Abstract
Background
A nurse practitioner-led colonoscopy surveillance service was introduced to improve appropriateness, validation and compliance with the National Patient Safety Agency safety alert and British Society of Gastroenterology consensus guidance for bowel cleansing agents.
Objective
To determine the clinical outcomes and efficacy of this new service.
Design and patients
A 4-month prospective audit of patients due to attend for surveillance colonoscopy.
Setting
Royal Liverpool University Hospital.
Intervention
A new nurse practitioner-led surveillance service reviewed all patients before listing.
Outcomes
Clinical outcomes, service efficiency and cost effectiveness.
Results
224 Patients (median age 68 years, 52% male, and median American Society of Anesthesiologists (ASA) 2) were assessed and 34% had medical factors influencing their colonoscopy. 37% patients were discharged without a colonoscopy, 17% deferred (median >2 years), 6% had died while on the register and the remaining (40%) had their procedure at the agreed interval. The 30-day and 6-month all-cause mortality was 0% for those fit for colonoscopy, compared with 5% and 14%, respectively, for those deemed unfit. The did-not-attend (DNA) rate was reduced from 7.6% to <1%. With 95 patients not requiring a colonoscopy a potential £40 000 saving to the primary care trust was made.
Conclusions
The nurse practitioner-led surveillance service has been invaluable for guideline adherence and medical management of patients before colonoscopy. In addition, it potentially avoided procedural all-cause mortality in these patients. It has proved to be efficacious with reduced DNA rates and over one-third of patients assessed did not require a colonoscopy.
Introduction
Two categories of guidance/guidelines have been issued recently, which have major implications for surveillance practice and processes for colonoscopy in the UK. The first, recently published by the British Society of Gastroenterology (BSG) and National Institute for Health and Clinical Excellence ruled on changes in surveillance intervals and categories 1 2 The second, issued by National Patient Safety Agency (NPSA) and the BSG consensus groups, provided guidance on the safety of oral bowel cleansing agents.3 4 These latter guidelines had a number of recommendations which should be considered before issuing oral bowel cleaning agents—namely, all patients should be clinically assessed and risk stratified for the appropriate bowel cleansing agent before their procedure; oral bowel cleansing agents should be prescribed and dispensed by authorised personnel; patients should give verbal and written information for use of the bowel preparation; renal function should be ascertained before the procedure; medication for diuretic patients should be reviewed; use of angiotensin receptor blockers, ACE inhibitors and the presence of specific comorbidities such as advanced liver disease, CCF and chronic kidney diseaseshould be ascertained. Compliance with these safety guidance has been particularly challenging for the surveillance patients requiring colonoscopy.5
Consequently, we introduced a new nurse practitioner-led colonoscopy surveillance service to improve compliance with these guidelines/guidance. In addition, this service manages the surveillance patients on the register, preassesses all patients, performs surveillance colonoscopy, collaborates with and educates the interested parties.
The aim of this prospective audit was to determine the clinical outcomes and efficacy of this new service.
Methods
A prospective audit was performed between 1 June 2010 and 30 September 2010 at the Royal Liverpool University Hospital. All patients due to be screened or undergo surveillance colonoscopy over this period were audited. (Patients in the Bowel Cancer Screening Programme were excluded as they belonged to a completely separate service.)
Patients were identified using the pre-existing surveillance register, which contained over 3500 patients. Referrals of patients due to be called for their surveillance colonoscopy in this period were diverted from this register to the nurse practitioner before a decision on listing for their procedure was made. A new nurse-led surveillance clinic was instituted using a standard protocol and proforma. All patients attending the clinic automatically had preassessment blood tests, including serum sodium, potassium and estimated glomerular filtration rate. Patients who were either vetted or had a telephone clinic review were sent for blood tests only if it was clinically required as stated by the BSG consensus guidance4 after their review. All patients referred for a repeat procedure immediately saw a nurse practitioner so that the histology could be followed up and the updated appropriate surveillance interval could be determined. Telephone clinics were established for long-distant patients and for a routine 30-day follow-up after the procedure. A virtual follow-up was arranged at 6 months to determine outcomes by reviewing electronic hospital information systems.
Data were prospectively collected onto a purpose built ACCESS database using medical case records, electronic patient records, endoscopy and histology databases and details from the patient consultation. The database was held on a secure hospital server and the audit was registered and approved by the trust audit department as a service evaluation audit.
Results
Patient demographics
A total of 224 patients were scheduled for a colonoscopy over this period, of whom 6% (n=13) had died but still remained on the re-call surveillance colonoscopy database. The median age of patients was 68 (SD 14), 52% were male and 54% had at least one significant comorbidity. The median American Society of Anesthesiologists (ASA) score was 2.
Surveillance categories
The patient's surveillance categories were polyp follow-up (37%) and inflammatory bowel disease (IBD) surveillance (27%), cancer surveillance (17%), family history (11%), genetic screening (7%) and acromegaly (1%).
Patient review
All 224 patients were reviewed before their procedure. The majority of patients were preassessed either by a face-to-face interview (64%) or by telephone (11%). All referrals for the remaining 25% were vetted by the nurse practitioner for appropriateness. In total, 53% were preassessed in the new nurse practitioner-led surveillance clinic and 11% in the nurse practitioner-led IBD clinic.
Medical factors potentially influencing decision to perform colonoscopy
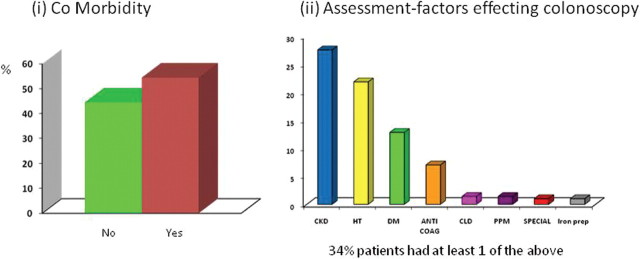
Over one-third (34%) of patients had at least one medical factor that might have influenced the decision to perform colonoscopy (see figure 1)—namely, chronic kidney disease (27.5%), hypertension and antihypertensive drugs (diuretics, angiotensin receptor blockers, ACE inhibitors) (21%) and diabetes mellitus (12.8%). Seven per cent were either receiving anticoagulants or clopidogrel, and 4.8%, required either a special consent, had advanced liver disease, a permanent pacemaker in situ, or were receiving long-term iron treatment.
Figure 1.
(A) The percentage comorbidity found in the surveillance population was >50%. (B) Medical factors detected by the nurse practitioner that might influence colonoscopy. Interesting, 34% of patients had at least one factor that would have influenced their colonoscopy.CKD, chronic kidney disease; CLD, clopidogrel; DM, diabetes mellitus; HT, hypertension; PPM, permanent pacemaker.
Service outcomes
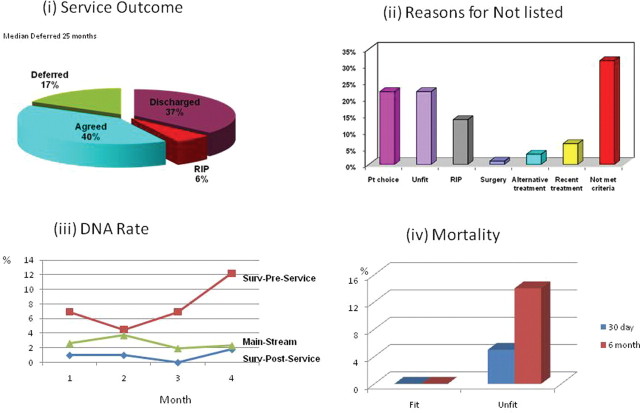
The overall service outcomes are shown in figure 2A. A total of 43% did not undergo a colonoscopy. Thirty-seven per cent because they were discharged without undergoing a colonoscopy and 6% had died.
Figure 2.
(A) Overall outcomes of the service, with the discharge of 37% of patients and deferral of 17% of patients with a median of 2 years. (B) Reasons why the procedure was not performed. (C) Did-not-attend (DNA) rates in the quarter 12 months ago before the service (surv-preservice), after the service (surv-post-service) and in the non-surveillance patients in the same quarter (mainstream). (D) The 30-day and 6-month mortality in patients assessed as being fit by the service and who went on to have a colonoscopy (0%) and those unfit and who did not go on to have a colonoscopy for whom the mortality was 5% and 14%, respectively.
Of the 95 patients not listed (see figure 2B), 31.5% did not meet the criteria of the new BSG guidelines (15 patients were under polyp surveillance, and seven each were in the IBD category and family history/genetic category). In 22%, patient choice was a factor: three patients turned down their invitation to telephone or clinic review, one woman was breast feeding, three patients wanted their surveillance done more locally, 12 patients declined because of their age and comorbidity and one had had a negative colonoscopy previously and did not want another (although two polyp-free colonoscopies were advised). Interestingly, of those who were already listed and were vetted only, inability to meet the slection criteria rather than ‘patient choice’ was the eliminating factor. Twenty-two per cent were assessed as unfit for the procedure, with a median age of 80, median ASA score 3, and they had at least two significant comorbidities or mobility problems, or both. In just over 6%, patients did not require their surveillance colonoscopy as they had had a recent procedure.
Seventeen per cent had their procedure deferred by a median of 25 months, of which 62% were changed in accordance with the new BSG guidelines, 17% were duplicate referrals (referrals generated from the clinic as well as the surveillance recall register), 13% were due to patient choice and 8% adjusted as they had undergone a recent procedure owing to new symptoms. Only 40% of patients had the colonoscopy at the originally agreed time.
Did-not-attend (DNA) rate
The DNA rate of the clinic was 7%. However, there was a considerable change in this rate for colonoscopy after the instigation of the service, with a mean DNA in the preceding year over the same quarter for the surveillance patients of 7.6% compared with <1% (0.94%) after instigation of the service. This compared with mean DNA rate of 2.6% in symptomatic (mainstream) patients in the same period (see figure 2C).
Cost-effectiveness
With a current Healthcare Resource Group tariff of £420, potential savings to the primary care trust would be approximately £40 000 over 4 months as 95 patients were discharged and did not undergo a colonoscopy. In addition the capacity of the service has improved with the removal of these patients and the deferral of 17% of patients by over 2 years for their scheduled procedure.
30-Day follow-up and mortality
Of those who were assessed as fit for a colonoscopy, there were no deaths within 30 days or 6 months after the procedure. In the 22% assessed as unfit for the procedure, three deaths (14%) occurred, of which one was within 30 days (5%) after the clinic review (figure 2D). All three were ASA 3.
Discussion
Our study has shown that a nurse practitioner-led surveillance service can have excellent outcomes on quality, safety, efficacy, and perhaps even avoid procedural mortality.
The nurse practitioner was appointed in April 2010, and before the start of the database and audit, time was spent on nurse education, collaboration between specialties, agreeing protocols and setting up clinics with an agreed proforma (figure 3). Most patients were reviewed by a face-to-face interview or by telephone. However, a quarter of patients were vetted and listed for their procedure without a clinic review because they had been given dates previously.
Figure 3.
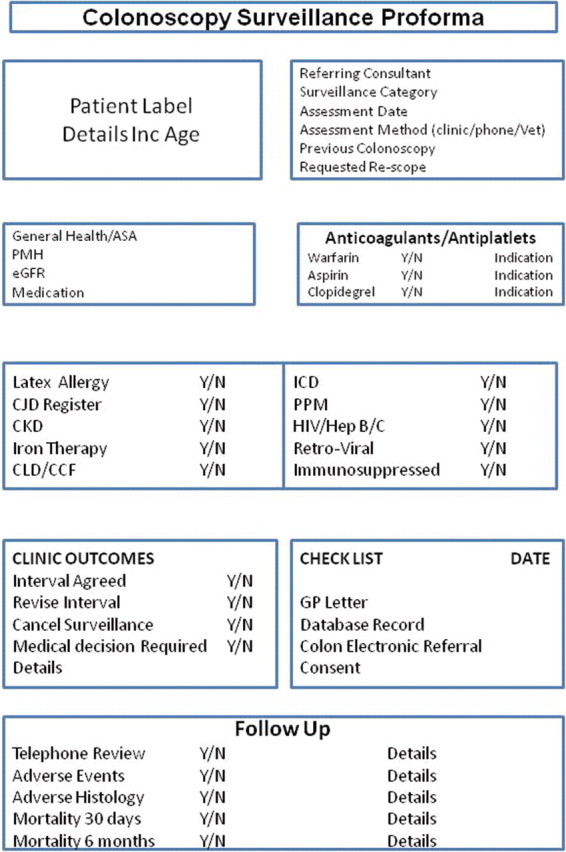
A customised version of the proforma used by the surveillance nurse practitioner. ASA, American Society of Anesthesiologists; CCF, congestive cardiac failure; CJD, Creutzfeldt-Jakob disease; CKD, chronic kidney disease; CLD, clopidogrel; eGFR, estimated glomerular filtration rate; ICD, implantable defibrillator ; PMH, pervious medical history; PPM, permanent pacemaker.
There was significant comorbidity in this surveillance population (>50%) and importantly, this new service highlighted that one-third of patients had at least one medical factor that would have influenced the colonoscopy and required risk stratification for bowel preparations or special provision before their test, such as stopping anticoagulants or iron. Moreover, the new service did not list 21 patients as they were considered unfit and the 6-month mortality in this group was considerable at 14% compared with 0% in the listed group. This illustrates the importance of this new service in improving safety by identifying patients at risk from bowel cleansing agents and also in potentially preventing procedural mortality by not listing unfit patients.
The new service had a significant impact in reducing the colonoscopy DNA rate in surveillance patients in comparison with those listed before the introduction of the service and was also significantly lower than in the symptomatic service; this trend is similar to the preassessment process in the national Bowel Cancer Screening Programme.6 Therefore, this new service improves efficiency by (1) identifying patients who need special consideration for colonoscopy before arrival so that special provisions can be made in advance, thus preventing delays or cancellations; (2) reducing the DNA rate; (3) improving the capacity of the service by filtering out inappropriate procedures.
Following the introduction of the NPSA guidance, the new surveillance guidelines and BSG consensus document4; we introduced a number of service redesign strategies for the lower gastrointestinal endoscopy service, of which the introduction of the nurse practitioner-led surveillance colonoscopy service was just one. Others have included: (1) collaboration with our renal doctors and pharmacy to change the bowel preparation protocol from exclusively use of picolax as an oral bowel cleansing agent for both flexible sigmoidoscopy and colonoscopy to a phosphate enema for the former and a polyethylene glycol-based preparation for colonoscopy5; (2) changing our method of referral to an electronic system with pathways, protocols, safety checks and bowel preparation prescription built in; (3) changing our administrative processes to include further safety checks before administration of the bowel preparation. The introduction of our new nurse practitioner-led surveillance service has been an integral part of compliance with the new surveillance category and intervals guidelines,1 2 and also has allowed compliance with the NPSA and BSG consensus guidelines for the safe use of oral bowel cleansing agents.3 4 It has allowed clinical validation and assessment of surveillance patients, prescription of the bowel preparation after assessment of fitness, preassessment renal and electrolyte measurements, risk stratification and bowel preparation by a healthcare professional.
The most significant outcome of this new service is that 43% of patients were assessed as not requiring their procedure. The most common reason was that they did not fit the criteria. Interestingly, the second most common reason was patient choice. It might be that this group of patients potentially would not attend for their procedure or that after a clinic consultation, they were better informed and decided that the risks outweighed the benefits of surveillance. Either way, these patients were identified early and thus improved the efficiency of the service by potentially reducing non-attendance and also improved quality. The service filtered out a significant number of patients who were unfit for bowel preparation or the procedure. The service may have prevented serious adverse events or cancellation of a procedure on the day of the test in these patients as well as potentially preventing procedural mortality.
The current Healthcare Resource Group tariff for colonoscopy is £420, and therefore, with 95 patients discharged directly from clinic without undergoing a colonoscopy savings to the primary care trust would be approximately £40 000 over 4 months, equating to £120 000 a year. The implications for the unit are improvement of capacity and waiting lists of the colonoscopy service by removal of these patients. In addition, 17% of patients had their procedure deferred by over 2 years which would improve these factors further.
In conclusion, we have shown that that an investment in a nurse practitioner-led surveillance service improves quality and safety of the service, and may even prevent procedure-related mortality as well as aiding compliance with the guidelines/guidance, and also improves efficiency of the colonoscopy service and is cost effective.
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cairns SR, Scholefield JH, Steele RJ, et al. : Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–90. [DOI] [PubMed] [Google Scholar]
- 2.NICE. Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn's Disease or Adenomas. London: NICE, 2011. [PubMed] [Google Scholar]
- 3.NPSA. Reducing Risk of Harm from Oral Bowel Cleansing Solutions. Lonfon: NPSA, 2009. [Google Scholar]
- 4.Royal college of Surgeons, BSG, BESGAR, Renal Association, Royal College of Radiologists. Consensus Guidelines for the Prescription and Administration of Oral Bowel Cleansing Agents, 2009. [Google Scholar]
- 5.Flanagan PK, Ahmed MS, Tin SM, et al. Service implications of implementing guidance for oral bowel cleansing agents in colonoscopy. J R Coll Physicians Edinb 2011;41:100–5. [DOI] [PubMed] [Google Scholar]
- 6.Merseyside and Cheshire. Bowel Cancer Screening, 2008. [Google Scholar]