Abstract
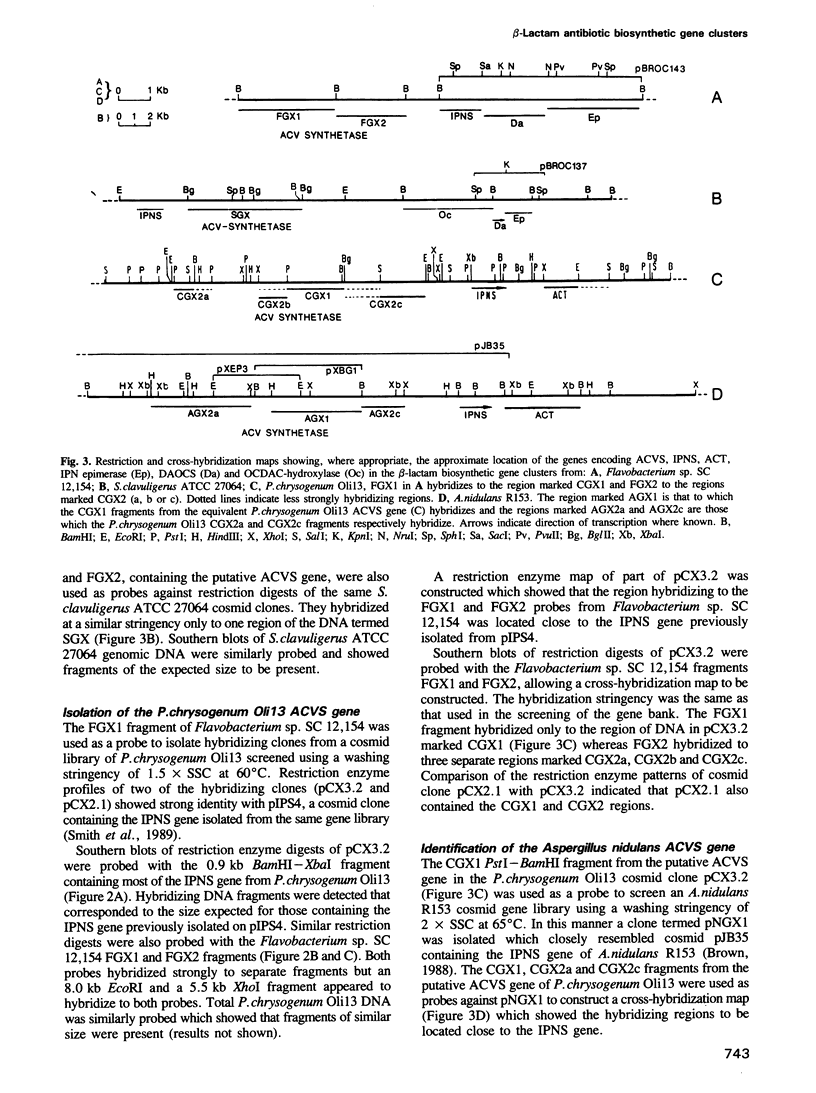
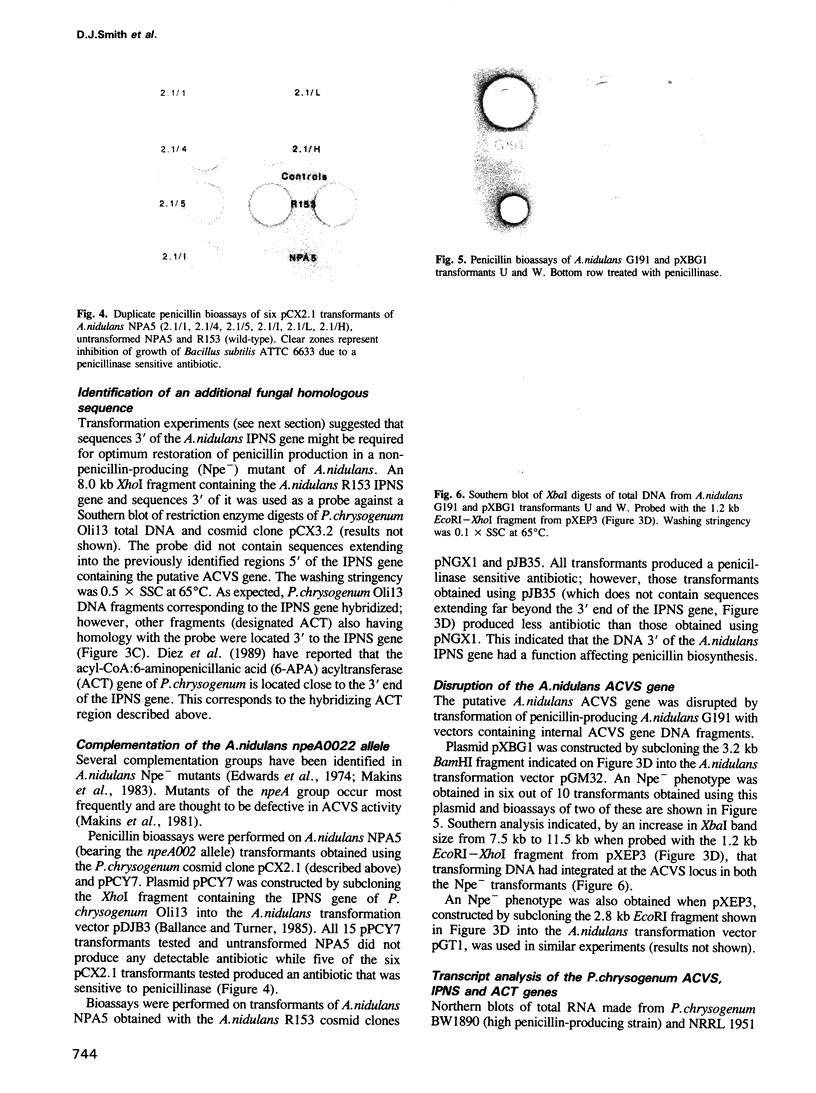
A cosmid clone containing closely linked beta-lactam antibiotic biosynthetic genes was isolated from a gene library of Flavobacterium sp. SC 12,154. The location within the cluster of the DNA thought to contain the gene for delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase (ACVS), the first step in the beta-lactam antibiotic biosynthetic pathway, was identified by a novel method. This DNA facilitated the isolation, by cross-hybridization, of the corresponding DNA from Streptomyces clavuligerus ATCC 27064, Penicillium chrysogenum Oli13 and Aspergillus nidulans R153. Evidence was obtained which confirmed that the cross-hybridizing sequences contained the ACVS gene. In each case the ACVS gene was found to be closely linked to other beta-lactam biosynthetic genes and constituted part of a gene cluster.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballance D. J., Turner G. Development of a high-frequency transforming vector for Aspergillus nidulans. Gene. 1985;36(3):321–331. doi: 10.1016/0378-1119(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Resistance, regulatory and production genes for the antibiotic methylenomycin are clustered. EMBO J. 1985 Jul;4(7):1893–1897. doi: 10.1002/j.1460-2075.1985.tb03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez B., Barredo J. L., Alvarez E., Cantoral J. M., van Solingen P., Groenen M. A., Veenstra A. E., Martín J. F. Two genes involved in penicillin biosynthesis are linked in a 5.1 kb SalI fragment in the genome of Penicillium chrysogenum. Mol Gen Genet. 1989 Sep;218(3):572–576. doi: 10.1007/BF00332426. [DOI] [PubMed] [Google Scholar]
- Edwards G. F., Holt G., Macdonald K. D. Mutants of Aspergillus nidulans impaired in penicillin biosynthesis. J Gen Microbiol. 1974 Oct;84(2):420–423. doi: 10.1099/00221287-84-2-420. [DOI] [PubMed] [Google Scholar]
- Frey J., Bagdasarian M., Feiss D., Franklin F. C., Deshusses J. Stable cosmid vectors that enable the introduction of cloned fragments into a wide range of gram-negative bacteria. Gene. 1983 Oct;24(2-3):299–308. doi: 10.1016/0378-1119(83)90090-2. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Queener S. W. Beta-lactam biosynthetic genes. Med Res Rev. 1989 Apr-Jun;9(2):245–264. doi: 10.1002/med.2610090206. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Analysis of penicillin N ring expansion activity from Streptomyces clavuligerus by ion-pair high-pressure liquid chromatography. Antimicrob Agents Chemother. 1983 Sep;24(3):307–312. doi: 10.1128/aac.24.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Partial purification and characterization of isopenicillin N epimerase activity from Streptomyces clavuligerus. Can J Microbiol. 1983 Nov;29(11):1526–1531. doi: 10.1139/m83-234. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kovacevic S., Weigel B. J., Tobin M. B., Ingolia T. D., Miller J. R. Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthetase. J Bacteriol. 1989 Feb;171(2):754–760. doi: 10.1128/jb.171.2.754-760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupka J., Shen Y. Q., Wolfe S., Demain A. L. Studies on the ring-cyclization and ring-expansion enzymes of beta-lactam biosynthesis in Cephalosporium acremonium. Can J Microbiol. 1983 May;29(5):488–496. doi: 10.1139/m83-078. [DOI] [PubMed] [Google Scholar]
- Leskiw B. K., Aharonowitz Y., Mevarech M., Wolfe S., Vining L. C., Westlake D. W., Jensen S. E. Cloning and nucleotide sequence determination of the isopenicillin N synthetase gene from Streptomyces clavuligerus. Gene. 1988;62(2):187–196. doi: 10.1016/0378-1119(88)90557-4. [DOI] [PubMed] [Google Scholar]
- Makins J. F., Allsop A., Holt G. Intergeneric cosynthesis of penicillin by strains of Penicillium chrysogenum, P. chrysogenum/notatum and Aspergillus nidulans. J Gen Microbiol. 1981 Feb;122(2):339–343. doi: 10.1099/00221287-122-2-339. [DOI] [PubMed] [Google Scholar]
- Makins J. F., Holt G., Macdonald K. D. The genetic location of three mutations impairing penicillin production in Aspergillus nidulans. J Gen Microbiol. 1983 Oct;129(10):3027–3033. doi: 10.1099/00221287-129-10-3027. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Normansell P. J., Normansell I. D., Holt G. Genetic and biochemical studies of mutants of Penicillium chrysogenum impaired in penicillin production. J Gen Microbiol. 1979 May;112(1):113–126. doi: 10.1099/00221287-112-1-113. [DOI] [PubMed] [Google Scholar]
- Nüesch J., Heim J., Treichler H. J. The biosynthesis of sulfur-containing beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:51–75. doi: 10.1146/annurev.mi.41.100187.000411. [DOI] [PubMed] [Google Scholar]
- Ramón D., Carramolino L., Patiño C., Sánchez F., Peñalva M. A. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the beta-lactam ring in Aspergillus nidulans. Gene. 1987;57(2-3):171–181. doi: 10.1016/0378-1119(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Shiffman D., Mevarech M., Jensen S. E., Cohen G., Aharonowitz Y. Cloning and comparative sequence analysis of the gene coding for isopenicillin N synthase in Streptomyces. Mol Gen Genet. 1988 Nov;214(3):562–569. doi: 10.1007/BF00330495. [DOI] [PubMed] [Google Scholar]
- Singh P. D., Ward P. C., Wells J. S., Ricca C. M., Trejo W. H., Principe P. A., Sykes R. B. Bacterial production of deacetoxycephalosporin C. J Antibiot (Tokyo) 1982 Oct;35(10):1397–1399. doi: 10.7164/antibiotics.35.1397. [DOI] [PubMed] [Google Scholar]
- Skatrud P. L., Queener S. W. An electrophoretic molecular karyotype for an industrial strain of Cephalosporium acremonium. Gene. 1989 May 30;78(2):331–338. doi: 10.1016/0378-1119(89)90235-7. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Bull J. H., Edwards J., Turner G. Amplification of the isopenicillin N synthetase gene in a strain of Penicillium chrysogenum producing high levels of penicillin. Mol Gen Genet. 1989 Apr;216(2-3):492–497. doi: 10.1007/BF00334395. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Edwards J., Earl A. J., Turner G. Cloning and heterologous expression of the penicillin biosynthetic gene cluster from penicillum chrysogenum. Biotechnology (N Y) 1990 Jan;8(1):39–41. doi: 10.1038/nbt0190-39. [DOI] [PubMed] [Google Scholar]
- Weigel B. J., Burgett S. G., Chen V. J., Skatrud P. L., Frolik C. A., Queener S. W., Ingolia T. D. Cloning and expression in Escherichia coli of isopenicillin N synthetase genes from Streptomyces lipmanii and Aspergillus nidulans. J Bacteriol. 1988 Sep;170(9):3817–3826. doi: 10.1128/jb.170.9.3817-3826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt H., von Döhren H., Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989 Mar 5;264(7):3680–3684. [PubMed] [Google Scholar]