996;89:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415–21. [DOI] [PubMed] [Google Scholar]
- 4.Nightingale JM, Reeves J. Knowledge about the assessment and management of undernutrition: a pilot questionnaire in a UK teaching hospital. Clin Nutr 1999;18:23–7. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval WM, Mueller HD. Nutrition education at the work site: a team approach. J Am Diet Assoc 1989;89:543–4. [PubMed] [Google Scholar]
- 6.Ward J, Close J, Little J, et al. Development of a screening tool for assessing risk of undernutrition in patients in the community. J Hum Nutr Diet 1998;11:323–30. [Google Scholar]
- 7.Jones B. ed. Annual BANS Report 2008: Artificial Nutrition Support in UK 2000–2007. http://www.bapen.org.uk/pdfs/bans_reports/bans_report_08.pdf (accessed 30 June 2010).
- 8.NPSA Alert. Early detection of complications after gastrostomy http://www.nrls.npsa.nhs.uk/alerts/?entryid45=73457 (accessed 30 June 2010).
- 9.NPSA Alert. Reducing harm caused by the misplacement of nasogastric feeding tubes. http://www.nrls.npsa.nhs.uk/alerts/?entryid45=59794&p=3 (accessed 30 June 2010).
- 10.Keohane PP, Jones BJ, Attrill H, et al. Effect of catheter tunnelling and a nutrition nurse on catheter sepsis during parenteral nutrition. A controlled trial. Lancet 1983;2:1388–90. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy JFR, Nightingale JMD. Cost savings of an adult hospital nutrition support Team Nutrition 2005;21:1127–33. [DOI] [PubMed] [Google Scholar]
- 12.Lennard-Jones JE. ed. A positive approach to nutrition as treatment. Report of a Working Group on the Role of Enteral and Parenteral Feeding in Hospital and at Home. London: Kings Fund Centre, 1992. [Google Scholar]
- 13.Powell-Tuck J. ed. Organisation of food and nutritional support in hospitals. Redditch: British Association for Parenteral and Enteral Nutrition, 2007. http://www.bapen.org.uk/ofnsh/OrganizationOfNutritionalSupportWithinHospitals.pdf (accessed 30 June 2010). [Google Scholar]
- 14.Nehme AE. Nutritional support of the hospitalised patient. JAMA 1980;243:1906–8. [PubMed] [Google Scholar]
- 15.Dalton MJ, Schepers G, Gee JP, et al. Consultative total parenteral nutrition teams: the effects on the incidence of total parenteral nutrition-related complications. J Parenter Enteral Nutr 1984;8:146–52. [DOI] [PubMed] [Google Scholar]
- 16.Traeger SM, Williams GB, Milliren G, et al. Total parenteral nutrition by a nutrition support team: improved quality of care. J Parenter Enteral Nutr 1986;10:408–12. [DOI] [PubMed] [Google Scholar]
- 17.Powers DA, Brown RO, Cowan GSM, et al. Nutritional support team vs nonteam management of enteral nutritional support in a veterans administration medical center teaching hospital. J Parenter Enteral Nutr 1986;10:635–8. [DOI] [PubMed] [Google Scholar]
- 18.Brown RO, Carlson SD, Cowan GSM, et al. Enteral nutritional support management in a university teaching hospital: team vs nonteam. J Parenter Enteral Nutr 1987;11:52–6. [DOI] [PubMed] [Google Scholar]
- 19.Fisher GG, Opper FH. An interdisciplinary nutrition support team improves quality of care in a teaching hospital. J Am Diet Assoc 1996:176–8. [DOI] [PubMed] [Google Scholar]
- 20.ChrisAnderson D, Heimburger DC, Morgan SL, et al. Metabolic complications of total parenteral nutrition: effects of a nutrition support service. J Parenter Enteral Nutr 1996;20:206–10. [DOI] [PubMed] [Google Scholar]
- 21.Png SJC, Chan S. Surgical nutritional team and its impact on total parenteral nutrition in the national university hospital, Singapore. Int J Clin Pract 1997;51:350–2. [PubMed] [Google Scholar]
- 22.Fettes SB, Lough M. An audit of the provision of parenteral nutrition in two acute hospitals: team versus non-team. Scot Med J 2000;45:121–5. [DOI] [PubMed] [Google Scholar]
- 23.Johansen N, Kondrup J, Plum LM, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr 2004;23:539–50. [DOI] [PubMed] [Google Scholar]
- 24.Faubion WC, Wesley JR, Khalidi N, et al. Total parenteral nutrition catheter sepsis: impact of the team approach. J Parenter Enteral Nutr 1986;10:642–5. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds N, McWhirter JP, Pennington CR. Nutrition support teams: an integral part of developing a gastroenterology service. Gut 1995;37:740–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mughal MM. Complications of intravenous feeding catheters. Br J Surg 1989;76:15–21. [DOI] [PubMed] [Google Scholar]
- 27.Stewart JAD, Mason DG, Smith N, et al. A mixed bag. An enquiry into the care of hospital patients receiving parenteral nutrition. A report by the National Confidential Enquiry into Patient Outcome Death 2010. http://www.ncepod.org.uk/2010report1/downloads/PN_report.pdf (accessed 30 June 2010).
- 28.Nutritional support in adults: oral supplements, enteral and parenteral feeding. National Collaborating Centre for Acute Care group producing NICE guidelines, 2006. www.nice.org.uk/nicemedia/live/10978/29981/29981.pdf (accessed 30 June 2010).
- 29.Puntis J, Beath S, Beattie M, et al. Intestinal failure: Recommendations for tertiary management of infants children. A Report by the Intestinal Failure Working Group, British Society of Paediatric Gastroenterology, Hepatology, and Nutrition, and the British Association of Paediatric Surgeons, 2007. http://www.bspghan.org.uk/working_groups/IFWGreportfinalMar2007.pdf (accessed 30 June 2010).
- 30.Agostoni C, Axelsson I, Colomb V, et al. ESPGHAN Committee on Nutrition. The need for nutrition support teams in pediatric units: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2005;41:8–11. [DOI] [PubMed] [Google Scholar]
- 31.Puntis JWL. Malnutrition in developed countries. Ann Nestlé [Engl] 2009;67:65–72. [Google Scholar]
- 32.National Patient Safety Agency. Patient Environment Action Teams (PEAT). 2009. http://www.nrls.npsa.nhs.uk/patient-safety-data/peat (accessed 30 June 2010).
- 33.O'Brien DD, Hodges RE, Day AT, et al. Recommendations of nutrition support team promote cost containment. J Parenter Enteral Nutr 1986:10:300–2. [DOI] [PubMed] [Google Scholar]
- 34.Tucker HN, Miguel SG. Cost containment through nutrition intervention. Nutr Rev 1996;54:111–21. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein M, Braitman LE, Levine GM. The medical and financial costs associated with termination of a nutrition support nurse. JPEN J Parenter Enteral Nutr 2000;24:323–7. [DOI] [PubMed] [Google Scholar]
- 36.Rakshit RC, Litchfield B, Nightingale JMD. Percutaneous endoscopic gastrostomy (PEG) insertion rate is falling but early mortality remains high. Gastroenterology 2008;134:A563. [Google Scholar]
- 37.A strategic framework for intestinal failure and home parenteral nutrition services for adults in England, 2008. http://www.ncg.nhs.uk/?dl_id=92 (accessed 30 June 2010).
- 38.A strategic framework for intestinal failure and home parenteral nutrition services for adults in England. Implementation plan, 2009. http://www.ncg.nhs.uk/?dl_id=330 (accessed 30 June 2010).
- 39.Stockdale AC, Williams B, Pennington CR. The role of the gastroenterologist in the provision of artificial nutrition support. Aliment Pharmacol Ther 1998;12:367–72. [DOI] [PubMed] [Google Scholar]
- 40.Howard P. Practical nutritional support: working together to make it happen. Proceed Nutr Soc 2001;60:415–18. [DOI] [PubMed] [Google Scholar]
- 41.Austin P, Stroud M. Prescribing adult intravenous nutrition. London: Pharmaceutical Press, 2007:P222–9. [Google Scholar]