Abstract
An increasing number of patients have chronic intestinal failure (IF) or other problems needing nutritional support. These patients need regular input from gastroenterologists, nutrition nurse specialists and dietitians, but traditionally these healthcare professionals see them separately. Here the authors describe their experience of a combined regional nutritional gastroenterology clinic and outline strategies that can avoid the need for home parenteral nutrition (HPN) or intravenous fluids in most cases. Over a 1-year period, 73 patients attended their clinic, with the majority (74%) coming from their own catchment area of 500 000. Of the 63 patients with IF, 49 had short bowel syndrome. 38 of the patients with IF (60%) could be managed with dietary and pharmacological modifications alone, while eight (13%) needed enteral tube feeding and 17 (27%) HPN or intravenous fluids. However, only nine (53%) of the 17 patients referred from other centres specifically for HPN instigation actually needed HPN or intravenous fluids. Patient satisfaction with the combined multidisciplinary clinic was high, with 85% of patients preferring to be seen within this model of outpatient care, although questionnaire response rates were low. The authors have therefore shown that a multidisciplinary nutritional gastroenterology clinic can provide effective patient-centred care and can minimise the need for invasive and costly intravenous nutritional support. Clinics of this type should be an integral part of the current plans to implement regional IF services.
Recent years have seen a steady increase in the number of outpatients with some degree of chronic intestinal failure (IF),1 characterised by a reduction in functioning gut mass resulting in the inability to maintain protein–energy, fluid, electrolyte or micronutrient balance.2 3 IF can occur secondary to surgical resection with resultant short bowel syndrome (SBS), impaired intestinal motility or impaired mucosal absorptive function, although in some cases a combination of these factors applies. It is important to appreciate that as with other organ dysfunction, for example renal failure, IF can be partial or complete and reversible or irreversible, and the severity and reversibility of IF in any individual case will dictate whether parenteral nutrition (PN) and/or intravenous fluid is needed to maintain health, or whether oral nutritional supplements, dietary modifications and/or oral pharmacological measures can suffice.
Patients with IF are complex and effective; management must include: treatment of the underlying medical or surgical condition when possible; optimisation of the patient's nutrition and fluid intake by the least invasive method available; and prevention or treatment of any complications that arise from artificial nutrition support when that is required.4
Multidisciplinary care of these patients is therefore essential and should ideally include review by gastroenterologists, dietitians and nutrition nurse specialists. Traditionally, however, these healthcare professionals see patients separately leading to several potential problems including a need for patients to make multiple hospital visits, and delays in or absence of communication between the healthcare professionals involved. To address these concerns a combined nutritional gastroenterology clinic was developed involving a consultant gastroenterologist with a subspecialty interest in nutrition, a specialist registrar gastroenterology trainee, a nutrition nurse specialist and a senior dietician. The new to follow-up ratio was 1:3, with 30 min slots for all patients. The clinic was predominantly run out of a single room but there were additional clinic rooms so patients could be seen together or separately as appropriate. The aim of this subspecialty gastroenterology clinic was to review patients with complex gastroenterological and nutritional problems, most of whom would have SBS, and in particular, to minimise the need for PN in patients with chronic irreversible but incomplete IF using the principles above. In this article we report our experience of this clinic over a 1-year period and discuss the relevance of our findings to the current Department of Health home intestinal failure network (HIFNET) initiatives to develop regional and national IF services.5
Methods
Referral criteria for the combined nutritional gastroenterology clinic were broad but included all patients with chronic IF ranging from those requiring dietary modifications and/or oral supplements to those requiring home enteral tube feeding or home parenteral nutrition (HPN). In addition, the clinic was occasionally used to review other patients requiring specialist clinical nutrition input (eg, eating disorder patients with very significant undernourishment).
Data collection and definitions
Clinical information was collected retrospectively from the case notes of patients attending the nutritional gastrointestinal clinic over a 1-year period between 1 August 2008 and 31 July 2009. Patients were identified from clinic lists generated by review of the patient administration system. As all patients under active follow-up receive at least a 6-monthly appointment it was estimated that the final dataset would represent virtually all patients actively managed by the nutrition clinic. Data collected included gender, age at last clinic appointment, local or tertiary referral, primary diagnosis, the cause of underlying IF (when appropriate) and type of nutritional support required.
Patient satisfaction surveys were sent out to all patients based on the AGA Patient Satisfaction Survey6 to determine overall patient satisfaction, modified to identify whether patients preferred combined multidisciplinary review to separate review, and whether this reduced the overall number of hospital visits required and thus impacted less on normal life. Domains of the patient satisfaction questionnaire included questions on the waiting time for clinic appointments, professionalism of the reception staff, whether patients felt that their concerns were listened to, how well the diagnosis, treatment plan and any procedures were explained, and whether the patient felt their health was improved as a result of the consultation. Patients were asked to indicate their level of satisfaction against a particular statement using a scale of 1–5, with 1 indicating that the patient was not at all satisfied and 5 indicating that the patient was very satisfied. Patients were also given the opportunity to make free text comments about the service and how this could be improved. Comments received were grouped into themes based on the questions asked in the patient satisfaction questionnaire.
Results
Baseline data and patient characteristics
Seventy-three patients had attended the nutritional gastrointestinal clinic during the 1-year audit period. The median age was 55 years (range 18–88 years) with a female to male ratio of 2.65 (53 women, 20 men).
The majority of patients lived within our own hospital's catchment area of approximately 500 000, with only 19 of 73 patients referred from other consultant gastroenterologists in the Wessex region. Sixty-three of the 73 patients attending the clinic had some degree of chronic irreversible IF, with the principal diagnoses listed in table 1. The predominant diagnoses underlying IF were inflammatory bowel disease and complications from abdominal surgery.
Table 1.
Principal diagnoses of nutrition clinic patients with IF
| n | % | |
|---|---|---|
| Inflammatory bowel disease | 23 | 36.5 |
| Surgical complications resulting in IF | 11 | 17.5 |
| Dysmotility | 4 | 6.3 |
| Malnutrition secondary to bariatric surgery | 4 | 6.3 |
| Mesenteric infarction | 3 | 4.8 |
| Scleroderma | 3 | 4.7 |
| Radiation enteritis | 2 | 3.2 |
| IF due to other causes | 13 | 20.6 |
| Total | 63 | 100 |
IF, intestinal failure.
The mechanism underlying the IF in the 63 patients was: SBS (77.7%); dysmotility (15.9%); intestinal obstruction (1.6%) and enterocutaneous fistulae (4.8%).
Table 2 summarises the type of nutritional support required in the IF patients referred to our nutritional gastroenterology clinic. Thirty-eight of the 63 patients were managed without the use of enteral or intravenous support, with only 14 requiring HPN and a further three requiring intravenous fluids. Seventeen patients were originally referred from other centres specifically to consider starting HPN, but in eight (47%) of these it was possible to avoid the need for any intravenous support using the approaches summarised in box 1.
Table 2.
Nutritional support required in patients with IF
| Local | Tertiary | Total | |
|---|---|---|---|
| Dietary management alone | 20 | 5 | 25 |
| Oral nutritional supplements alone | 6 | 0 | 6 |
| Glucose/saline solution | 6 | 1 | 7 |
| Enteral tube feeding | 6 | 2 | 8 |
| Intravenous fluids | 1 | 2 | 3 |
| Home parenteral nutrition | 7 | 7 | 14 |
IF, intestinal failure.
Box 1. —Approaches to be considered in the management of patients with IF.
-
▶
Assess length and function of remaining bowel
-
▶
Exclude/treat other causes that could contribute to IF (eg, active IBD, small bowel overgrowth, entero-enteral fistula)
-
▶
Minimise rapid transit using anti-motility drugs (eg, high dose loperamide 2–8mg four times a day ±codeine phosphate 30–60 mg four times a day)
-
▶
Add antisecretory drugs if high output stoma for example, omeprazole 40 mg once daily
-
▶
Reduce oral hypotonic fluids (may need restriction to as little as 500 ml/day with supplementary glucose/saline - see below)
-
▶
Advise patients on the use of glucose/saline solution (eg, St Mark's solution/double strength dioralyte) up to 1 or occasionally 1.5 l/day
-
▶
Ensure full dietary assessment and provide nutrition support as required allowing for malabsorption secondary to short bowel
-
▶
Monitor nutritional, hydration and electrolyte status particularly aiming to normalise urinary sodium levels in stoma patients
Nutritional support in non-IF patients
Ten patients attending the nutritional gastroenterology clinic did not have IF, with four having eating disorders resulting in significant malnutrition and six having poor oral intake secondary to neurological conditions. Five of these patients required long-term enteral tube feeding while three required oral nutritional supplements, and two were managed by dietary modifications only.
Patient satisfaction
Unfortunately, only 20 of the 73 patients completed the patient satisfaction questionnaire—a response rate of 27%. Nevertheless, general patient satisfaction scores were high across all domains, with a mean score of 4.42 and a range of 4.1–4.65. The lowest ranking score was for waiting time, with free text comments including that it was difficult to get an appointment within a reasonable time, and because the clinic was held at a fixed time of day it could be difficult to attend the appointment. Highest scores were recorded for how well the patient felt their care was managed, the personal manner of the team and how well procedures, diagnoses and treatments were explained.
Combined multidisciplinary review was felt to be advantageous by most patients, with 17 patients (85%) agreeing or strongly agreeing that they preferred to be seen in the nutritional gastroenterology clinic compared with a general gastroenterology clinic or other outpatient setting. Sixteen patients (80%) felt that the nutrition clinic review reduced the number of times they had to attend clinic, while 14 (70%) felt that being reviewed in the nutrition clinic impacted less on their life than being reviewed in separate clinics.
Discussion
This study reports our experience of managing complex IF and other problems related to nutritional support over a 1-year period. The patient demographics and underlying diagnoses of our cohort with IF are similar to those previously published in other single and multicentre studies.7 8 Referrals to this clinic were predominantly from local clinicians, with relatively few tertiary referrals, which were primarily patients thought to need long-term intravenous support, for example HPN. In reality, however, this was frequently not the case because the management of those ‘HPN referrals’ before their attendance at our clinic was often suboptimal, especially for patients with SBS who had frequently been encouraged to ‘drink more fluid’—a step that often worsens gastrointestinal losses and thus leads to an increased risk of dehydration, salt depletion and the need for intravenous support.
By using the measures listed in box 1 we were able significantly to improve nutritional status and reduce the level of nutritional support required in many cases. This was particularly relevant for tertiary referrals in which we were able to prevent the need to start HPN in nearly half of the patients referred expressly to commence this invasive and costly therapy. Our data therefore raise questions about the level of expertise in district general hospitalgastroenterologists in relation to IF, a problem that is probably due to the current lack of structured nutrition training.9
Combined multidisciplinary review of patients was essential to the success of the nutritional gastroenterology service as it enabled effective communication between the gastroenterologists, dietitians and nutrition nurse specialists, and allowed a clear, optimal management plan to be formulated for each patient. Furthermore, it allowed more effective education of the patient, especially with the development of our IF-related patient information leaflets on SBS and other topics.10 The combination of individualised dietetic advice and patient information leaflets has previously been shown to improve patient outcomes.11 Combined review also reduced the number of clinic appointments that a patient needed, and although our patient satisfaction data were limited, they did strongly suggest that patients prefer a combined multidisciplinary approach that reduces the impact on their life and enables them to understand their condition better. The very disappointing response rates for our patient satisfaction survey may have been due to the fact that patients were sent the questionnaires by post and asked to return them in a prepaid envelope, and in future we plan to seek patient feedback immediately after clinic review in order to improve response rates.
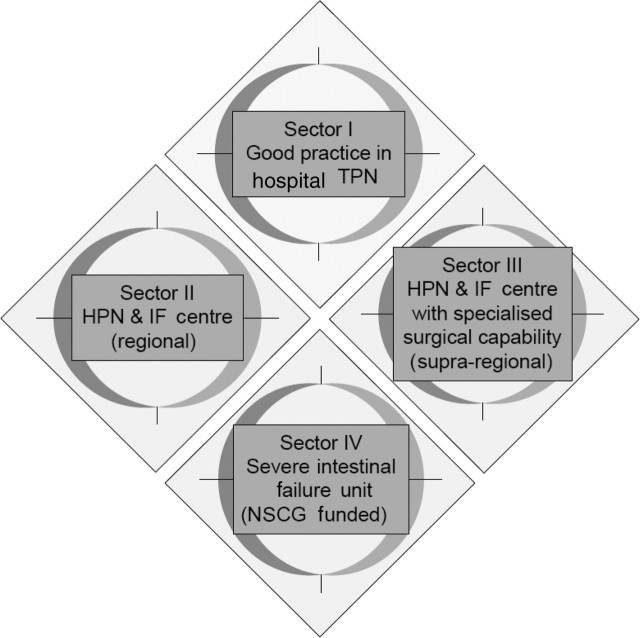
Recently, the Department of Health recommended a framework for IF care in England outlined in figure 1, and regional specialist commissioners have now been asked to implement these arrangements.5 In the proposed HIFNET framework, sector 1 acute hospitals look after the majority of short-term IF patients, while sector 2 hospitals (regional IF centres) or sector 3 hospitals (regional IF centres with specialist IF surgery expertise) undertake the management of longer-term established IF patients, referring them on to a sector 4 hospital (one of the two existing national IF units) as necessary. The HIFNET recommendations, however, are primarily intended to improve care for IF patients requiring intravenous nutritional support, while the data from our audit show that the majority of those who could benefit from attending a multidisciplinary nutritional gastrointestinal clinic either have degrees of IF that do not warrant such support or other gastrointestinal-related nutritional problems. Furthermore, as the majority of the patients attending our specialist clinic actually came from within our immediate catchment area, it seems likely that similar clinics, perhaps run less frequently, could be of benefit in most sector 1 hospitals, that is most district general hospitals.
Figure 1.
Proposed network structure. HPN, home parenteral nutrition; IF, intestinal failure; IV, intravenous; NSCG, National Specialised Commissioning Group; TPN, total parenteral nutrition.
In view of the above, we therefore suggest that when HIFNET proposals for regional IF services are implemented, not only should a combined multidisciplinary nutritional gastroenterology clinic, similar to that reported here, be integral to all sector 2 and 3 regional IF services, but that each region should also determine how best to provide wider multidisciplinary nutritional gastroenterology care. This might be through the running of a single large sector 2/3 regional clinic, but may also be through running a regular, albeit less frequent, multidisciplinary clinic at sector 1 hospitals, either as a satellite staffed by the regional sector 2/3 unit, or a truly local clinic staffed by sector 1 clinicians and other healthcare professionals who have attended the regional multidisciplinary clinic to gain specific experience, and who then work to defined regional standards. Both of the latter models of care would reduce overall distances that patients have to travel, while providing patients with adequate levels of expertise and knowledge, and whereas in the past it may have been difficult to envisage adequate enthusiasm or expertise for such clinics to work, recent moves to improve standards of training in clinical nutrition for all trainees, along with information that many future gastroenterologists do have subspecialty interest in this area,12 suggest that it should be possible. It is also important to have a regional multiprofessional network to encourage networking of care and improve knowledge and expertise.
In summary, we have shown that a combined multidisciplinary specialist nutritional gastroenterology clinic works well and enables the vast majority of patients with IF to be managed without recourse to PN. The service is preferred by patients and reduces the number of hospital appointments needed.
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Smith T, Micklewright A, Hirst A, et al. eds. Annual BANS Report 2009: Artificial Nutrition Support in the UK 2000–2008. A Report by the British Artificial Nutrition Survey (BANS). Redditch: The British Association for Parenteral and Enteral Nutrition (BAPEN), 2009. [Google Scholar]
- 2.Nightingale JM. Management of patients with a short bowel. World J Gastroenterol 2001;7:741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Keefe SJ, Buchman AL, Fishbein TM, et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 2006;4:6–10. [DOI] [PubMed] [Google Scholar]
- 4.Nightingale JM. The medical management of intestinal failure: methods to reduce the severity. Proc Nutr Soc 2003;62:703–10. [DOI] [PubMed] [Google Scholar]
- 5.National Specialised Commissioning Group. A strategic framework for intestinal failure and home parenteral nutrition services for adults in England. April 2008. http:///.ncg.nhs.uk/?d1_id=92 (Accessed 25 Jan 2010).
- 6.AGA Institute Centre for Quality in Healthcare Practice. AGA Patient Satisfaction Survey: Outpatient Office. 2007. http://www.gastro.org/practice/quality-initiatives/performance-measures/AGAI_Patient_Satisfaction_Surveys_Office.doc (Accessed 12 Sept 2009).
- 7.Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999;117:1043–50. [DOI] [PubMed] [Google Scholar]
- 8.Vantini I, Benini L, Bonfante F, et al. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis 2004;36:46–55. [DOI] [PubMed] [Google Scholar]
- 9.Harvey JAH, Nield PJ. Nutritional training in gastroenterology. Frontline Gastroenterol 2010;1:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Southampton General Hospitals Dietician and Nutrition Support Team. SUHT Patient information leaflet: short bowel syndrome. 2008. http://www.shifnet.org.uk/guidelines.htm (Accessed 14 Nov 2009).
- 11.Culkin A, Gabe SM, Madden AM. Improving clinical outcome in patients with intestinal failure using individualised nutritional advice. J Hum Nutr Diet 2009;22:290–8; quiz 300–1. [DOI] [PubMed] [Google Scholar]
- 12.Nield PJ. Are we now in a position to offer workplace based training in nutrition to our gastroenterology trainees across the UK. Frontline Gastroenterol 2010;1:19–24. [Google Scholar]