Abstract
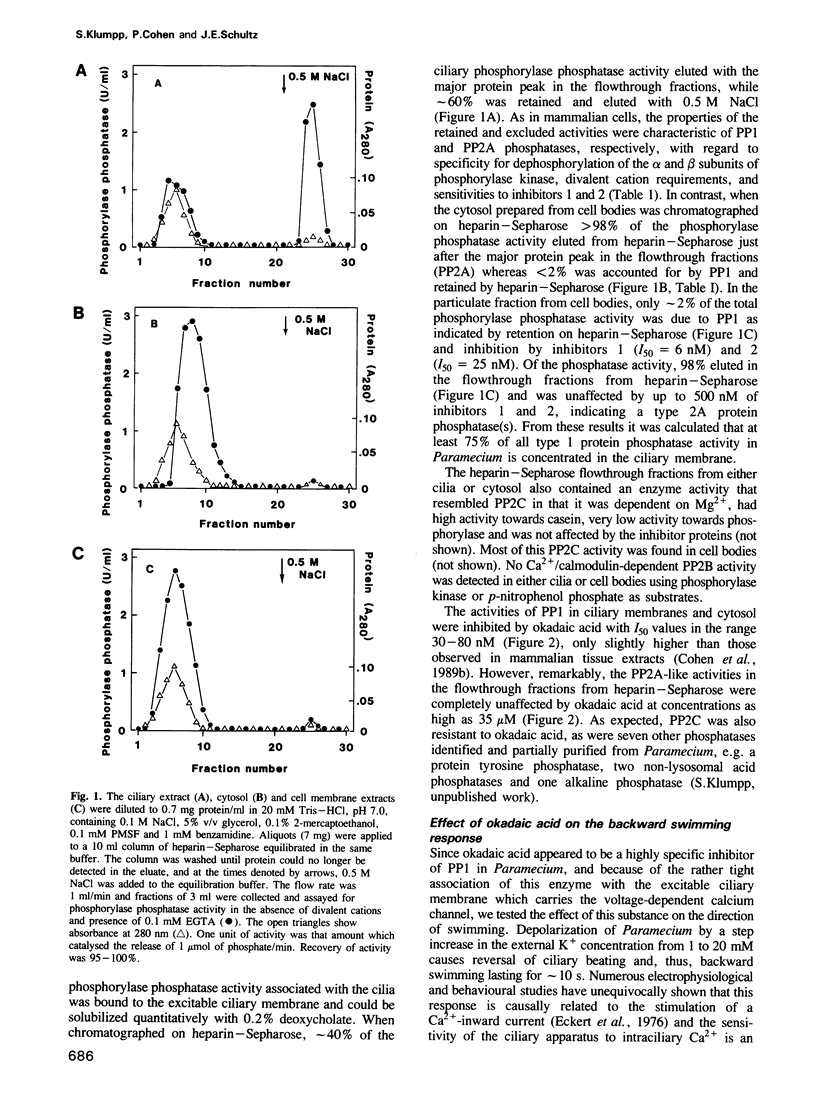
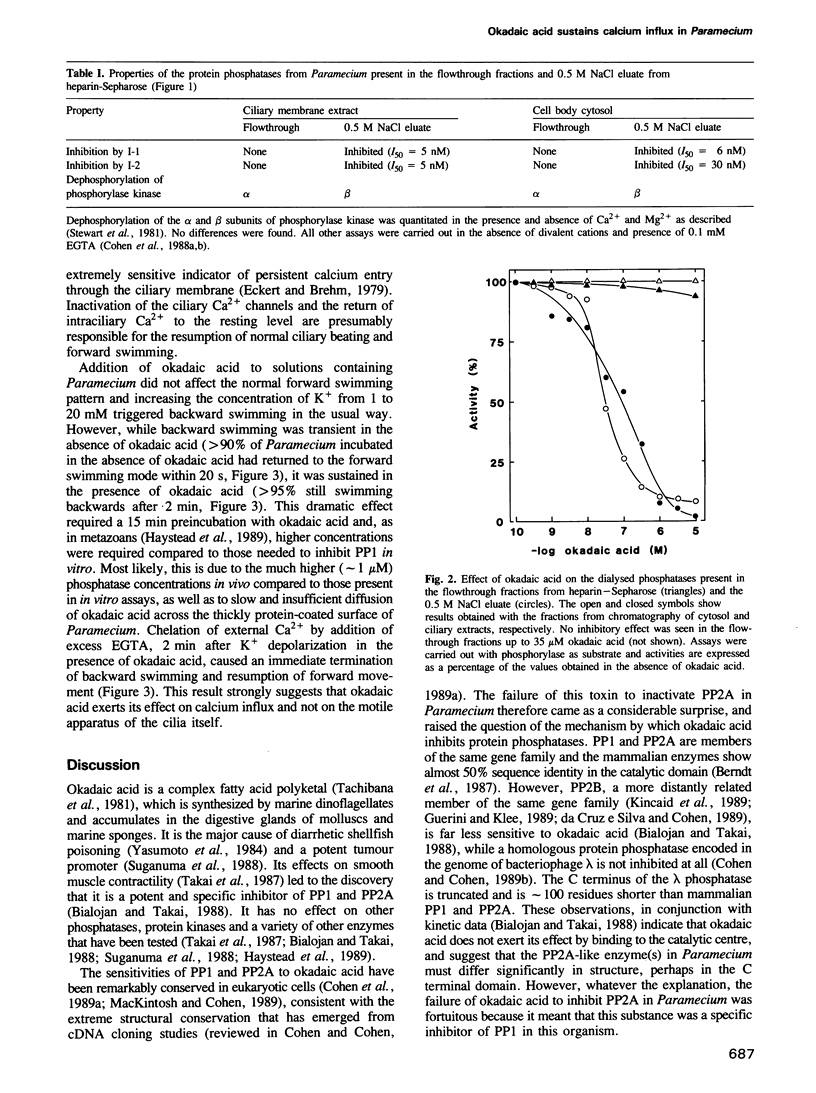
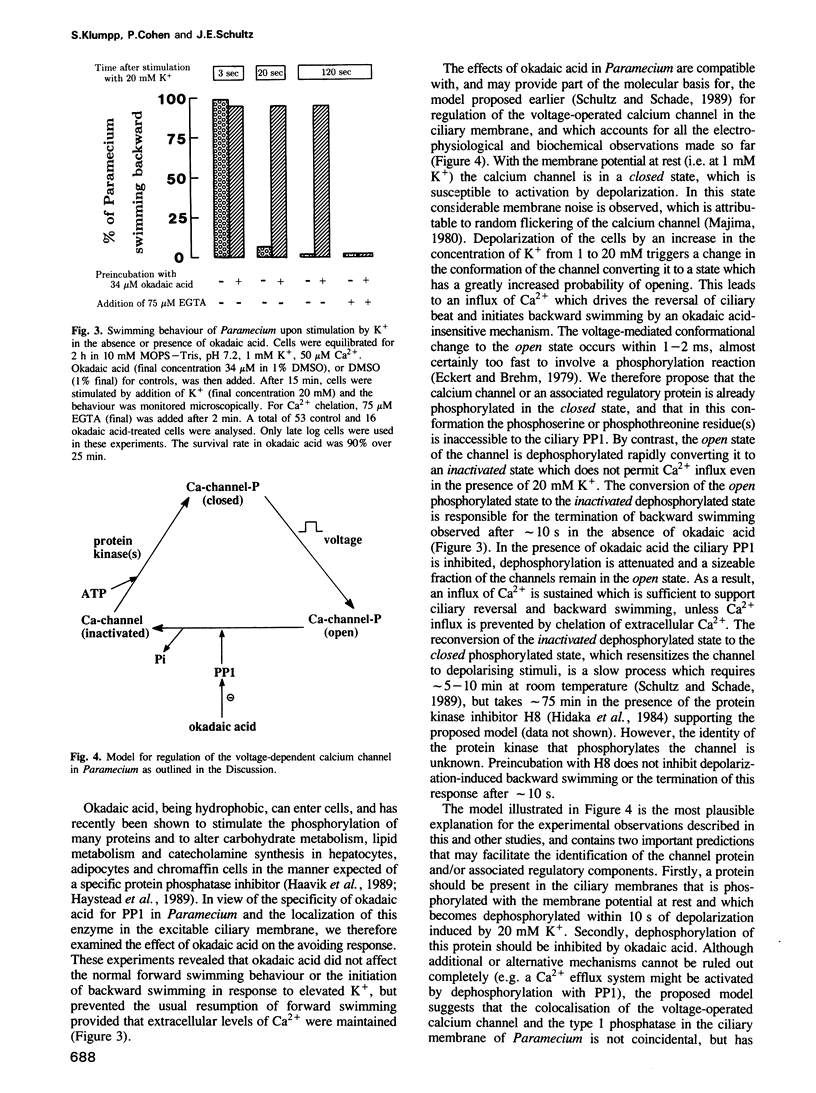
Backward swimming is a stereotypic behavioural response of Paramecium. It is triggered by depolarizing stimuli, which open calcium channels in the excitable ciliary membrane. The influx of Ca2+ causes the reversal of ciliary beat and initiates backward swimming. Here, we demonstrate that the protein phosphatase inhibitor okadaic acid does not affect the normal forward swimming pattern of Paramecium, but greatly extends the duration of backward swimming as initiated by depolarization caused by a rise in extracellular K+. Chelation of external Ca2+ results in an immediate resumption of forward swimming. The results suggest that the voltage-operated calcium channel is inactivated by a dephosphorylation event, and that okadaic acid blocks this dephosphorylation without any effect on the motile apparatus of the cilia. In addition, Paramecium is unique among eukaryotic cells, in that okadaic acid inhibits just one protein phosphatase, namely a type 1 enzyme, 75% of which is tightly associated with the excitable ciliary membrane. The type 2A protein phosphatases in Paramecium are unaffected by okadaic acid. The results indicate that protein phosphatase 1 is the enzyme responsible for the dephosphorylation and closure of the calcium channel in Paramecium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. T., Cohen P. Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem J. 1989 Jun 15;260(3):931–934. doi: 10.1042/bj2600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. T., Schelling D. L., da Cruz e Silva O. B., Barker H. M., Cohen P. The major type-1 protein phosphatase catalytic subunits are the same gene products in rabbit skeletal muscle and rabbit liver. Biochim Biophys Acta. 1989 Jun 1;1008(1):125–128. doi: 10.1016/0167-4781(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Cohen P., Alemany S., Hemmings B. A., Resink T. J., Strålfors P., Tung H. Y. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Foulkes J. G., Holmes C. F., Nimmo G. A., Tonks N. K. Protein phosphatase inhibitor-1 and inhibitor-2 from rabbit skeletal muscle. Methods Enzymol. 1988;159:427–437. doi: 10.1016/0076-6879(88)59042-0. [DOI] [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cohen P., Schelling D. L., Stark M. J. Remarkable similarities between yeast and mammalian protein phosphatases. FEBS Lett. 1989 Jul 3;250(2):601–606. doi: 10.1016/0014-5793(89)80804-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Erdödi F., Csortos C., Bot G., Gergely P. Separation of rabbit liver latent and spontaneously active phosphorylase phosphatases by chromatography on heparin-sepharose. Biochem Biophys Res Commun. 1985 Apr 30;128(2):705–712. doi: 10.1016/0006-291x(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik J., Schelling D. L., Campbell D. G., Andersson K. K., Flatmark T., Cohen P. Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett. 1989 Jul 17;251(1-2):36–42. doi: 10.1016/0014-5793(89)81424-3. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W., Mieskes G., Söling H. D. Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem. 1987 Jun 1;165(2):261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Schultz J. E. Paramecium: a model system for the study of excitable cells. Trends Neurosci. 1988 Jan;11(1):27–32. doi: 10.1016/0166-2236(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Nightingale M. S., Martin B. M. Characterization of a cDNA clone encoding the calmodulin-binding domain of mouse brain calcineurin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8983–8987. doi: 10.1073/pnas.85.23.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majima T. Membrane potential fluctuation in Paramecium. Biophys Chem. 1980 Feb;11(1):101–108. doi: 10.1016/0301-4622(80)85012-5. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Hemmings B. A., Cohen P., Goris J., Merlevede W. The MgATP-dependent protein phosphatase and protein phosphatase 1 have identical substrate specificities. Eur J Biochem. 1981 Mar 16;115(1):197–205. doi: 10.1111/j.1432-1033.1981.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A., Bialojan C., Troschka M., Rüegg J. C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987 Jun 8;217(1):81–84. doi: 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- Thiele J., Klumpp S., Schultz J. E., Bardele C. F. Differential distribution of voltage-dependent calcium channels and guanylate cyclase in the excitable ciliary membrane from Paramecium tetraurelia. Eur J Cell Biol. 1982 Aug;28(1):3–11. [PubMed] [Google Scholar]


