Abstract
Objective
Stability and selectivity are important when restoring long-term, functional sensory feedback in individuals with limb-loss. Our objective is to demonstrate a chronic, clinical neural stimulation system for providing selective sensory response in two upper-limb amputees.
Approach
Multi-contact cuff electrodes were implanted in the median, ulnar, and radial nerves of the upper-limb.
Main results
Nerve stimulation produced a selective sensory response on 19 of 20 contacts and 16 of 16 contacts in subjects 1 and 2, respectively. Stimulation elicited multiple, distinct percept areas on the phantom and residual limb. Consistent threshold, impedance, and percept areas have demonstrated that the neural interface is stable for the duration of this on-going, chronic study.
Significance
We have achieved selective nerve response from multi-contact cuff electrodes by demonstrating characteristic percept areas and thresholds for each contact. Selective sensory response remains consistent in two upper-limb amputees for 1 and 2 years, the longest multi-contact sensory feedback system to date. Our approach demonstrates selectivity and stability can be achieved through an extraneural interface, which can provide sensory feedback to amputees.
Keywords: functional electrical stimulation (FES), nerve electrodes, peripheral nerve, selective stimulation, upper extremity neuroprostheses, sensory feedback, prosthetics
1. Introduction
An estimated 2 million people in the United States live with limb-loss [1]. Individuals with upper-limb loss highly desire natural sensory feedback in their prostheses [2, 3]. A permanent sensory feedback system may lead to improved control of the prosthetic hand [4, 5], increased sense of self [6], and elimination of uncomfortable phantom limb sensation [7, 8]. Although recent advances have demonstrated the feasibility of sensory feedback [9, 10], these studies have not demonstrated stability using a long-term neural interface in humans. For clinical acceptance, a nerve interface must be stable and capable of selectively producing sensory percepts at multiple locations. Stability and selectivity are important when restoring long-term and functional sensory feedback in physically active amputees.
Researchers have long investigated neural interfaces to provide natural sensory feedback to amputees. Early extra-neural stimulation research with single-contact nerve cuff electrodes on the median nerve in amputees provided a sense of paresthesia or a proprioceptive sensation of fist clenching [11]. Intraneural stimulation of the median, ulnar, and radial nerves elicited unusual throbbing and paresthesia, and the stability of the electrode response was challenging [12]. In 2003, a microelectrode array was implanted in a median nerve of a normal human but stimulation recruited motor response, which was interpreted as sensory feedback [13]. Recently, distinct and graded pressure sensation on the phantom hand was demonstrated using intrafascicular electrodes implanted in median and ulnar nerves of a human amputee [14]. Touch and tingling sensations were elicited from approximately 50% of multi-contact intrafascicular electrodes in another study [7]. The long term safety and stability of intrafascicular electrodes remain unknown because these trials were four weeks or less in duration and often reported an upward trend in stimulation threshold [7, 9, 14].
We have demonstrated a chronic, clinical neural stimulation system for selectively restoring sensation in two subjects with upper-limb loss [15]. Our approach uses multi-contact cuff electrodes implanted around peripheral nerves. At 18 and 24 months in two subjects, the system is also the longest neural-interfacing sensory stimulation system in humans, to date. These results also mark the first successful, chronic human implant of the flat interface nerve electrode (FINE) [16]. The FINE takes advantage of the natural structure of peripheral nerves to achieve fascicle-selective stimulation [17–20]. The Case Western Reserve University (CWRU) self-sizing spiral electrode [21], which has been used extensively in restoring motor control in SCI patients [22, 23], is also used in this study to provide sensory feedback. Based on pre-clinical trials with the FINE and previous clinical trials with the spiral, we expected the stimulation thresholds, electrode impedance, and number and location of percepts to remain stable for more than one year.
2. Methods
2.1. Surgical implantation
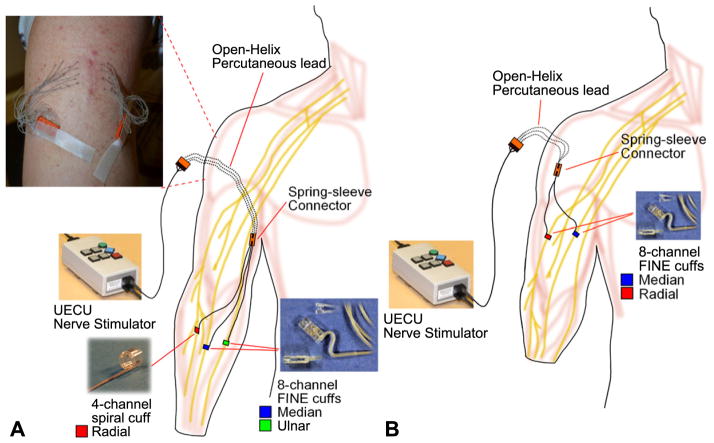
During an outpatient procedure, orthopedic surgeons implanted multi-contact nerve cuff electrodes on the peripheral nerves of two amputee subjects. At the time of implant, subject 1 is a 46 year old male who has a unilateral wrist disarticulation from work-related trauma. The subject was 19 months post-amputation. In the mid-forearm, 8-contact FINEs were implanted around the median and ulnar nerves and a 4-contact CWRU spiral electrode was implanted around the radial nerve. Cuff leads were routed subcutaneously to percutaneous leads [22, 24, 25] in the upper arm (figure 1(A)). The intraluminal size of the FINEs was 10 mm × 1.5 mm. The spiral cuff was 4 mm in diameter. Peripheral nerve studies in human cadavers [26] guided the sizes of available cuffs, from which the surgeons selected during the implant. The advantages of the FINE over the spiral electrode are the higher number of stimulating contacts and a design that achieves fascicle-selective stimulation [16]. However, a spiral was selected for the radial nerve due to the small nerve size and superficial nature of the implant location.
Figure 1.
(A) Nerve cuff electrode implanted in the forearm of subject 1. The electrode leads are connected to percutaneous leads via spring-sleeve connectors [25]. The open-helix percutaneous leads are passed individually through the skin (inset) so that the skin will grow into the open-helix, preventing pistoning of the lead and reducing the risk of bacterial infection. Stimulation is provided from the external stimulator, the universal External Control Unit (UECU). (B) In subject 2, the electrodes are implanted in the mid-upper arm.
At the time of implant, subject 2 is a 47 year old male with a mid-forearm amputation from work-related trauma. The subject was 93 months post-amputation. Eight-contact FINES were implanted on the median, ulnar and radial nerves. However, post-surgical x-ray imaging of the electrode contact orientation revealed that the ulnar FINE had opened, loosing intimate contact around the nerve. All electrodes were implanted in the arm proximal to the elbow and connected to percutaneous leads that exited through the upper arm (figure 1(B)). The intraluminal sizes of the FINEs were 10 mm × 1.0 mm on the median and ulnar nerves, and 10 mm × 1.5 mm on the radial nerve.
The implanted electrodes were allowed to stabilize for three weeks before experimental sessions began, based on clinician recommendation. However, thresholds were measured beginning at week 2 to evaluate the health of the nerve. At weeks 2–4, a compressive sock controlled post-surgical swelling of the implanted limb. Subjects were provided with percutaneous site maintenance instructions and dressing supplies. The percutaneous site was cleaned daily with a disinfecting agent, typically rubbing alcohol, and protected with a waterproof bandage (3M™ Tegaderm™ or similar). Clinical staff examined the percutaneous site at every experimental session. Redness at the percutaneous site was treated with topical antibiotics and, if deemed appropriate, oral antibiotics. Over the entire implant period, there were no incidents of painful, unpleasant, or unusual phantom sensations unrelated to stimulation that would have indicated excessive pressure or impingement of the nerve by the electrodes. The percutaneous leads and implanted components have remained free of infection.
The study protocol was approved by the Internal Review Board at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center and under a Food and Drug Administration Investigational Device Exemption. Implanted components (cuff electrodes, percutaneous leads, connectors) were manufactured by Ardiem Medical (Indiana, PA) and sterilized with ethylene oxide by Moog (Buffalo, NY).
2.2. Nerve stimulation
Subjects participated in nerve stimulation sessions once every 1 to 2 weeks for subject 1 and twice every 3 weeks for subject 2, based on subject availability. In each session, stimulation was individually applied through each contact. The duration of stimulation varied depending on the experiment, but typically lasted for less than 30 s. We provided a stimulus train of monopolar, bi-phasic, charge-balanced, cathodic-first square pulses. Anodic return was through a surface electrode on the dorsal surface of the arm immediately proximal to the elbow. For all trials, the subject was blinded to the stimulation parameters. Stimulation was limited to less than a charge density of 0.5 μC mm−2 [27, 28] and limited to less than 50% of the duty cycle [29].
Sensory perception thresholds were determined on every contact during weeks 2 through 8 in subject 1. Threshold was determined using the Single-Interval Adjustment Matrix, an unbiased, adaptive staircase method [30]. The parameters were set for a target performance of 50% (t = 0.5), which is the maximal difference between hit rate and false positive rate, with true stimulation provided 50% of the time. Fifty percent of the trials were catch trials with no stimulation and were randomly intermixed with stimulation trials to prevent subject bias. The threshold search was defined as complete at 12–16 reversals. Stimulation was applied for 1 s and repeated upon subject request. Stimulation frequency was held constant at 20 Hz. To prevent uncomfortable sensation from overly intense stimulation, the stimulus pulse amplitude (PA) and pulse width (PW) were incremented by 0.1 mA and 10 μs steps, respectively, until the rough threshold was determined. Then PA was held constant at one step (0.1 mA) below the rough threshold while the adaptive staircase method was used to determine a precise PW threshold with a 1 μs resolution.
For all subsequent sessions with subject 1 and all sessions with subject 2, threshold was determined using patterned stimulation intensity (PSI). In the full-scale modulation pattern, the width of the pulses in the pulse train (f0 = 100 Hz) followed a slow (fmod = 1 Hz) sinusoidal envelope, which typically gives rise to a natural, pulsing perception absent of paresthesia [15]. The PW varied between 0 μs and a maximum of B μs, where B is the measured threshold. Stimulation was applied in 5 s trials and repeated on subject request. The threshold PA level was determined by setting the peak of the sinusoidal PW to the maximum stimulation range (255 μs) and incrementing the PA stepwise by 0.1 mA until sensation was perceived, thus approximating the rheobase current. Then PA was held constant, while a binary search method was used to determine a precise PW threshold to 5 μs precision. Typically, the threshold was determined within 3–4 reversals. In addition, no catch trials were included because neither subject ever reported false positives in response to a catch trial at initial threshold evaluation.
Following stimulation, the subject was asked to describe any perceived sensation and sketch the percept area on a hand diagram. The percept area drawings were digitized for later analysis.
Impedance measures were taken between pairs of electrodes within each cuff using non-perceptible 0.3 mA and 50 μs pulses at 20 and 100 Hz. Frequencies were selected based on typical parameters used during functional testing with sensory feedback. The mean of eight measures of the resulting peak voltage drop between each pair of contacts was measured to calculate the impedance.
Unless otherwise noted, ANOVA was used to test significance with α = 0.05.
3. Results
3.1. Sensory locations and modalities
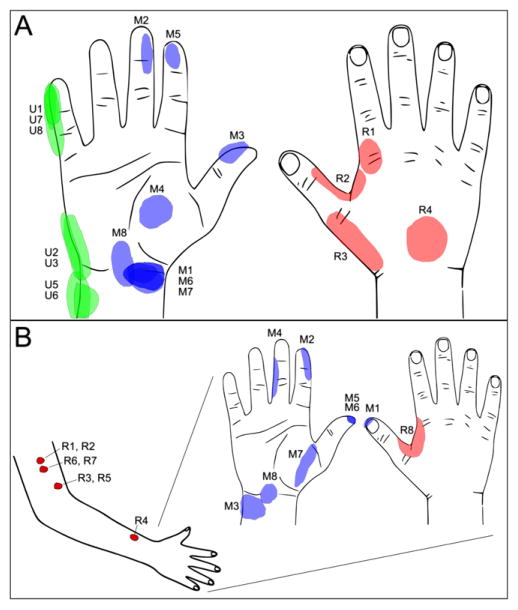
At two weeks post-operation, stimulation provided sensation response on the phantom limb from 19 of the 20 available contacts in subject 1. The one exception, Ulnar-4 (U4), was likely off the nerve as the FINE was larger than the nerve. Electrode contacts were each selective to a specific percept area, as shown at threshold stimulation levels indicated in figure 2(A). The location of sensory percepts aligns with expected areas of innervation for the median, ulnar, and radial nerves. Although percept areas may overlap at threshold stimulation levels, each contact elicited uniquely-expanding percept areas at suprathreshold stimulation levels. Contacts were also selective for ‘depth’ of sensation. For example, although three contacts on the median nerve cuff (M1, M6, M7) had overlapping percept area at the wrist, M1 and M7 produced a superficial, skin-surface sensation whereas M6 had a deep sensation.
Figure 2.
(A) In subject 1, threshold stimulation provides sensory response in approximately 15 unique percept areas on the phantom hand which cover classic innervation patterns for the median (M1-8), ulnar (U1-8), and radial (R1-4) nerves. (B) In subject 2, typical percept areas covered approximate median and radial innervation patterns of the hand and on the arm in approximately 10 unique percept areas.
The perceived modality of sensation from stimulation depended on the stimulation waveform and electrode contact [15]. Initial testing, up to week 8, using constant parameter stimulation evoked paresthesia (tingling) on all contacts. Various stimulation waveforms were explored over the chronic study period until we were able to repeatedly produce a natural tactile sensation absent of paresthesia using a sinusoidal (1 Hz) PW-modulated waveform. Channels were selective to specific tactile sensations including pressure (86%), vibration or tapping (7%), and light moving touch (7%) [15]. Proprioception was not observed in subject 1. However, when eliciting a pressure perception on the tips of digit 1 and 2 (M3, M5), the subject sometimes voluntarily reported that his perceived hand was in the shape of the ‘okay’ position with the thumb and index finger pinched together. Across all stimulation trials, motor recruitment was not observed in subject 1, which is consistent with the implant location being distal to motor axon branching.
Initially, 10 of the 16 available contacts produced sensation in subject 2. However, the number of active channels increased to 16 of 16 by week 27 (figure 2(B)). Each contact elicited a characteristic percept area. Stimulation through the median nerve cuff produced perceptive fields matching classical touch receptor innervations for the median nerve. During stimulation through the radial cuff, one contact (R8) repeatedly and two contacts (R3 and R4) occasionally produced sensation correlating with radial innervation of the perceived hand, while the other contacts produced sensations on the residual limb, mostly on the skin of the arm (figure 2(B)). Stimulation with the sinusoidal (1 Hz) PW-modulated waveform initially produced perceptions described as tactile pressure, ‘a cold edge’, or ‘a jet of water moving across the hand.’ Over multiple visits, the sensations evoked during median nerve stimulation resolved to tactile pressure (75% of channels) similar to that reported by subject 1. However the radial FINE primarily elicited a sensation of vibration (75% of channels) [15]. One channel (M4) led to both tactile pressure and the proprioceptive sensation of middle finger flexion at threshold stimulation levels. Visual confirmation and palpation indicated muscle twitch of a residual forearm muscle during perception of proprioception.
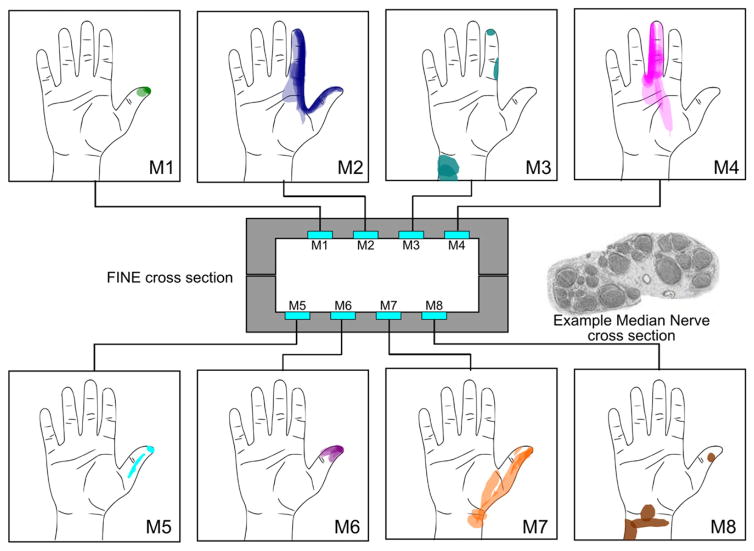
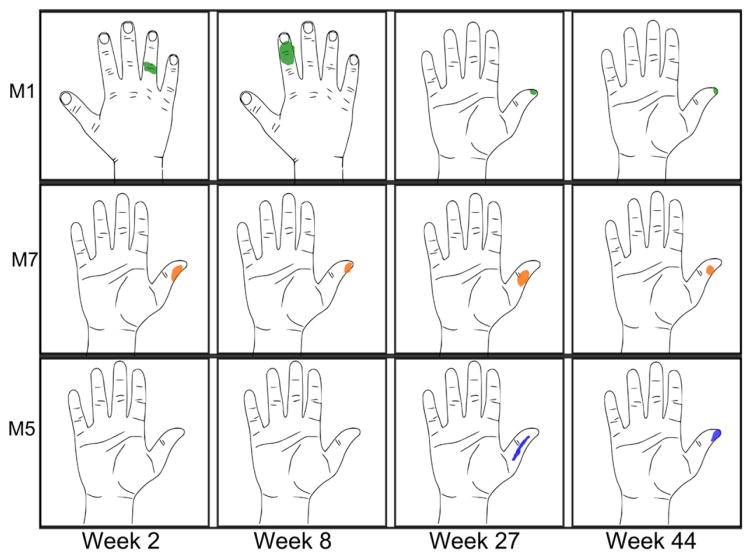
The perceptive areas of the stimulating channels resolved into stable and repeatable locations in both subjects. Figure 3 shows drawings from week 2 to week 56 (14 trials, overlaid) in subject 2. Interestingly, in subject 2, two channels in the median FINE (M1, M8) initially elicited percept areas on the dorsum (M1 shown in figure 4), which are classical ulnar and radial innervation zones. Over time, these percept areas shifted and have since settled to characteristic median nerve innervation areas (figure 4, top row). The sensory location evoked by channels M2, M4, and M7 remains consistent with those found during the first experimental session (figure 4, middle row). Additional channels recruited percept areas by week 27 (figure 4, bottom row).
Figure 3.
The cross section of the FINE implanted on the median nerve of subject 2 and the corresponding, channel-specific percept areas are shown. The example nerve cross section shown is from a comparable location taken from human histology studies [31]; it is not from subject 2. Multiple measures, including suprathreshold responses up to week 56, are shown for each channel. Most channels produce sensation in stable and characteristic percept areas, suggesting that fascicles at the level of the implant retain somatotopic organization. A similar drawing is not available for subject 1 because the exact order of channels in his cuff electrodes is unknown.
Figure 4.
Patterns of percept areas over time in subject 2. M1 showed a shift over time toward proper median innervation, settling on the thumb. Most channels remained in the same percept area since the first experimental session (for example, M7). Initially, 5 of 8 channels elicited sensation (week 2). However all channels elicited sensation by week 27 (example M5).
3.2. Sensory thresholds
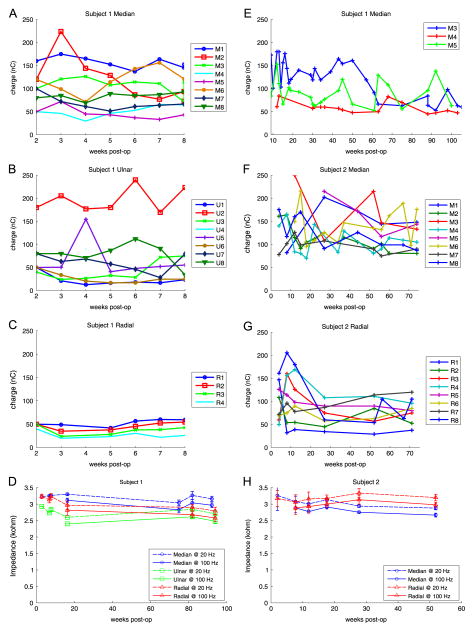
The mean threshold for eliciting perceptual sensation in our amputee subjects is 69.1 ± 36.0 nC and 109.7 ± 43.2 in subject 1 and 2, respectively. In subject 1, the threshold for sensation perception was determined for all 20 contacts from week 2 through 8 (figures 5(A)–(C)). Mean percept thresholds were 95.5 ± 42.5, 70.7 ± 59.2, and 40.7 ± 12.4 nC for the median, ulnar and radial nerves, respectively. Thresholds for median, ulnar and radial nerves were statistically different from each other (t-test, p ≤ 0.017). Linear regression on each contact over time produced a slope that was either not significantly different than 0 (n = 18, p ≥ 0.103) or significantly decreasing (n = 1, p = 0.044) suggesting no significant increase in threshold over the first 8 weeks. From week 8 to 105, threshold measurements were only taken on M3, M4, and M5 because they produced the most functionally-relevant perceptive fields on fingertip and palmar areas and the process was too time intensive to repeat for all channels. Mean perceptive threshold for the median nerve was 96.7 ± 36.8 nC. The thresholds continued decreasing (M3, p < 0.001) or not changing with time (M4, M5, p ≥ 0.09). In subject 2, thresholds on all channels were tracked for week 4 to 74 (figure 5). The mean cuff thresholds were 125.9 ± 41.5 and 120.4 ± 32.5 nC for the median and radial nerves respectively. Median and radial nerve thresholds were significantly different (t-test, p = 0.033). Again, similar trends were observed with most thresholds either not significantly changing (n = 12, p ≥ 0.20) or significantly decreasing (n = 3, p ≤ 0.015). The threshold associated with a single channel in the radial FINE (R7) was found to increase over time (slope = 0.64 nC/week, p = 0.016).
Figure 5.
Threshold and Impedance measurements over time indicate stable neural interface. Perceptual thresholds were measured for each contact for the first 8 weeks of the median (A), ulnar (B), and radial (C) nerve cuffs of subject 1. Threshold measures using a different stimulation waveform, patterned stimulation intensity, are shown up to 105 weeks (E). In subject 2, threshold measures were recorded up to 74 weeks on every contact in the median (F) and radial (G) nerve cuffs. Impedance measures also suggest stable neural interfaces in subject 1 (D) and subject 2 (H).
Impedance measures up to 94 weeks in subject 1 and up to 52 weeks in subject 2 are shown in figure 5. In subject 1, the mean channel impedances were 3.12 ± 0.15, 2.66 ± 0.15, and 2.91 ± 0.22 kΩ for the median, ulnar, and radial nerve cuffs respectively. In subject 2, the mean channel impedances were 2.92 ± 0.21 and 3.09 ± 0.19 kΩ for the median and radial nerve cuffs, respectively. Decreasing impedances were correlated with time (p < 0.001) for the median nerve cuffs of both subjects and the ulnar nerve cuff in subject 1 with Pearson’s correlation coefficients of −0.51, −0.73, and −0.49, respectively. Radial electrodes impedances did not change significantly over time in either subject (p > 0.42).
4. Discussion
An ongoing challenge in the field of neural interfaces is to produce selective neural stimulation with an interface that is stable for chronic, long-term clinical application. Selectivity would seem to be best achieved with a one-to-one interface with axons. Thus, current neural engineering dogma suggests that the greatest selectivity is achieved with the most intimate of interfaces: intrafascicular electrodes [7, 9, 10, 14], micro-electrode arrays [13], and regeneration electrodes [32]. While these technologies have advanced the field of neuromodulation, they have not studied long-term performance in humans. Although they are less intimate, we have demonstrated that nerve cuffs provide stable and selective stimulation for at least 24 months. Such a system is essential for translating neuromodulation to the clinical setting.
The FINE was preferred over the spiral cuff electrode for this study because the FINE achieves a greater selectivity. The original design intent of the FINE was to ‘reshape’ the nerve bundle such that the individual fascicles are arranged akin to a computer ribbon cable. Then, extraneural stimulation along the rectangular circumference would selectively stimulate each fascicle. However, human cadaver histological studies have found that peripheral nerves are naturally elongated in shape [26]. Thus, the FINE is a cuff that maintains the natural shape of the nerve. Although the spiral has demonstrated stability in chronic human studies [22], the current study represents the first example of such with the FINE.
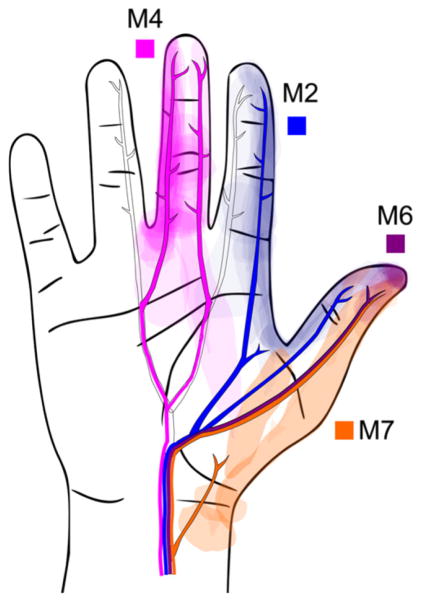
Fascicular organization of the peripheral nerve is evident from the selective, sensory response from nerve stimulation. Both of our subjects exhibit selective percept area stimulation with approximately 15 and 10 unique percept areas from 19 and 16 active contacts in subject 1 and subject 2, respectively. Consistently unique percept areas and qualities imply that specific subsections of the nerve are being activated from stimulation on specific cuff contacts. Given the precept areas and the known locations of contacts within the cuffs, we may infer the underlying fascicular organization within the nerve bundle. For example, contacts M1, M5, and M6, which are either immediately adjacent to or directly across the cuff from each other, produce sensation on the thumb tip of subject 2. This suggests that these three contacts may be activating the same population of axons within the same fascicle (figure 3). Some contacts elicit a sensation in the same locations but with different perceptual qualities (superficial versus deep sensation on M6 and M7, subject 1). The selectivity of sensory recruitment is perhaps surprising, but gratifying for an external cuff implanted in the proximal arm such as in subject 2. Figure 6 shows an overlay of suprathreshold sensory recruitment locations and the textbook neural anatomy of the proper palmar digital nerve branches of the median nerve [33], suggesting that the axons of these distal branches remain clustered in the proximal arm. The results build on the emerging understanding of neural anatomy that the nerve bundle retains a high level of somatic organization along the entirety of the peripheral nerve [34, 35], despite the plexiform branching and joining described by Sunderland [36].
Figure 6.
All percept areas, including suprathreshold response, elicited from week 2 to 56 for contacts M2, M6, M4, M7 in subject 2 is shown with an overlay of the proper palmar digital nerves of the median nerve adapted from the textbook neuroanatomy. Reproduced with permission from [33], Copyright 2001 Elsevier. Selective activation can be inferred by the pattern of recruitment and the relationship to underlying neuroanatomy.
The mean threshold for eliciting perceptual sensation in our amputee subjects is 69.1 ± 36.0 nC and 109.7 ± 43.2 in subject 1 and 2, respectively. This is, on average, greater than the 25 ± 17 nC found in previous chronic motor recruitment studies with a spiral nerve cuff implanted on upper extremity nerves in humans [37], and 21 ± 18and 41 ± 36 nC found during intraoperative studies with the FINE cuff implanted on lower extremity nerves in humans [18, 38]. A chronic implant is expected to have higher thresholds than an acute implant due to the additional impedance of the encapsulation tissue. Encapsulation tissue may have had a greater effect on threshold measures in the FINE than the spiral, as the FINEs in our study exhibited a much wider variance of thresholds than the spiral. One possible explanation is that contacts near the center of the rectangular cuff will be closer to the nerve than contacts near the edge of the cuff, assuming the nerve is centered within the cuff. Although our nerves may be offset from the center, the difference between the lowest and highest threshold contacts is consistent with outer to inner contact threshold differences from previous FINE studies in cats [39]. In fact, the lowest individual contact thresholds for the FINE cuffs are similar to the spiral cuff thresholds, suggesting certain FINE contacts are as close to the nerve as can be achieved with the spiral cuff. A proper fit is important for optimum nerve response to the FINE, which may also explain the differences in threshold. During the implant procedure of subject 1, the surgeons commented that the ulnar nerve was slightly smaller than the chosen cuff size. This may explain contact U2’s high threshold and the inactive U4 contact as they are likely to be on the edge of the nerve. Additionally, the difference from previous studies may also be attributed to motor recruitment threshold being determined by EMG recordings whereas sensation thresholds are being determined by conscious subject perception, a completely different neural pathway involving higher-order central processing. Lastly, axonal degeneration may have occurred after amputation [40–42], and may have had a greater effect in subject 2. During the implantation surgery of subject 2, who was 8 years post amputation, it was noted that the median and ulnar peripheral nerves were smaller than expected from histological studies [26].
We have demonstrated that multi-contact, cuff electrodes are suitable for stable, chronic nerve interfaces. Of all the active channels (n = 35) from both subjects, 97% have no significant change (n = 30) or trend toward a decrease (n = 4) in threshold over time. Contact-to-contact impedances remain stable. The threshold and impedance measures suggest a stable electrode interface (figure 5). The current study is limited to data collected over weekly to monthly measures due to subject availability. For reliable neuroprostheses, futures investigations should also demonstrate consistent response day-to-day [43] and within the day of active use, which are important features of our next study: at home use of a sensory feedback system. Thus far, our study has not shown an increase in threshold and/or impedance, which would have indicated continued tissue encapsulation, nerve impairment, and/or electrode degradation. In early chronic studies in human, intraneural interfaces have had challenges with failing electrode channels [13] and rising stimulation threshold [7, 9]. However, chronic intraneural research is preliminary in humans and research continues to progress toward long-term stability [44, 45].
Channel recruitment over time increased to nearly 100% of available channels in subject 2 by week 27 (figure 4, bottom row). The result is unique in that prior intrafascicular nerve interfaces described a loss in available channels over time, either from mechanical failure [13] or a reactive environment leading to worsening recording or stimulating capability [7, 46]. The mechanism for the recruitment of additional channels in our cuff electrodes is unclear, which may be peripherally or centrally-mediated. In the peripheral case, the stimulation characteristic of the neural interface may improve through a tighter fit of the electrode to the nerve. Axonal retrograde degeneration occurs after amputation [40–42]. Tissue activity may lead to regeneration via functional hyperaemia, but it is unknown if occasional electrical stimulation leads to sufficient activity to encourage nerve regeneration and improve fit of the electrode to the nerve. Alternatively, increased encapsulation around the outside of the nerve cuff may influence impedance [47], causing a high impedance shell that focuses stimulation charge to the target area. In the central case, brain plasticity [48] may increase the sensitivity to electrically-stimulated input over time.
The percept areas of both subjects reflected classical innervation patterns of median, ulnar, and radial nerves. This suggests cortical reorganization in long-term amputees does not interfere with sensory restoration from nerve stimulation, which is consistent with previous reports [49]. Unexpectedly, early stimulation sessions of the median nerve in subject 2 resulted in sensations from classically ulnar and radial innervated locations on the perceived hand. In contrast, subject 1 has always reported locations consistent with innervation patterns of the stimulated median, ulnar or radial nerve. The non-median nerve locations (from stimulating channels M1, M8) in subject 2 shifted during the initial period of study and ultimately stabilized in median nerve locations by week 27. The cortical representation, which may have expanded [50] to include ulnar and radial percept areas, may have reduced to the original cortical representation of the median nerve during stimulation of the previously ‘quiet’ afferent pathways.
One channel produces perceptive fields that alternate between two distinct locations, suggesting that the contact borders between two groupings of axons (figure 3, M3). If the nerve bundle and fascicles within are somotatopically-organized, the change in percept area suggests that recruitment alternates between two fascicles. Based on modeling studies on the influence of neighboring fascicles, two fascicles may be moving slightly into and out of the stimulus field such that one or the other, but not both, results with activated axons [20].
The number of percept areas on the hands of both subjects suggest that our approach is a viable method for a sensory-enhanced prosthetic hand to provide multi-point sensory restoration. In addition, both subjects reported fingertip percept areas, which are functionally important in grasp patterns. The fingertip recruitment of our study is consistent with intrafascicular interface studies [43] and is likely due to a high innervation density of the digit tips. A sensory feedback system using our approach can provide multiple discrete percept area options, increasing the likelihood of matched somatotopic feedback, and possibly restoring sensation across the whole hand.
Direct neural stimulation to provide sensory feedback may be more natural than sensory substitution methods, which usually require training to associate mismatched mode and somatotopic sensation. Sensory substitution has been shown to reduce the force applied to objects via grasping pressure feedback [51, 52] and to provide positional feedback about hand opening [5]. However, sensory substitution systems have been commercially unsuccessful, possibly because the mismatched mode and somatotopic feedback requires additional cognitive load and may be distracting [52].
Using all available contacts in a wide variety of locations, is it easy to imagine implementing a sensor-enhanced prosthetic hand and feedback system capable of improving activities of daily living, providing natural touch, and enhancing embodiment of prosthetic limbs. However, the current study is limited to the resulting stability and selectivity of the FINE and spiral cuff. Additional issues are important to consider for the end application of useful sensory feedback in amputees. We have detailed various modalities of perception, graded control of sensation intensity, and demonstrated delicate object manipulation with the cuff electrode approach [15]. Proprioception is an important aspect of sensory feedback; however, it is unknown by which mechanism proprioception is best achieved. Recent studies of direct neural stimulation with intrafascicular electrodes reported proprioception in several subjects [10, 14, 43, 49], whereas other subjects have none [7, 9, 10]. The nature of proprioception as a sensation elicited by a patterned population of afferents, including muscle, tendon and skin-stretch receptors, may explain why proprioception is generally difficult to achieve solely through afferent nerve stimulation [53, 54]. Future investigation will continue to examine the nature of the proprioception response and the effect of a sensory feedback-enabled prosthesis on functional task performance, such as object size and compliance discrimination [10] and shape discrimination [9].
5. Conclusion
We have achieved selective nerve respoe nse from multi-contact cuff electrodes by demonstrating characteristic percept areas and stimulating thresholds for each contact. We demonstrated stability in percept area, threshold, and impedance measures from five electrode cuffs chronically implanted in two human amputee subjects for more than 1 and 2 years. Our approach demonstrates selectivity and stability can be achieved through an extraneural interface, which can provide sensory feedback to amputees. The technology is not only applicable for amputees, but may also be applied in other clinical, peripheral, neural prosthesis applications as well, including pain therapy.
Acknowledgments
Special thanks to Joyce Tyler for provided occupational therapy support and Melissa Schmitt for clinical and study coordinator support. This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research & Development Service, Merit Review #A6156R.
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Biddiss E, Beaton D, Chau T. Consumer design priorities for upper limb prosthetics. Disabil Rehabil Assist Technol. 2007;2:346–57. doi: 10.1080/17483100701714733. [DOI] [PubMed] [Google Scholar]
- 3.Pylatiuk C, Schulz S, Döderlein L. Results of an internet survey of myoelectric prosthetic hand users. Prosthet Orthot Int. 2007;31:362–70. doi: 10.1080/03093640601061265. [DOI] [PubMed] [Google Scholar]
- 4.Riso RR. Strategies for providing upper extremity amputees with tactile and hand position feedback—moving closer to the bionic arm. Technol Health Care. 1999;7:401–9. [PubMed] [Google Scholar]
- 5.Witteveen H, Droog E, Rietman J, Veltink P. Vibro-and electrotactile user feedback on hand opening for myoelectric forearm prostheses. IEEE Trans Biomed Eng. 2012;59:2219–26. doi: 10.1109/TBME.2012.2200678. [DOI] [PubMed] [Google Scholar]
- 6.Marasco PD, Kim K, Colgate JE, Peshkin MA, Kuiken TA. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain. 2011;134:747–58. doi: 10.1093/brain/awq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossini PM, et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol. 2010;121:777–83. doi: 10.1016/j.clinph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Flor H, Denke C, Schaefer M, Grüsser S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357:1763–4. doi: 10.1016/S0140-6736(00)04890-X. [DOI] [PubMed] [Google Scholar]
- 9.Raspopovic S, et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med. 2014;6:222ra19. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 10.Horch K, Meek S, Taylor TG, Hutchinson DT. Object discrimination with an artificial hand using electrical stimulation of peripheral tactile and proprioceptive pathways with intrafascicular electrodes. IEEE Trans Neural Syst Rehabil Eng. 2011;19:483–9. doi: 10.1109/TNSRE.2011.2162635. [DOI] [PubMed] [Google Scholar]
- 11.Clippinger FW, Avery R, Titus BR. A sensory feedback system for an upper-limb amputation prosthesis. Bull Prosthet Res. 1974;10–22:247–58. [PubMed] [Google Scholar]
- 12.Anani A, Körner L. Discrimination of phantom hand sensations elicited by afferent electrical nerve stimulation in below-elbow amputees. Med Prog Technol. 1979;6:131–5. [PubMed] [Google Scholar]
- 13.Warwick K, Gasson M, Hutt B, Goodhew I, Kyberd P, Andrews B, Teddy P, Shad A. The application of implant technology for cybernetic systems. Arch Neurology. 2003;60:1369–73. doi: 10.1001/archneur.60.10.1369. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng. 2005;13:468–72. doi: 10.1109/TNSRE.2005.856072. [DOI] [PubMed] [Google Scholar]
- 15.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014;6:257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyler DJ, Durand DM. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann Biomed Eng. 2003;31:633–42. doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 17.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiefer MA, Freeberg M, Pinault GJC, Anderson J, Hoyen H, Tyler DJ, Triolo RJ. Selective activation of the human tibial and common peroneal nerves with a flat interface nerve electrode. J Neural Eng. 2013;10:056006. doi: 10.1088/1741-2560/10/5/056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiefer MA, Tyler DJ, Triolo RJ. Probabilistic modeling of selective stimulation of the human sciatic nerve with a flat interface nerve electrode. J Comput Neurosci. 2012;33:179–90. doi: 10.1007/s10827-011-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinberg Y, Schiefer MA, Tyler DJ, Gustafson KJ. Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Trans Neural Syst Rehabil Eng. 2008;16:572–81. doi: 10.1109/TNSRE.2008.2010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng. 1988;35:905–16. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 22.Polasek KH, Hoyen HA, Keith MW, Kirsch RF, Tyler DJ. Stimulation stability and selectivity of chronically implanted multicontact nerve cuff electrodes in the human upper extremity. IEEE Trans Neural Syst Rehabil Eng. 2009;17:428–37. doi: 10.1109/TNSRE.2009.2032603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher L, Tyler D, Anderson J, Triolo R. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. J Neural Eng. 2009;6:046010. doi: 10.1088/1741-2560/6/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutson JS, Naples GG, Peckham PH, Keith MW. Electrode fracture rates and occurrences of infection and granuloma associated with percutaneous intramuscular electrodes in upper-limb functional electrical stimulation applications. J Rehabil Res Dev. 2002;39:671–83. [PubMed] [Google Scholar]
- 25.Letechipia JE, Peckham PH, Gazdik M, Smith B. Inline lead connector for use with implanted neuroprosthesis. IEEE Trans Biomed Eng. 1991;38:707–9. doi: 10.1109/10.83572. [DOI] [PubMed] [Google Scholar]
- 26.Brill N, Tyler DJ. Quantification of human upper extremity nerves and fascicular anatomy. Muscle Nerve. doi: 10.1002/mus.25534. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polasek K, Schiefer M. Intraoperative evaluation of the spiral nerve cuff electrode on the femoral nerve trunk. J Neural Eng. 2009;6:1–12. doi: 10.1088/1741-2560/6/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng. 1992;39:424–6. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]
- 29.Agnew WF, McCreery DB, Yuen TG, Bullara LA. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection of parameters. Ann Biomed Eng. 1989;17:39–60. doi: 10.1007/BF02364272. [DOI] [PubMed] [Google Scholar]
- 30.Kaernbach C. A single interval adjustment matrix (SIAM) procedure for unbiased adaptive testing. J Acoust Soc Am. 1990;88:2645–55. doi: 10.1121/1.399985. [DOI] [PubMed] [Google Scholar]
- 31.Brill N, Tyler DJ. PhD Dissertation. Case Western Reserve University; 2014. Optimization of high density nerve cuff stimulation in upper extremity nerves. [Google Scholar]
- 32.Clements IP, Mukhatyar VJ, Srinivasan A, Bentley JT, Andreasen DS, Bellamkonda RV. Regenerative scaffold electrodes for peripheral nerve interfacing. IEEE Trans Neural Syst Rehabil Eng. 2013;21:554–66. doi: 10.1109/TNSRE.2012.2217352. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins DB. Hollinshead’s Functional Anatomy of the Limbs and Back. 8. Philadelphia, PA: Saunders; 2001. p. 200. [Google Scholar]
- 34.Hallin RG, Wu G. Novel information on peripheral tactile mechanisms in man acquired with concentric needle electrode microneurography. Behav Brain Res. 2002;135:11–8. doi: 10.1016/s0166-4328(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 35.Prodanov D, Nagelkerke N, Marani E. Spatial clustering analysis in neuroanatomy: applications of different approaches to motor nerve fiber distribution. J Neurosci Methods. 2007;160:93–108. doi: 10.1016/j.jneumeth.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Sunderland S. The intraneural topography of the radial, median and ulnar nerves. Brain. 1945;68:243–98. doi: 10.1093/brain/68.4.243. [DOI] [PubMed] [Google Scholar]
- 37.Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2007;15:76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 38.Schiefer MA, Polasek KH, Triolo RJ, Pinault GCJ, Tyler DJ. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J Neural Eng. 2010;7:26006. doi: 10.1088/1741-2560/7/2/026006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 40.Chu N. Long-term effects of finger amputation on stump skin sensibility and digital nerve conduction. Muscle Nerve. 1996;19:1049–51. doi: 10.1002/(SICI)1097-4598(199608)19:8<1049::AID-MUS14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Aitken JT, Thomas PK. Retrograde changes in fibre size following nerve section. J Anat. 1962;96:121–9. [PMC free article] [PubMed] [Google Scholar]
- 42.McComas AJ, Sica RE, Banerjee S. Long-term effects of partial limb amputation in man. J Neurolgy Neurosurgery Psychiatry. 1978 May;41:425–32. doi: 10.1136/jnnp.41.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhillon GS, Krüger TB, Sandhu JS, Horch KW. Effects of short-term training on sensory and motor function in severed nerves of long-term human amputees. J Neurophysiol. 2005;93:2625–33. doi: 10.1152/jn.00937.2004. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence SM, Larsen JO, Horch KW, Riso R, Sinkjaer T. Long-term biocompatibility of implanted polymer-based intrafascicular electrodes. J Biomed Mater Res. 2002;63:501–6. doi: 10.1002/jbm.10303. [DOI] [PubMed] [Google Scholar]
- 45.Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51:146–57. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 46.McConnell GC, Rees HD, Levey AI, Gutekunst C-A, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 47.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2006;117:447–54. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Björkman A, Weibull A, Rosén B, Svensson J, Lundborg G. Rapid cortical reorganisation and improved sensitivity of the hand following cutaneous anaesthesia of the forearm. Eur J Neurosci. 2009;29:837–44. doi: 10.1111/j.1460-9568.2009.06629.x. [DOI] [PubMed] [Google Scholar]
- 49.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J Hand Surg Am. 2004;29:605–15. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Moore CE, Schady W. Investigation of the functional correlates of reorganization within the human somatosensory cortex. Brain. 2000;123:1883–95. doi: 10.1093/brain/123.9.1883. [DOI] [PubMed] [Google Scholar]
- 51.Panarese A, Edin BB, Vecchi F, Carrozza MC, Johansson RS. Humans can integrate force feedback to toes in their sensorimotor control of a robotic hand. IEEE Trans Neural Syst Rehabil Eng. 2009;17:560–7. doi: 10.1109/TNSRE.2009.2021689. [DOI] [PubMed] [Google Scholar]
- 52.Pylatiuk C, Kargov A, Schulz S. Design and evaluation of a low-cost force feedback system for myoelectric prosthetic hands. J Prosthet Orthot. 2006;18:57–61. [Google Scholar]
- 53.Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–71. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallin RG, Carlstedt T, Wu G. Population behaviour of human cutaneous mechanoreceptive units. Behav Brain Res. 2002;135:19–26. doi: 10.1016/s0166-4328(02)00150-x. [DOI] [PubMed] [Google Scholar]