Abstract
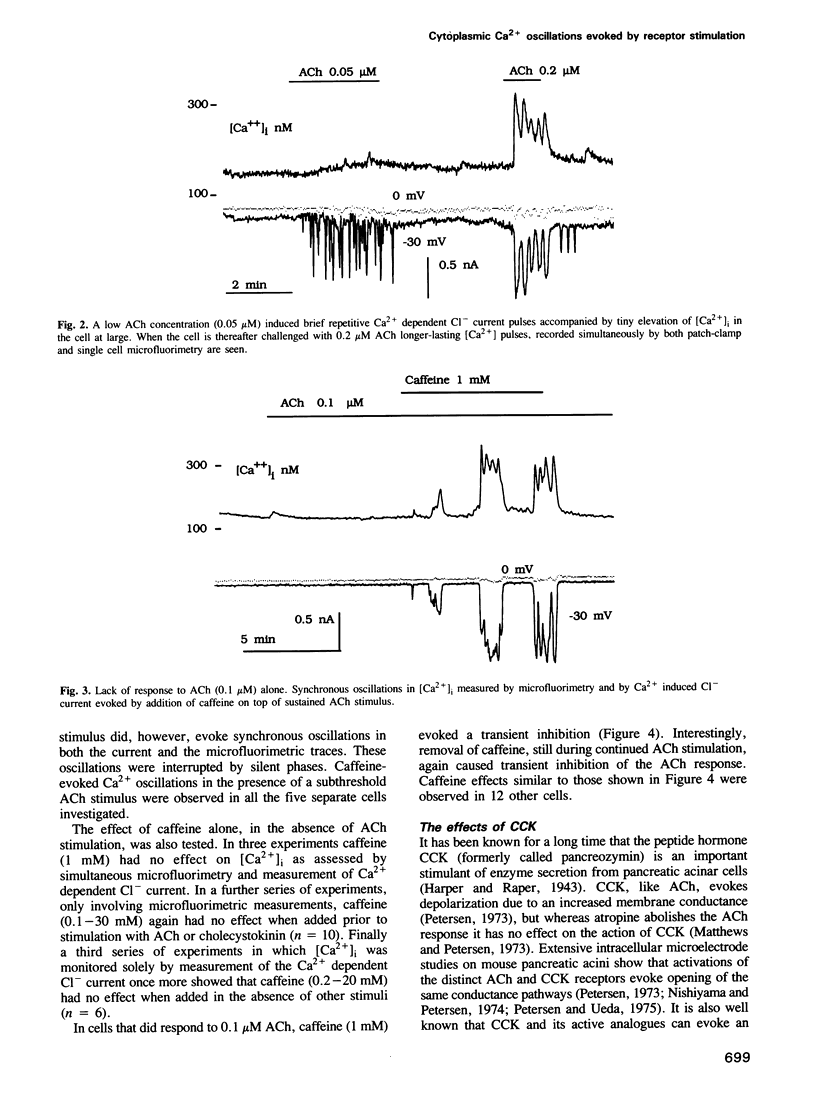
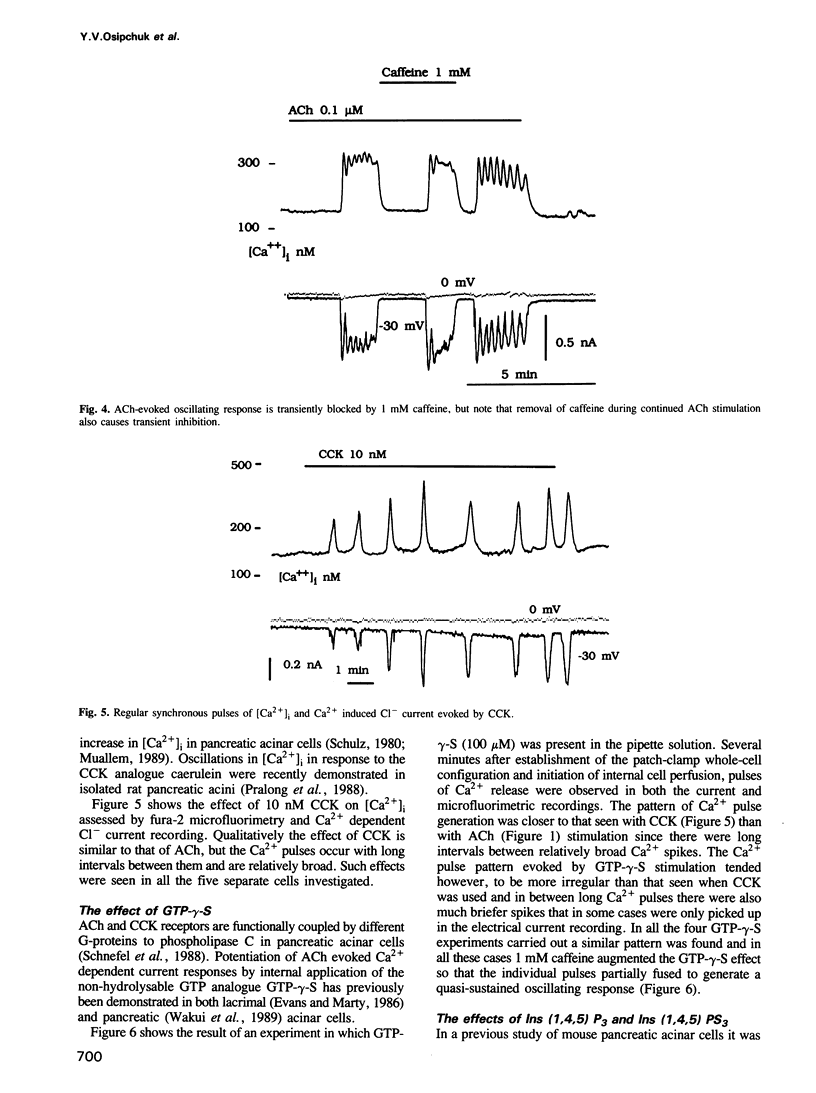
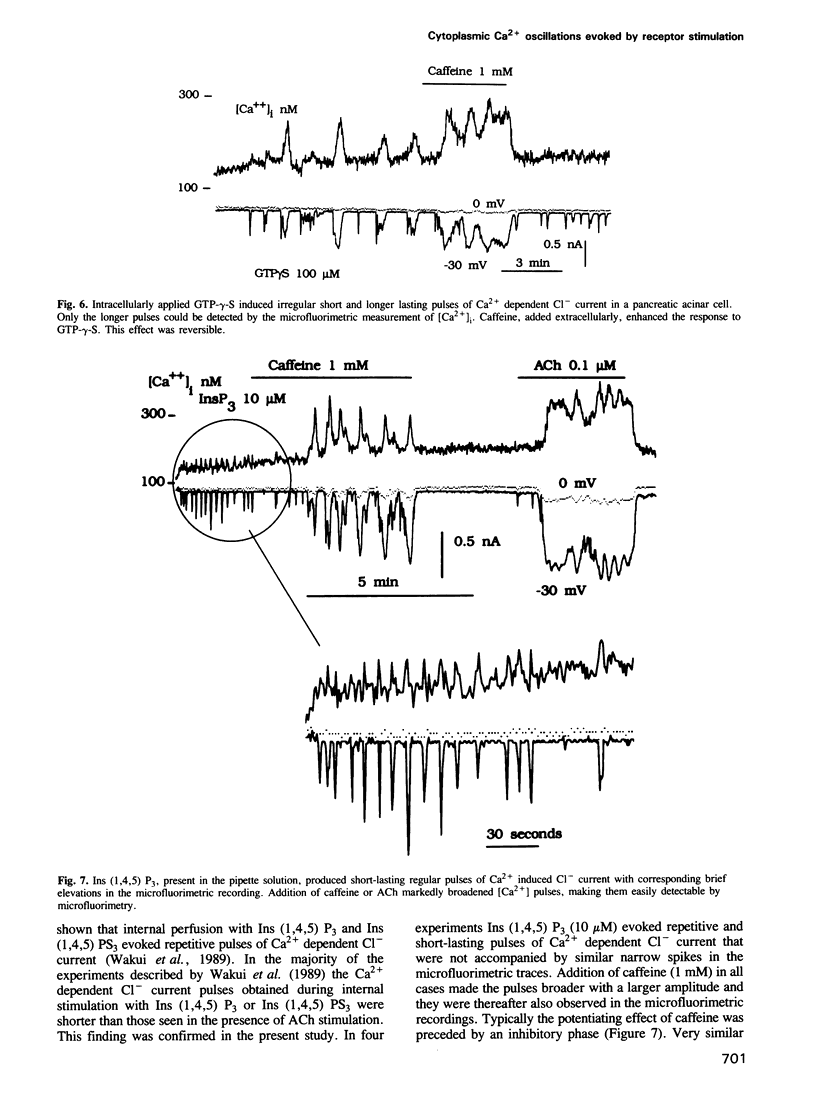
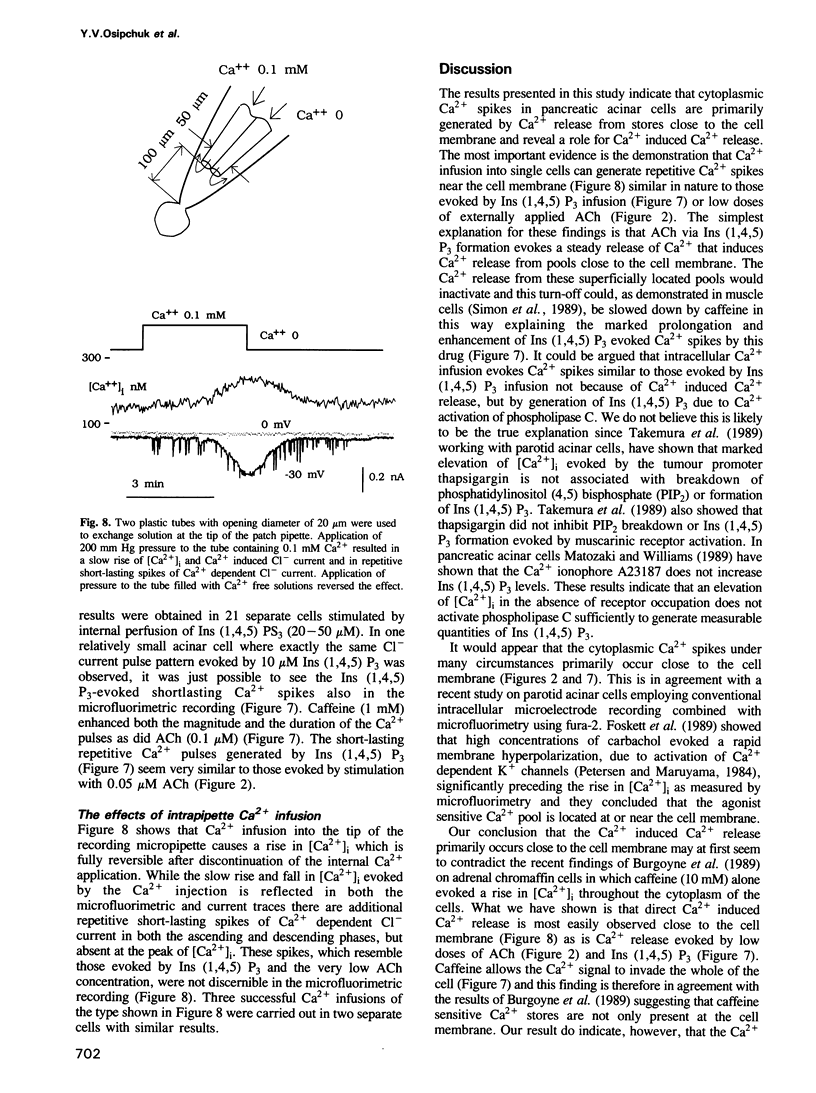
The effects of acetylcholine (ACh), cholecystokinin (CCK), internally applied GTP-gamma-S, inositol trisphosphate [Ins (1,4,5) P3] or Ca2+ on the cytoplasmic free Ca2+ concentration [( Ca2+]i) were assessed by simultaneous microfluorimetry (fura-2) and measurement of the Ca2(+)-dependent Cl- current (patch-clamp whole-cell recording) in single internally perfused mouse pancreatic acinar cells. ACh (0.1-0.2 microM) evoked an oscillating increase in [Ca2+]i measured in the cell as a whole (microfluorimetry) which was synchronous with oscillations in the Ca2(+)-dependent Cl- current reporting [Ca2+]i close to the cell membrane. In the same cells a lower ACh concentration (0.05 microM) evoked shorter repetitive Cl- current pulses that were not accompanied by similar spikes in the microfluorimetric recording. When cells did not respond to 0.1 microM ACh, caffeine (1 mM) added on top of the sustained ACh stimulus resulted in [Ca2+]i oscillations seen synchronously in both types of recording. CCK (10 nM) also evoked [Ca2+]i oscillations, but with much longer intervals between slightly broader Ca2+ pulses. Internal perfusion with 100 microM GTP-gamma-S evoked [Ca2+]i oscillations with a similar pattern. Ins (1,4,5) P3 (10 microM) evoked repetitive shortlasting spikes in [Ca2+]i that were only seen in the Cl- current traces, except in one small cell where these spikes were also observed synchronously in the microfluorimetric recording. Caffeine (1 mM) broadened these Ca2+ pulses. [Ca2+]i was also directly changed, bypassing the normal signalling process, by infusion of a low or high Ca2+ solution into the pipette.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate and inositol 1,4,5-trisphosphate act by different mechanisms when controlling Ca2+ in mouse lacrimal acinar cells. FEBS Lett. 1989 Jul 17;251(1-2):43–48. doi: 10.1016/0014-5793(89)81425-5. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K., Gunter-Smith P. J., Melvin J. E., Turner R. J. Physiological localization of an agonist-sensitive pool of Ca2+ in parotid acinar cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):167–171. doi: 10.1073/pnas.86.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Acetylcholine-like effects of intracellular calcium application in pancreatic acinar cells. Nature. 1977 Jul 14;268(5616):147–149. doi: 10.1038/268147a0. [DOI] [PubMed] [Google Scholar]
- Jauch P., Petersen O. H., Läuger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94(2):99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- Laugier R., Petersen O. H. Effects of intracellular EGTA injection on stimulant-evoked membrane potential and resistance changes in pancreatic acinar cells. Pflugers Arch. 1980 Jul;386(2):147–152. doi: 10.1007/BF00584202. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. C., Yule D. I., Dunne M. J., Gallacher D. V., Petersen O. H. Vasopressin directly closes ATP-sensitive potassium channels evoking membrane depolarization and an increase in the free intracellular Ca2+ concentration in insulin-secreting cells. EMBO J. 1989 Dec 1;8(12):3595–3599. doi: 10.1002/j.1460-2075.1989.tb08532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Williams J. A. Multiple sources of 1,2-diacylglycerol in isolated rat pancreatic acini stimulated by cholecystokinin. Involvement of phosphatidylinositol bisphosphate and phosphatidylcholine hydrolysis. J Biol Chem. 1989 Sep 5;264(25):14729–14734. [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard P., Vacher P., Dufy B., Winiger B. P., Schlegel W. Thyrotropin-releasing hormone-induced rise in cytosolic calcium and activation of outward K+ current monitored simultaneously in individual GH3B6 pituitary cells. J Biol Chem. 1988 Dec 25;263(36):19570–19576. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Muallem S. Calcium transport pathways of pancreatic acinar cells. Annu Rev Physiol. 1989;51:83–105. doi: 10.1146/annurev.ph.51.030189.000503. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Mechanism of action of pancreozymin and acetylcholine on pancreatic acinar cells. Nat New Biol. 1973 Jul 18;244(133):73–73. doi: 10.1038/newbio244073a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Philpott H. G. Mouse pancreatic acinar cells: the anion selectivity of the acetylcholine-opened chloride pathway. J Physiol. 1980 Sep;306:481–492. doi: 10.1113/jphysiol.1980.sp013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: effect of acetylcholine, pancreozymin, gastrin and secretin on membrane potential and resistance in vivo and in vitro. J Physiol. 1975 May;247(2):461–471. doi: 10.1113/jphysiol.1975.sp010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong W. F., Wollheim C. B., Bruzzone R. Measurement of cytosolic free Ca2+ in individual pancreatic acini. FEBS Lett. 1988 Dec 19;242(1):79–84. doi: 10.1016/0014-5793(88)80989-x. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Chanson M., Trautmann A. Calcium and secretagogues-induced conductances in rat exocrine pancreas. Pflugers Arch. 1988 Jan;411(1):53–57. doi: 10.1007/BF00581646. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Schnefel S., Banfic H., Eckhardt L., Schultz G., Schulz I. Acetylcholine and cholecystokinin receptors functionally couple by different G-proteins to phospholipase C in pancreatic acinar cells. FEBS Lett. 1988 Mar 28;230(1-2):125–130. doi: 10.1016/0014-5793(88)80655-0. [DOI] [PubMed] [Google Scholar]
- Schulz I. Messenger role of calcium in function of pancreatic acinar cells. Am J Physiol. 1980 Nov;239(5):G335–G347. doi: 10.1152/ajpgi.1980.239.5.G335. [DOI] [PubMed] [Google Scholar]
- Schulz I., Thévenod F., Dehlinger-Kremer M. Modulation of intracellular free Ca2+ concentration by IP3-sensitive and IP3-insensitive nonmitochondrial Ca2+ pools. Cell Calcium. 1989 Jul;10(5):325–336. doi: 10.1016/0143-4160(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Simon B. J., Klein M. G., Schneider M. F. Caffeine slows turn-off of calcium release in voltage clamped skeletal muscle fibers. Biophys J. 1989 Apr;55(4):793–797. doi: 10.1016/S0006-3495(89)82878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Petersen O. H. Patch-clamp study of single-channel and whole-cell K+ currents in guinea pig pancreatic acinar cells. Am J Physiol. 1988 Sep;255(3 Pt 1):G275–G285. doi: 10.1152/ajpgi.1988.255.3.G275. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thévenod F., Dehlinger-Kremer M., Kemmer T. P., Christian A. L., Potter B. V., Schulz I. Characterization of inositol 1,4,5-trisphosphate-sensitive (IsCaP) and -insensitive (IisCaP) nonmitochondrial Ca2+ pools in rat pancreatic acinar cells. J Membr Biol. 1989 Jul;109(2):173–186. doi: 10.1007/BF01870856. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Gallacher D. V. Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett. 1988 Nov 7;239(2):358–362. doi: 10.1016/0014-5793(88)80951-7. [DOI] [PubMed] [Google Scholar]


