Abstract
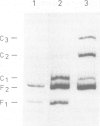
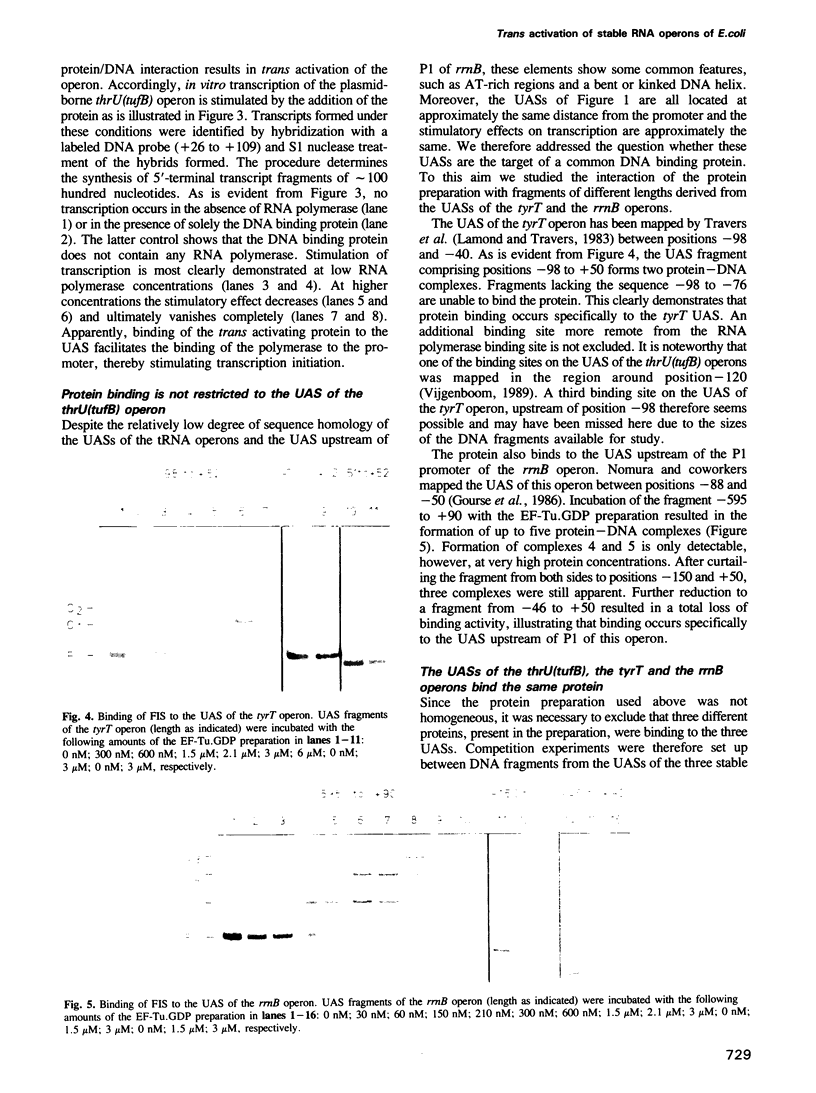
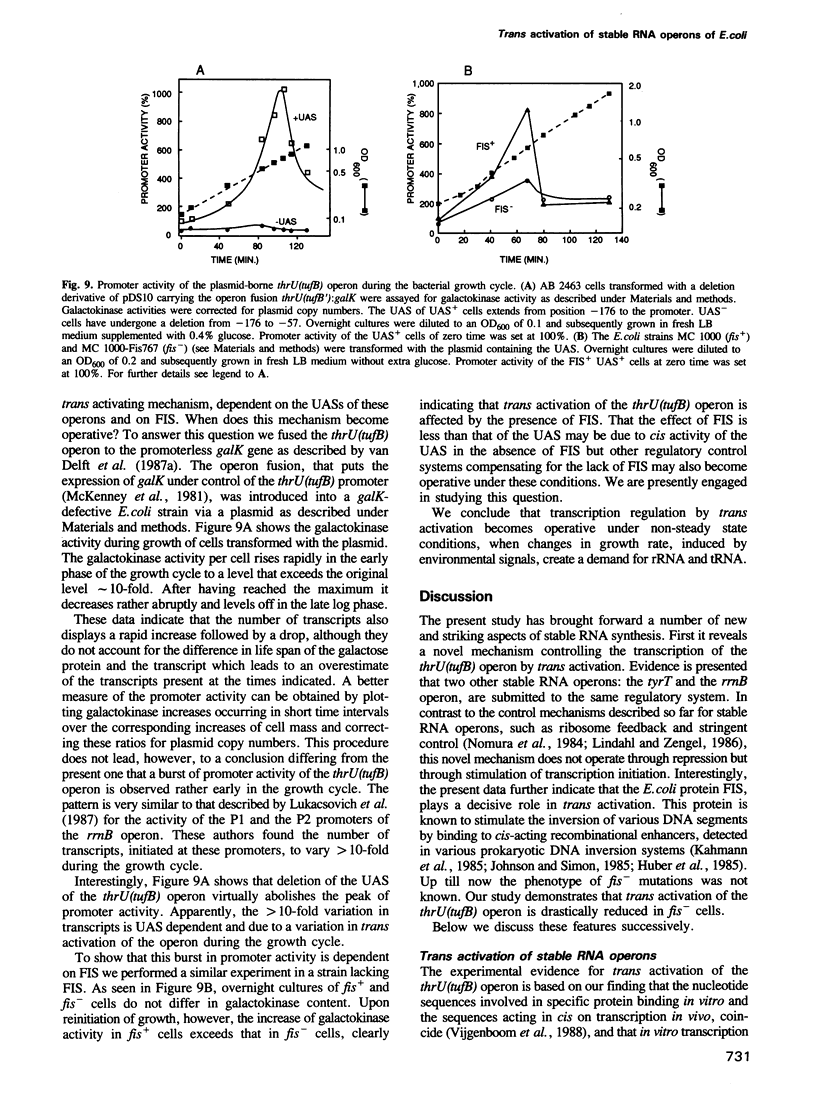
The thrU(tufB) operon of Escherichia coli is endowed with a cis-acting region upstream of the promoter, designated UAS for Upstream Activator Sequence. A protein fraction has been isolated that binds specifically to DNA fragments of the UAS, thus forming three protein-DNA complexes corresponding to three binding sites on the UAS. It stimulates in vitro transcription of the operon by facilitating the binding of the RNA polymerase to the promoter. All three protein-DNA complexes contain one and the same protein. Dissociation constants for the three complexes have been determined, the lowest being in the sub-nanomolar range. The protein also binds to the UAS of the tyrT operon and to the UAS upstream of the P1 promoter of the rrnB operon, suggesting that transcription of the three operons, if not of more stable RNA operons, is activated by a common trans activator. We demonstrate that the E.coli protein FIS (Factor for Inversion Stimulation) also binds to the UAS of the thrU(tufB) operon forming three protein-DNA complexes. A burst of UAS- and FIS-dependent promoter activity is observed after reinitiation of growth of stationary cultures in fresh medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Hatfield G. W. Effects of promoter strengths and growth conditions on copy number of transcription-fusion vectors. J Biol Chem. 1984 Jun 25;259(12):7399–7403. [PubMed] [Google Scholar]
- Bauer B. F., Kar E. G., Elford R. M., Holmes W. M. Sequence determinants for promoter strength in the leuV operon of Escherichia coli. Gene. 1988;63(1):123–134. doi: 10.1016/0378-1119(88)90551-3. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Duester G., Elford R. M., Holmes W. M. Fusion of the Escherichia coli tRNALeu1 promoter to the galK gene: analysis of sequences necessary for growth-rate-dependent regulation. Cell. 1982 Oct;30(3):855–864. doi: 10.1016/0092-8674(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Gaal T., Barkei J., Dickson R. R., deBoer H. A., deHaseth P. L., Alavi H., Gourse R. L. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J Bacteriol. 1989 Sep;171(9):4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Haffter P., Bickle T. A. Purification and DNA-binding properties of FIS and Cin, two proteins required for the bacteriophage P1 site-specific recombination system, cin. J Mol Biol. 1987 Dec 20;198(4):579–587. doi: 10.1016/0022-2836(87)90201-4. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. E., Iida S., Arber W., Bickle T. A. Site-specific DNA inversion is enhanced by a DNA sequence element in cis. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3776–3780. doi: 10.1073/pnas.82.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner P., Arber W. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 1989 Feb;8(2):577–585. doi: 10.1002/j.1460-2075.1989.tb03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner P., Haffter P., Iida S., Arber W. Bent DNA is needed for recombinational enhancer activity in the site-specific recombination system Cin of bacteriophage P1. The role of FIS protein. J Mol Biol. 1989 Feb 5;205(3):493–500. doi: 10.1016/0022-2836(89)90220-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ball C. A., Pfeffer D., Simon M. I. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci U S A. 1988 May;85(10):3484–3488. doi: 10.1073/pnas.85.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Simon M. I. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985 Jul;41(3):781–791. doi: 10.1016/s0092-8674(85)80059-3. [DOI] [PubMed] [Google Scholar]
- Kahmann R., Rudt F., Koch C., Mertens G. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell. 1985 Jul;41(3):771–780. doi: 10.1016/s0092-8674(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Koch C., Kahmann R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15673–15678. [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Genetically separable functional elements mediate the optimal expression and stringent regulation of a bacterial tRNA gene. Cell. 1985 Feb;40(2):319–326. doi: 10.1016/0092-8674(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Requirement for an upstream element for optimal transcription of a bacterial tRNA gene. Nature. 1983 Sep 15;305(5931):248–250. doi: 10.1038/305248a0. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Lukacsovich T., Boros I., Venetianer P. New regulatory features of the promoters of an Escherichia coli rRNA gene. J Bacteriol. 1987 Jan;169(1):272–277. doi: 10.1128/jb.169.1.272-277.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacsovich T., Gaal T., Venetianer P. The structural basis of the high in vivo strength of the rRNA P2 promoter of Escherichia coli. Gene. 1989 May 15;78(1):189–194. doi: 10.1016/0378-1119(89)90328-4. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Moitoso de Vargas L., Koch C., Kahmann R., Landy A. Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway. Cell. 1987 Sep 11;50(6):901–908. doi: 10.1016/0092-8674(87)90516-2. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Van Delft J. H., Schmidt D. S., Bosch L. The tRNA-tufB operon transcription termination and processing upstream from tufB. J Mol Biol. 1987 Oct 20;197(4):647–657. doi: 10.1016/0022-2836(87)90471-2. [DOI] [PubMed] [Google Scholar]
- Vijgenboom E., Bosch L. Transfer of plasmid-borne tuf mutations to the chromosome as a genetic tool for studying the functioning of EF-TuA and EF-TuB in the E. coli cell. Biochimie. 1987 Oct;69(10):1021–1030. doi: 10.1016/0300-9084(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Vijgenboom E., Nilsson L., Bosch L. The elongation factor EF-Tu from E. coli binds to the upstream activator region of the tRNA-tufB operon. Nucleic Acids Res. 1988 Nov 11;16(21):10183–10197. doi: 10.1093/nar/16.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft J. H., Mariñon B., Schmidt D. S., Bosch L. Transcription of the tRNA-tufB operon of Escherichia coli: activation, termination and antitermination. Nucleic Acids Res. 1987 Nov 25;15(22):9515–9530. doi: 10.1093/nar/15.22.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]