Abstract
Imaging of Crohn's disease of the small bowel is gradually moving away from barium fluoroscopy and towards cross-sectional modalities. This review explores the strengths and limitations of various techniques, and focuses on several current questions in small bowel imaging, such as the comparison between oral ingestion or nasojejunal intubation and enteroclysis for introduction of contrast, the use of computerised tomography (CT) versus magnetic resonance (MR) and the likely changes over the next decade.
Over the last decade, rapid technological advances in cross-sectional imaging modalities have combined with increased numbers of scanners to enable multiple new means of imaging the small bowel. The previously established position of small bowel barium enteroclysis (SBE) and barium follow-through (BFT) is gradually being shifted towards MR enteroclysis and enterography, and CT enteroclysis and enterography. This article will explore several current questions in imaging of small bowel Crohn's, evaluating the relative merits of the different imaging options, plus their limitations and disadvantages. Ultrasound and wireless capsule endoscopy (WCE) for investigation of the small bowel will be covered in separate articles.
Current important questions in radiological imaging of small bowel Crohn's include:
When should we use conventional barium techniques and when cross-sectional techniques?
When should we employ MR enteroclysis or enterography as opposed to CT enteroclysis or enterography?
Should we use nasojejunal intubation or oral ingestion of contrast?
How do ultrasound and capsule endoscopy fit in?
When should we use conventional barium techniques and when cross-sectional techniques?
The recently updated European evidence-based Consensus on the diagnosis and management of Crohn's disease states that ‘For suspected Crohn's disease, ileocolonoscopy and biopsies from the terminal ileum as well as each colonic segment to look for microscopic evidence of Crohn's disease are first line procedures to establish the diagnosis. Irrespective of the findings at ileocolonoscopy, further investigation is recommended to examine the location and extent of any Crohn's disease in the upper gastrointestinal tract or small bowel.’1 Imaging is used at the initial diagnosis and clinical relapse of Crohn's, mainly in an outpatient setting.1 2 The technical factors affecting the performance of different imaging modalities are summarised in table 1, and table 2 summarises the effect of this on diagnosing features of Crohn's. Barium techniques outperform cross-sectional in fine mucosal detail (figures 1 and 2). One must, however, question the clinical relevance of this advantage. The earliest signs of Crohn's terminal ileitis may be less visible on MR and CT than fluoroscopy, but are generally available to direct inspection at endoscopy, which is even more sensitive than barium and generally performed in combination with small bowel radiology.3–5
Table 1.
Technical factors affecting the performance of different radiological modalities
| Barium | MR | CT | |
|---|---|---|---|
| Spatial resolution (fine mucosal detail) | +++ | ++ | ++ |
| Contrast resolution (soft tissue visibility) | + | +++ | ++ |
| Use of intravenous contrast (enables assessment of bowel enhancement pattern) | No | Yes | Yes |
| Missed pathology due to overlapping loops | Yes | No | No |
Table 2.
| SBE/BFT | MR enteroclysis/enterography | CT enteroclysis/enterography | |
|---|---|---|---|
| Mucosal detail | +++ | ++ | ++ |
| Transmural inflammation | + | +++ | +++ |
| Fistulation | + | +++ | +++ |
| Extra-luminal changes | − | +++ | +++ |
| Disease activity | + | ++ | ++ |
BFT, barium follow-through; SBE, small bowel barium enteroclysis.
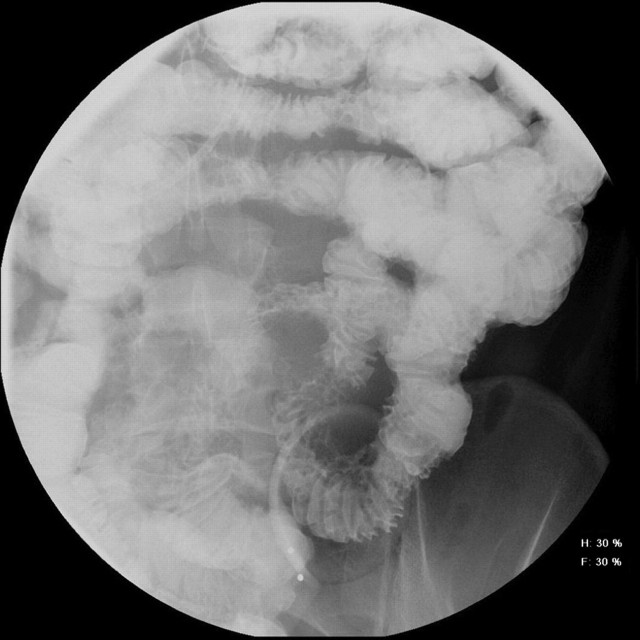
Figure 1.
Excellent mucosal detail on a barium enteroclysis demonstrates nodular jejunal Crohn's.
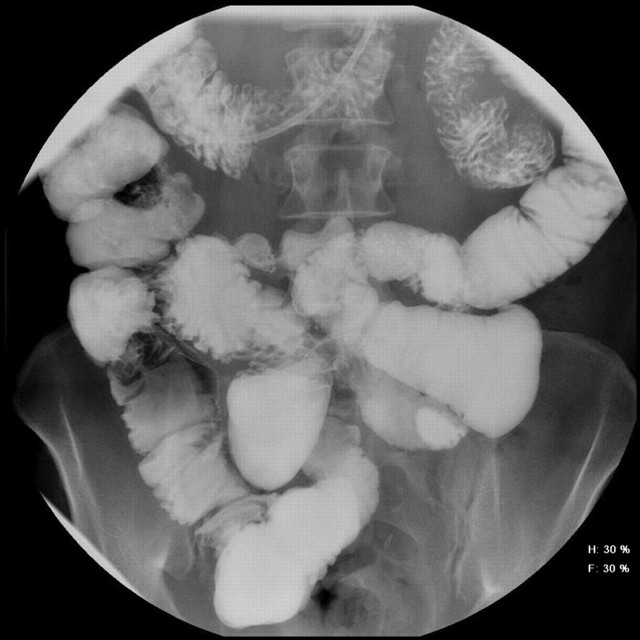
Figure 2.
Excellent mucosal detail on a barium enteroclysis, but the tight jejunal stricture and ulceration are obscured by overlapping bowel loops (same patient as figure 3).
Cross-sectional modalities outperform barium techniques in the clinically critical distinction between active, inflammatory and chronic fibrotic disease, and in detection of complications, such as fistula and abscess, because of their ability to see beyond the bowel mucosa and assess the degree and pattern of contrast enhancement (figures 3–9).6–17 Table 3 lists the features associated with active, inflammatory or with chronic, fibrotic disease.
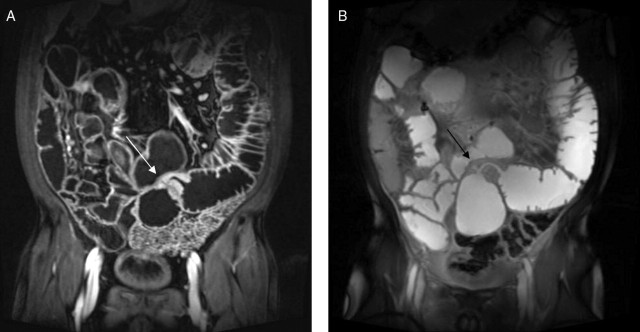
Figure 3.
Coronal MR enteroclysis: (A) T1 fat-saturated, contrast-enhanced and (B) T2 images demonstrating a tight jejunal stricture with a deep ulcer (arrows) (same patient as figure 2).
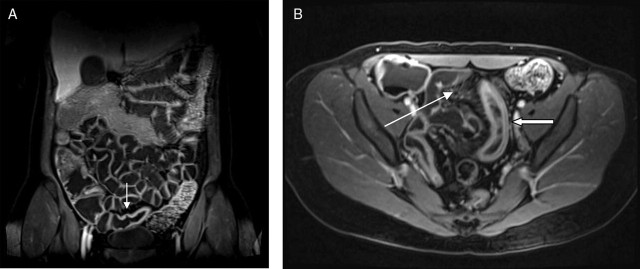
Figure 4.
(A) Coronal and (B) axial T1 fat-saturated, contrast-enhanced T1 MR enteroclysis images demonstrating thickened ileal wall with laminar enhancement (thick arrow), congestion of the vasa vasorum (comb sign, long, thin arrow) and deep ulcers (short, thin arrow), features of active, inflammatory disease.
Figure 5.
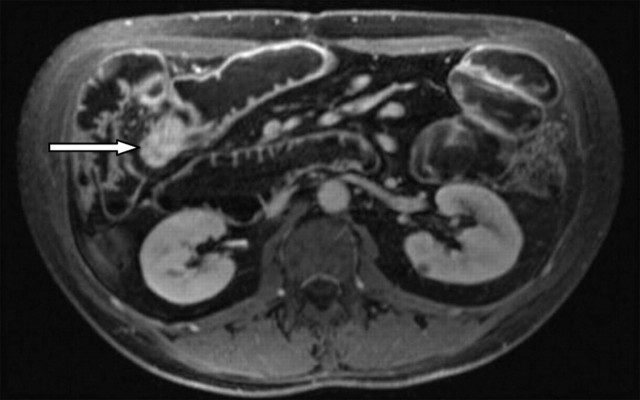
CT enteroclysis contrast-enhanced axial image demonstrating laminar enhancement of thickened terminal ileal wall (arrow), features of active inflammatory disease.
Figure 6.
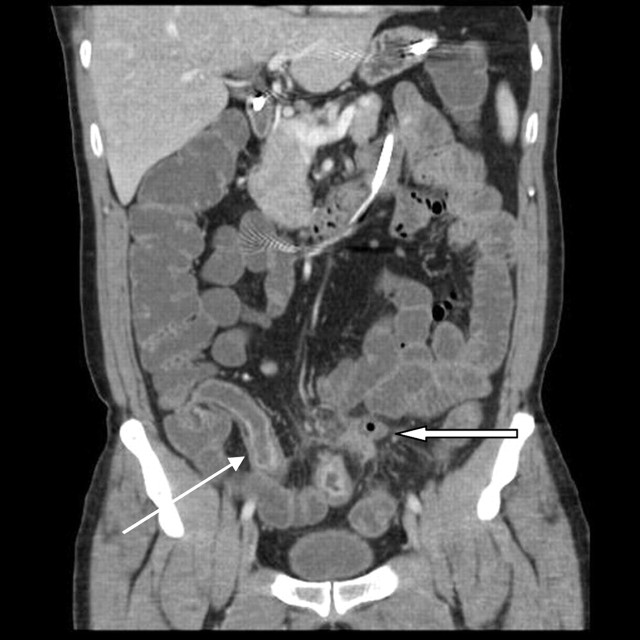
Coronal reformatted image of contrast-enhanced CT enteroclysis demonstrating laminar enhancement of thickened terminal ileal wall (thin arrow), with a small abscess in the mesentery (thick arrow).
Figure 7.
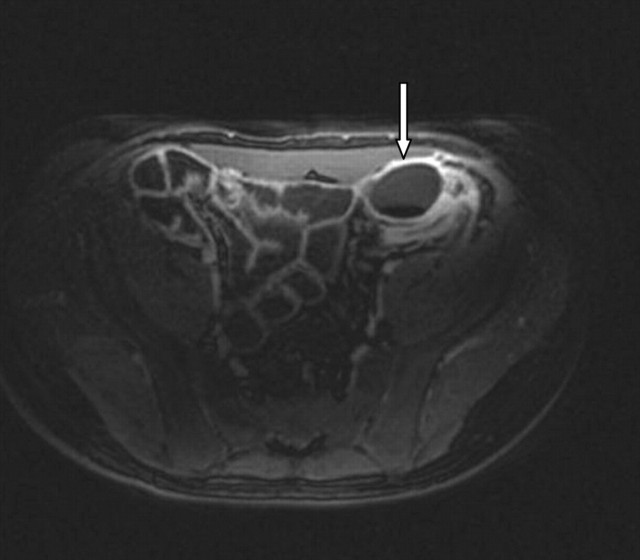
Axial T1 fat-saturated, contrast-enhanced image of a pericolic abscess (arrow) detected on an MR enteroclysis looking for small bowel Crohn's.
Figure 8.
Axial T1 fat-saturated, contrast-enhanced MR enteroclysis image demonstrating a sinus (arrow) into the jejunal mesentery.
Figure 9.
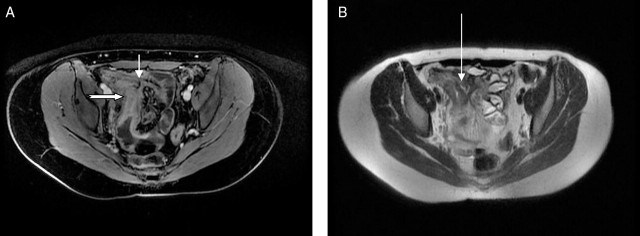
Axial MR enteroclysis demonstrating an ileo-ileal fistula. (A) T1 fat-saturated, contrast-enhanced image demonstrating an inflamed terminal ileum (thick arrow) with a fistula passing to another ileal loop (short, thin arrow). (B) T2 image demonstrating a trace of fluid within the fistula (long, thin arrow).
Table 3.
Features at cross-sectional imaging associated with active, inflammatory and chronic, fibrotic disease patterns7 12–17 50 52
| Active, inflammatory | Chronic | |
|---|---|---|
| Wall thickening >4 mm | + | + |
| Wall oedema* | + | |
| Wall submucosal fat* | + | |
| Increased wall enhancement | + (marked, often stratified) | + (mild) |
| Ulceration | + | |
| Mesenteric vascular engorgement (comb sign) | + | |
| Mesenteric oedema | + | |
| Abnormally enhancing mesenteric nodes | + |
MR is superior to CT for distinguishing between wall oedema and fat.
Cross-sectional techniques, at least with enteroclysis, are superior to barium in detection of disease proximal to the terminal ileum (figures 2 and 3).18
From a patient's perspective, the preparation is similar for most of these examinations. Fasting for a few hours prior to the procedure, often combined with a restricted diet the day before, is common. CT and MR involve usually two intravenous injections (buscopan and intravenous contrast), which are not used at fluoroscopy. Renal impairment (relative contra-indication to intravenous contrast) is seldom a problem in this patient cohort. In addition, the pragmatics of moving a patient from fluoroscopy, if performing enteroclysis, to CT or MR for image acquisition generally means a longer time spent at the hospital for the cross-sectional modalities. For the oral techniques, barium tastes somewhat better than most of the substances used for MR and CT. These do not seem to be significant problems for most patients.
If the cross-sectional modalities have so many advantages over barium, what is limiting their employment? Many gastroenterologists and surgeons may have trained in an era of barium radiology and may be more confident in interpreting these images, so they prefer to request SBE/BFT. However, increasing exposure of medical students and junior doctors means that they demonstrate increasing competence in interpreting cross-sectional anatomy, and it is likely that this will translate into a preference in their requesting behaviour as consultants over the next decade (radiation doses are discussed in the next section).
The major limitation in shift to cross-sectional modalities is due to a combination of local radiological expertise, access to scanner time and funding. The traditional specialisation of radiologists according to modality, such as fluoroscopy, has been increasingly replaced by specialisation by system, such as gastrointestinal radiology, over the last decade. Current trainees completing training with gastrointestinal specialisation are likely to be fully conversant with CT and MR, as well as ultrasound. Fluoroscopy is actually dwindling. Being trained in the fluoroscopic appearances of small bowel Crohn's and the CT/MR appearances of fistulae and abscesses means that employing CT and MR to look for the same findings is a relatively easy step. There are no good data on consistency of quality in this field, but reported inter-observer agreement for MR enteroclysis, MR enterography and CT enterography is good.7 8 19 20 There are technical requirements for performance of small bowel imaging on CT and MR (multislice CT, good gradients and software on MR), but most departments have adequate equipment for one or both.
The costing and funding of radiological procedures is an obscure art! The ‘cost’ of performing an investigation is derived locally within trusts, and depends on many factors, such as numbers of procedures performed, equipment and staff time. A shift from, for example, SBE to MR enteroclysis could result in each remaining SBE actually ‘costing’ more: the equipment and staff are still present even if the room is used less frequently. However, National Health Service (NHS) finance structures tend not to reflect these changes rapidly. In our department, the cost of an MR enteroclysis (£268) is slightly higher than an MR enterography or BFT (both £241). An SBE is lower at £195 and a CT enteroclysis costs just £133. These numbers could be radically different in different departments. For example, in one other district general hospital in England, small bowel enema and BFT both cost £94, CT enteroclysis £131 and MR enterography £195, the other examinations not being offered (personal communication, Craig Forster, Head of Radiology, Royal United Hospital Bath NHS Trust). Diagnostic imaging, after a brief ‘fling’ of unbundling in the 2009–2010 financial year, is now re-bundled into inpatient and outpatient tariffs. There is no specific Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures (OPCS)code for MR enteroclysis or enterography. An MRI ‘requiring extensive patient repositioning and/or more than one contrast agent’ attracts reimbursement of £298 multiplied by the local Market Forces Factor (a sort of ‘London weighting’ for fees). This would be the reimbursement from the primary care trust for a general practitioner direct access MR enteroclysis. However, since small bowel imaging is a specialist area, it tends not to be done via general practitioner direct access, and so is bundled into the in/outpatient tariff (£268 for the first gastroenterology appointment). Hence, the combination of the local cost of each procedure, plus limitations in access with waiting time targets, will dictate the ability of departments to provide these examinations, even assuming local expertise.
In the emergency setting, with suspected perforation, abscess or high grade small bowel obstruction, a conventional contrast-enhanced CT of the abdomen is adequate and most easily organised.
When should we employ MR enteroclysis or enterography as opposed to CT enteroclysis or enterography?
There is little literature on the performance of MR enteroclysis or enterography compared with CT enteroclysis or enterography.21–23 What evidence is available suggests little difference in Crohn's disease. Horsthuis et al23 performed a large meta-analysis of prospective studies employing ultrasound, MR, scintigraphy or CT in inflammatory bowel disease. This included studies not specifically involving enteroclysis or enterography techniques. On a per-patient basis, the mean sensitivity estimates for the diagnosis of inflammatory bowel disease were high (84.3–93%) for all four modalities. Mean per-patient specificity estimates were high (92.8–95.6%) and not significantly different for US, MR and CT. CT was, however, less sensitive and less specific on a per-bowel-segment basis.
The main and critical difference between MR and CT is in radiation dose. MR enterography uses no ionising radiation whatsoever. MR enteroclysis requires only a trivial dose for fluoroscopic guidance of nasojejunal intubation (0.005–0.01 mSv in our institution, personal data, 2005). CT enteroclysis and enterography involve a large radiation dose, which will vary with scanner and technique but usually lies around 10–15 mSv.24 This should be compared with the dose from BFT and SBE of around 1.5–6 mSv.25 26 The lifetime risk of induction of a fatal cancer from irradiation of an adult patient is 1 in 20 000 per mSv and may vary with the age and sex of the patient.27 28 The cumulative effective radiation dose was over 75 mSv in 15% Crohn's patients in one study.29 Thus, while a shift from barium techniques to MR practically removes the risks of the radiation, a shift instead to CT increases radiation burden dramatically. Taking into consideration the necessity of repeated examinations over the years in often young patients, this is a very strong argument in favour of MR techniques, and a counsel of caution in transferring all barium examinations to CT where MR access is limited.
In an acutely unwell patient, with clinical suspicion of a perforation or abscess, the requirement for prompt imaging may necessitate CT, and a contrast-enhanced CT with oral contrast should suffice. CT enteroclysis or enterography could be useful in the minority of patients who are claustrophobic, or have other contra-indications to MR such as metallic foreign bodies.
Should we use nasojejunal intubation or oral ingestion of contrast?
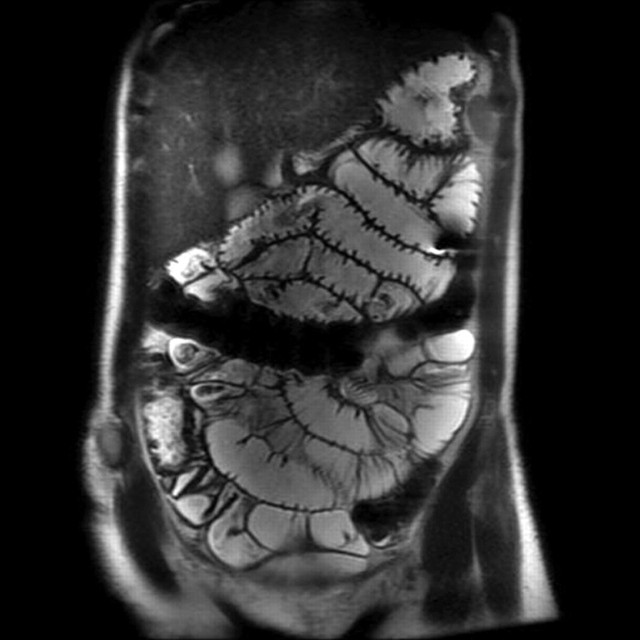
There is no doubt that most patients dislike nasojejunal intubation.30 There is also no doubt that demonstration of abnormality of the small bowel is made with more confidence in distended bowel, and that jejunal distension, at least, is significantly better using enteroclysis techniques (figures 10–12).6 19 31 32 However, on a per patient basis, the clinical significance of this difference is difficult to demonstrate.19 33 34 This could be because there is no clinical significance, but is more likely because the number of patients with, for example, isolated and non-obstructive (obstructing lesions will usually be adequately demonstrated without intubation) jejunal Crohn's disease is very low, and the existing studies have not been powered to detect this difference. Of note, Horsthuis et al's23 meta-analysis did demonstrate a significantly higher sensitivity of MR enteroclysis above MR enterography in diagnosing inflammatory bowel disease. It then becomes something of a philosophical question as to whether the discomfort of intubation is justified. Pragmatists may favour oral ingestion, while purists may favour intubation. Most departments will carry over their historical fluoroscopic preference for either nasojejunal intubation or oral ingestion to CT and MR examination of the small bowel.
Figure 10.
Coronal T2 image from an MR enteroclysis demonstrating excellent jejunal distension and detail.
Figure 11.
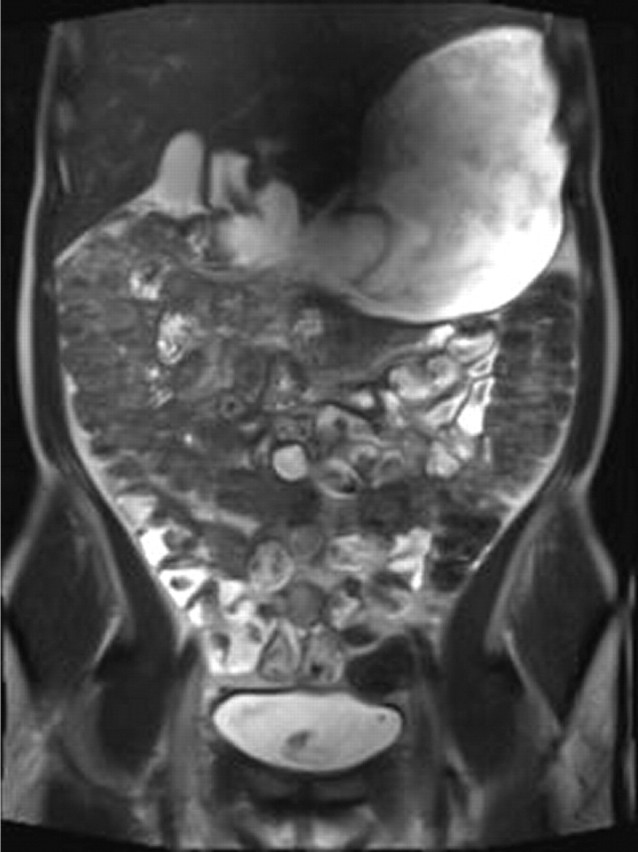
Coronal T2 image from an MR enterographydemonstrating collapsed jejunal loops, which could obscure pathology. Much of the ingested contrast is in the stomach and colon.
Figure 12.

Coronal T2 image from an MR enteroclysis demonstrating nodular jejunal Crohn's in well-distended jejunal loops (arrow). MRE, magnetic resonance enteroclysis/enterography.
Classically, it has been thought that Crohn's disease affects small bowel proximal to the terminal ileum in about 10% of patients.1 However, WCE detects more disease here than other modalities.35–37 Given the difficulty of detection, the true prevalence here is not clear. In one study comparing SBE and MR enteroclysis, jejunal disease was detected in approximately half the patients.18 The clinical importance of detection of mild, non-obstructive jejunal disease should be used to guide the decision to perform enteroclysis or enterography.
There are few data available on which tests are actually being performed in the UK. A recent postal survey of UK centres' use of small bowel imaging investigations yielded only a 27% response rate.38 The results are, therefore, difficult to extrapolate. BFT remains the most commonly employed investigation for suspected Crohn's and follow-up, with CT for suspected extra-luminal complications. MR and ultrasound are used less frequently, mainly in younger patients. CT enteroclysis and MR enteroclysis are available in only 11% of responding departments, mainly teaching hospitals. If radiologists with a special interest are more likely to respond to postal surveys, then the real dissemination of these techniques nationwide could be even lower.
How do ultrasound and capsule endoscopy fit in?
Ultrasound is a very user dependent modality, and convincing illustrative demonstration of pathology to referrers is much more difficult than with other radiological techniques. However, in expert hands, ultrasound is capable of demonstrating the features of Crohn's and is especially reliable for terminal ileitis, less so for proximal lesions. This is discussed in a separate article in this edition. Ultrasound expertise for small bowel imaging does tend to be limited to a few enthusiastic centres, and lateral dissemination has been slow.
WCE is more sensitive than barium, CT or MR for the demonstration of early mucosal abnormalities in Crohn's.35–37 39 However, the clinical significance of minor mucosal lesions identified at WCE is sometimes uncertain, with frequent findings in asymptomatic individuals.40 WCE does appear more sensitive in detection of lesions proximal to the terminal ileum.35 37 41 The risk of capsule impaction at strictures can be avoided by prior radiological exclusion of strictures or by a dummy capsule run. One advantage of the radiological examinations is the elegant demonstration of the anatomical distribution of disease not gained at WCE. However, these should be seen as complementary examinations. Where radiology has been equivocal, or unexpectedly normal, a WCE can clarify and reveal more subtle changes.
Summary and the future
Barium radiology has probably had its day. There is little prospect of significant technological progress, and radiology trainees are becoming more competent in other modalities. Fluoroscopy remains a useful problem solving technique, but routine imaging of the small bowel should move to cross-sectional modalities over the next decade. In departments where there is relatively easy access to CT and MR scanner time for body investigations, and where local health finances support patient-centred best practice, then the advantages of demonstration of transmural and extraintestinal abnormalities plus more accurate distinction between active, inflammatory and chronic, fibrotic disease mean that we should choose MR enteroclysis or enterography and CT enteroclysis or enterography above SBE/BFT in most Crohn's patients, at initial diagnosis and staging of extent and in recurrence/relapse. Some progress is being made in low dose techniques to reduce the radiation from CT examinations.42 However, the reduced radiation burden from MR means this should be the technique of choice. MR will develop further, with definition of the roles of MR fluoroscopic sequences to define stricture morphology, as well as diffusion and perfusion techniques. CT should ideally be confined to imaging of suspected acute complications, and patients in whom MR is contraindicated. The reality, of limited access to scanner time and funding difficulties, means that many departments currently either do not offer the cross-sectional techniques, or only in selected patients, such as the very young with complex disease requiring multiple investigations. One should certainly endeavour not to perform double investigations, with a barium study to look at the mucosa and a CT to exclude an abscess, but combine into one cross-sectional study. It must be hoped that future costs will truly reflect the expense, but also the value, of more specialised radiological procedures, enabling a genuine ‘market’, perhaps with a minority of centres offering a tertiary service at a sustainable rate.
Acknowledgments
I am grateful to Sue Altman, Programme Manager, Department of Commissioning and Performance, for information regarding NHS financing structures.
Footnotes
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
Contributors: HB is the sole contributor to this article.
References
- 1.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 3.Lipson A, Bartram CI, Williams CB, et al. Barium studies and ileoscopy compared in children with suspected Crohn's disease. Clin Radiol 1990;41:5–8. [DOI] [PubMed] [Google Scholar]
- 4.Tribl B, Turetschek K, Mostbeck G, et al. Conflicting results of ileoscopy and small bowel double-contrast barium examination in patients with Crohn's disease. Endoscopy 1998;30:339–44. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JK, Cawdron R, Zealley I, et al. Prospective comparison of small bowel meal with pneumocolon versus ileo-colonoscopy for the diagnosis of ileal Crohn's disease. Am J Gastroenterol 2004;99:1321–9. [DOI] [PubMed] [Google Scholar]
- 6.Masselli G, Casciani E, Polettini E, et al. Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn's disease. Eur Radiol 2008;18:438–47. [DOI] [PubMed] [Google Scholar]
- 7.Maccioni F, Bruni A, Viscido A, et al. MR imaging in patients with Crohn disease: value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology 2006;238:517–30. [DOI] [PubMed] [Google Scholar]
- 8.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology 2009;251:751–61. [DOI] [PubMed] [Google Scholar]
- 9.Masselli G, Casciani E, Polettini E, et al. Assessment of Crohn's disease in the small bowel: prospective comparison of magnetic resonance enteroclysis with conventional enteroclysis. Eur Radiol 2006;16:2817–27. [DOI] [PubMed] [Google Scholar]
- 10.Rieber A, Wruk D, Potthast S, et al. Diagnostic imaging in Crohn's disease: comparison of magnetic resonance imaging and conventional imaging methods. Int J Colorectal Dis 2000;15:176–81. [DOI] [PubMed] [Google Scholar]
- 11.Rieber A, Aschoff A, Nüssle K, et al. MRI in the diagnosis of small bowel disease: use of positive and negative oral contrast media in combination with enteroclysis. Eur Radiol 2000;10:1377–82. [DOI] [PubMed] [Google Scholar]
- 12.Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology 2009;252:712–20. [DOI] [PubMed] [Google Scholar]
- 13.Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn's disease. AJR Am J Roentgenol 2001;177:1325–32. [DOI] [PubMed] [Google Scholar]
- 14.Bodily KD, Fletcher JG, Solem CA, et al. Crohn Disease: mural attenuation and thickness at contrast-enhanced CT Enterography – correlation with endoscopic and histologic findings of inflammation. Radiology 2006;238:505–16. [DOI] [PubMed] [Google Scholar]
- 15.Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, et al. Assessment of Crohn's disease activity in the small bowel with MR and conventional enteroclysis: preliminary results. Eur Radiol 2004;14:1017–24. [DOI] [PubMed] [Google Scholar]
- 16.Florie J, Wasser MN, Arts-Cieslik K, et al. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn's disease. AJR Am J Roentgenol 2006;186:1384–92. [DOI] [PubMed] [Google Scholar]
- 17.Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut 2009;58:1113–20. [DOI] [PubMed] [Google Scholar]
- 18.Ochsenkühn T, Herrmann K, Schoenberg SO, et al. Crohn disease of the small bowel proximal to the terminal ileum: detection by MR-enteroclysis. Scand J Gastroenterol 2004;39:953–60. [DOI] [PubMed] [Google Scholar]
- 19.Negaard A, Paulsen V, Sandvik L, et al. A prospective randomized comparison between two MRI studies of the small bowel in Crohn's disease, the oral contrast method and MR enteroclysis. Eur Radiol 2007;17:2294–301. [DOI] [PubMed] [Google Scholar]
- 20.Booya F, Fletcher JG, Huprich JE, et al. Active Crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology 2006;241:787–95. [DOI] [PubMed] [Google Scholar]
- 21.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn's disease. AJR Am J Roentgenol 2009;193:113–21. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt S, Lepori D, Meuwly JY, et al. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of ‘sign-by-sign’ correlation. Eur Radiol 2003;13:1303–11. [DOI] [PubMed] [Google Scholar]
- 23.Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64–79. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe TA, Gaca AM, Delaney S, et al. Radiation doses from small-bowel follow-through and abdominopelvic MDCT in Crohn's disease. AJR Am J Roentgenol 2007;189:1015–22. [DOI] [PubMed] [Google Scholar]
- 25.Hart D, Haggett PJ, Boardman P, et al. Patient radiation doses from enteroclysis examinations. Br J Radiol 1994;67:997–1000. [DOI] [PubMed] [Google Scholar]
- 26.Mettler FA, Jr, Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248:254–63. [DOI] [PubMed] [Google Scholar]
- 27.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 2007:37:1–332. [DOI] [PubMed] [Google Scholar]
- 28.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol 2008;81:362–78. [DOI] [PubMed] [Google Scholar]
- 29.Desmond AN, O'Regan K, Curran C, et al. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut 2008;57:1524–9. [DOI] [PubMed] [Google Scholar]
- 30.Negaard A, Sandvik L, Berstad AE, et al. MRI of the small bowel with oral contrast or nasojejunal intubation in Crohn's disease: randomized comparison of patient acceptance. Scand J Gastroenterol 2008;43:44–51. [DOI] [PubMed] [Google Scholar]
- 31.Mazzeo S, Caramella D, Belcari A, et al. Multidetector CT of the small bowel: evaluation after oral hyperhydration with isotonic solution. Radiol Med 2005;109:516–26. [PubMed] [Google Scholar]
- 32.Maglinte DD, Kelvin FM, O'Connor K, et al. Current status of small bowel radiography. Abdom Imaging 1996;21:247–57. [DOI] [PubMed] [Google Scholar]
- 33.Toms AP, Barltrop A, Freeman AH. A prospective randomised study comparing enteroclysis with small bowel follow-through examinations in 244 patients. Eur Radiol 2001;11:1155–60. [DOI] [PubMed] [Google Scholar]
- 34.Schreyer AG, Geissler A, Albrich H, et al. Abdominal MRI after enteroclysis or with oral contrast in patients with suspected or proven Crohn's disease. Clin Gastroenterol Hepatol 2004;2:491–7. [DOI] [PubMed] [Google Scholar]
- 35.Hara AK, Leighton JA, Heigh RI, et al. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology 2006;238:128–34. [DOI] [PubMed] [Google Scholar]
- 36.Liangpunsakul S, Chadalawada V, Rex DK, et al. Wireless capsule endoscopy detects small bowel ulcers in patients with normal results from state of the art enteroclysis. Am J Gastroenterol 2003;98:1295–8. [DOI] [PubMed] [Google Scholar]
- 37.Gölder SK, Schreyer AG, Endlicher E, et al. Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis 2006;21:97–104. [DOI] [PubMed] [Google Scholar]
- 38.Hafeez R, Greenhalgh R, Rajan J, et al. Use of small bowel imaging for the diagnosis and staging of Crohn's disease – a survey of current UK practice. Br J Radiol 2011;84:508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn's disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut 2005;54:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 2005;3:133–41. [DOI] [PubMed] [Google Scholar]
- 41.Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn's disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut 2005;54:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kambadakone AR, Prakash P, Hahn PF, et al. Low-dose CT examinations in Crohn's disease: impact on image quality, diagnostic performance, and radiation dose. AJR Am J Roentgenol 2010;195:78–88. [DOI] [PubMed] [Google Scholar]
- 43.Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, et al. Imaging of small intestinal Crohn's disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol 2006;16:1915–25. [DOI] [PubMed] [Google Scholar]
- 44.Umschaden HW, Szolar D, Gasser J, et al. Small-bowel disease: comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology 2000;215:717–25. [DOI] [PubMed] [Google Scholar]
- 45.Raptopoulos V, Schwartz RK, McNicholas MM, et al. Multiplanar helical CT enterography in patients with Crohn's disease. AJR Am J Roentgenol 1997;169:1545–50. [DOI] [PubMed] [Google Scholar]
- 46.Sailer J, Peloschek P, Reinisch W, et al. Anastomotic recurrence of Crohn's disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol 2008;18:2512–21. [DOI] [PubMed] [Google Scholar]
- 47.Maglinte DD, Chernish SM, Kelvin FM, et al. Crohn disease of the small intestine: accuracy and relevance of enteroclysis. Radiology 1992;184:541–5. [DOI] [PubMed] [Google Scholar]
- 48.Cirillo LC, Camera L, Della Noce M, et al. Accuracy of enteroclysis in Crohn's disease of the small bowel: a retrospective study. Eur Radiol 2000;10:1894–8. [DOI] [PubMed] [Google Scholar]
- 49.Barloon TJ, Lu CC, Honda H, et al. Does a normal small-bowel enteroclysis exclude small-bowel disease? A long-term follow-up of consecutive normal studies. Abdom Imaging 1994;19:113–15. [DOI] [PubMed] [Google Scholar]
- 50.Malagò R, Manfredi R, Benini L, et al. Assessment of Crohn's disease activity in the small bowel with MR-enteroclysis: clinico-radiological correlations. Abdom Imaging 2008;33:669–75. [DOI] [PubMed] [Google Scholar]
- 51.Hara AK, Alam S, Heigh RI, et al. Using CT enterography to monitor Crohn's disease activity: a preliminary study. AJR Am J Roentgenol 2008;190:1512–16. [DOI] [PubMed] [Google Scholar]
- 52.Maccioni F, Viscido A, Broglia L, et al. Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging 2000;25:219–28. [DOI] [PubMed] [Google Scholar]