Abstract
Between 1940 and 2004, more than 335 emerging infectious disease events were reported in the scientific literature. The majority (60%) of these events involved zoonoses, most of which (72%) were of wildlife origin or had an epidemiologically important wildlife host. Because this trend of increasing emerging diseases likely will continue, understanding the pathogenesis, transmission, and diagnosis of these diseases in the relevant wildlife host is paramount. Achieving this goal often requires using wild animals as research subjects, which are vastly different from the traditional livestock or laboratory animals used by most universities and institutions. Using wildlife in infectious disease research presents many challenges but also provides opportunities to answer questions impossible to address by using traditional models. Cervid species, especially white-tailed deer (Odocoileus virginianus), elk (Cervus canadensis), and red deer (Cervus elaphus), are hosts or sentinels for several important pathogens, some of which are zoonotic. The long history of infectious disease research using white-tailed deer, conducted at ever-increasing levels of sophisticated biosecurity, demonstrates that this type of research can be conducted safely and that valuable insights can be gained. The greatest challenges to using wildlife in infectious disease research include animal source, facility design, nutrition, animal handling, and enrichment and other practices that both facilitate animal care and enhance animal wellbeing. The study of Mycobacterium bovis infection in white-tailed deer at the USDA's National Animal Disease Center serves to illustrate one approach to address these challenges.
Abbreviations: NADC, National Animal Disease Center; SCWDS, Southeast Cooperative Wildlife Disease Study; WTD, white-tailed deer
Introduction
The One Health Initiative emphasizes the interdependency of human, livestock, and wildlife health. Specifically, the initiative asserts that the physical health of humans, livestock and wildlife are linked through shared diseases.11 Between 1940 and 2004, more than 335 emerging infectious disease events were reported in the scientific literature. The majority (60%) of these events involved zoonoses, most of which (72%) had an epidemiologically important wildlife host.48 More than 90% of animal-related research at universities and other institutions involves traditional laboratory animals (that is, rats and mice).64 Today, understanding the pathogenesis, transmission and diagnosis of emerging diseases often requires research involving wild animals, either free-ranging or captive, that are vastly different from laboratory animals or traditional livestock.
Cervid species, specifically white-tailed deer (WTD; Odocoileus virginianus) red deer (Cervus elaphus), and elk (Cervus canadensis), are important hosts for several zoonotic pathogens (for example, Brucella abortus, Mycobacterium bovis, hepatitis E virus).74,76,107 These species also serve as sentinels for other zoonotic pathogens (for example, enterohemorrhagic Escherichia coli, Yersinia enterocolitica, Listeria monocytogenes).27 Furthermore, some diseases such as brucellosis and tuberculosis are transmitted between cervids and livestock, making these important diseases at the wildlife–livestock–human interface. Experimental infection of cervids with zoonotic pathogens requires specialized facilities and practices to prevent pathogen release and to ensure personnel safety. In addition, pathogens such as B. abortus are considered biologic select agents and require intense biosecurity measures beyond standard practices.4
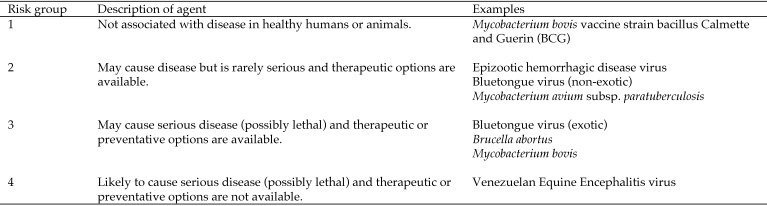
National biosafety guidelines categorize infectious agents into 4 ascending levels of risk (Figure 1). These designations are based on the pathogen's ability to infect and cause disease in humans or animals, severity of disease, and availability of preventive or therapeutic options.118 These risk criteria are used to define corresponding biosafety levels of physical containment. Each of the 4 biosafety levels of containment describes the level of protection in terms of the practices, equipment, and facilities necessary for handling an agent of the corresponding risk level. These criteria also apply to the housing of animals infected with such agents. In situations where highly infective agricultural agents and large animals such as cows, pigs, bison, and deer are used, requirements beyond typical BSL3 practices are required. This advanced BSL3 designation is known as BSL3Ag.118
Figure 1.
Recommended risk group classifications and examples of agents experimentally administered to WTD.
The following paragraphs describe published research using white-tailed deer. Some of the reported studies were conducted prior to the formal introduction of risk factors and biosafety levels of containment. As such, the descriptions of research facilities are those used at the time and are not necessarily facilities that would be appropriate today.
Infectious Disease Research Involving WTD in BSL1 Environments
Pre1990 studies with WTD included infection trials with Leptospira pomona in 1962, Salmonella meleagridis in 1970, Anaplasma marginale in 1971, Venezuelan equine encephalomyelitis virus in 1972, Fasciola hepatic in 1974, Fascioloides magna in 1979, Jamestown Canyon and Keystone viruses in 1979, malignant catarrhal fever in 1981 and 1982, and Mycobacterium avium subsp. paratuberculosis in 198320,40,53,93,98,115-117 (Figure 2). Descriptions of containment facilities for each of these studies generally are not provided in the literature or are only minimally described; therefore, the animals can be assumed to have been housed in outdoor pens consistent with BSL1 containment. The study using Anaplasma marginale was done at a field laboratory operated by USDA in Nuevo Laredo, Tamaulipas, Mexico—presumably as a precaution given that the tick vector (Boophilus annulatus), although endemic in Mexico, was essentially eradicated from the United States at that time. Research on chronic wasting disease (CWD) at the Colorado Division of Wildlife's Wildlife Research Center from 2010 to 2013 housed CWD-inoculated deer in—presumably—outdoor “biosecure paddocks.” During these studies, some deer were held for short periods of time in metabolic cages for urine and feces collection.45,60,99
Figure 2.
Experimental infection studies involving WTD in BSL1 or BSL2 biocontainment.
Infectious Disease Research Involving WTD in BSL2 Environments
The Southeastern Association of Fish and Wildlife Agencies founded the Southeast Cooperative Wildlife Disease Study (SCWDS) in Athens, GA, in 1957 to examine the cause of widespread die-offs of WTD. Objectives—then and now—are to determine the causes of disease in wildlife, effects of disease on wildlife populations, and interrelationships between wildlife, domestic livestock, and human infectious diseases. At the SCWDS, experimental infection trials in BSL2 type containment have included such notable pathogens as E. coli 0157:H7;19 epizootic hemorrhagic disease virus,23-26,88,89,100,103,104,106 bluetongue virus,41,42 and multiple agents of anaplamosis,65,108,109 borreliosis,54,63,73 and ehrlichiosis8-10,112,122,123 (Figure 2). For the past 50 years and longer, the SCWDS has been a leader in the development of experimental biology approaches for the study of infectious diseases in WTD.
Experimental infection studies with endemic strains of bluetongue virus were performed under BSL2 containment as early as 1967 at the University of Wisconsin (Madison, WI) and later at the SCWDS in the mid 1990s41,106,114 (Figure 2). Richard E Shope demonstrated the viral etiology of epizootic hemorrhagic disease in WTD and detailed the pathologic manifestations of the disease.102 Biocontainment for experimental infection trials performed by Shope at the Rockefeller Institute (Trenton, NJ) consisted of individual pens on a cement floor deeply bedded with straw or hay in a sturdy wooden frame lined with a 14-gauge welded wire of 2×1-in. mesh covered with a plastic “insect-proof” mesh screen. Studies with a California serovar of bluetongue virus (BTV8) at the University of Wisconsin used similar biocontainment measures, described as “a Rockefeller-type isolation building.”114 These early studies by Shope provided a framework for experimental biology approaches using WTD. Fletch and Karstad extended Shope's findings by demonstrating that disseminated intravascular coagulation was a key pathophysiologic feature of experimental epizootic hemorrhagic disease in WTD.20 Later, multiple studies performed at SCWDS in BSL2 environments provided insights into the pathogenesis, vector biology, clinical signs, and immune responses of WTD infected with epizootic hemorrhagic disease virus.23-26,89,103,104,106
Ruder and colleagues demonstrated the vector competence and susceptibility of WTD to a nonendemic serotype of epizootic hemorrhagic disease virus (EHDV7); this work highlighted the importance of serotype-specific diagnostic tests during hemorrhagic disease outbreaks.100,101 In 1972, Hoff and Trainer infected 3 WTD with an attenuated Trinidad vaccine strain of Venezuelan equine encephalomyelitis virus by using various routes of inoculation; studies were conducted in “tight isolation facilities at the University of Wisconsin Charmany Research Center.”40
Throughout the past 20 y and longer, numerous studies have been performed at the SCWDS under BSL2 containment on tickborne pathogens involving WTD including Anaplasma spp., Ehrlichia spp., and Borrelia spp.8-10,54,63,65,73,108,109,112,122,123 (Figure 2). Over the past 10 y, experimental infection studies with Mycobacterium avium subsp. paratuberculosis,77 the prion agent of chronic wasting disease (CWD),29,31,35,36,37 and bovine viral diarrhea virus96,97 have been performed under BSL2 containment at the National Animal Disease Center (NADC) in Ames, IA.
Studies on CWD using WTD began at Colorado State University in the late 1990s. The first long-term study, which was almost 2 y in duration, was published in 2006.57 Numerous subsequent studies using samples from the original study or similar biocontainment protocols produced seminal papers on the presence of infectious prions in saliva and blood, transmission through environmental exposure, the presence of infectious prions in B cells and platelets, and aerosol transmission of infectious prions.12,16,17,28,30,32,38,39,55-57 Biosecurity measures included “showering in procedures, wearing of Tyvek clothing, face masks, head covers, and footwear.”57,59
Other BSL2 studies involving WTD include those on CWD at the University of Wisconsin and the USDA's National Wildlife Research Center (Ft Collins, CO); bovine viral diarrhea at Auburn University, Purdue University, and the Sybille Conservation Education and Wildlife Research Center in Wyoming;46,47,70,84,85 adenovirus hemorrhagic disease at the University of California in Davis;121 La Crosse virus at the University of Wisconsin;75 E. coli 0157:H7 hemorrhagic disease at SCWDS;19 Parelaphostrongylus tenuis infection at the University of New Brunswick, Canada;14,15 and Ehrlichia chaffeensis at Oklahoma State University (Stillwater, OK).3,44,67,68 (Figure 2).
Infectious Disease Research Involving in BSL3 Environments
The first published reports in peer-reviewed journals regarding the use of WTD in BSL3-type biocontainment facilities were experimental infection studies with rinderpest and peste des petits ruminants viruses33,34 that were performed at Plum Island Animal Disease Center (PIADC) in Greenport, NY in 1975 (Figure 3). Studies on rinderpest and peste des petits ruminants viruses demonstrated the susceptibility of WTD to both viruses, highlighting the significant concern about the potential role of wildlife in the propagation of foreign animal diseases. In these studies, deer were “handled under observation in strict isolation during the course of the experiment.” At this time, safety procedures and equipment at the facility included “air-tight animal rooms; air-sealed (gasket) doors; and automatic wash-down airlocks.”52 The center's biosafety standard was presented to the American Biologic Safety Association for consideration in defining the 4 levels of biocontainment used for animal studies with infectious agents.5 In 1987, studies at Plum Island demonstrated that WTD develop a rapid onset of neurologic disease and pulmonary edema after intravenous inoculation of Cowdria ruminantium (now termed Ehrlichia ruminantium)-infected blood.6 The authors concluded that WTD “could play a major role in the spread and maintenance of this organism if it were ever introduced into the United States.”
Figure 3.
Experimental infection studies involving WTD in BSL3 biocontainment.
More recently, WTD have been used in BSL3 containment for extensive studies on the pathogenesis and diagnosis of Mycobacterium bovis infection;72,77-83,105 pathogenesis and deer-to-cattle transmission of foot-and-mouth disease virus;62 and pathogenesis and patterns of viremia after experimental infection with a Northern European strain of bluetongue virus (BTV8)13 (Figure 3). These studies were performed in BSL3 or BSL3Ag high-containment facilities at NADC, National Centre for Foreign Animal Disease (Winnipeg, Manitoba, Canada), and the BSL3 Animal Disease Laboratory at Colorado State University (Fort Collins, CO), respectively.
Using WTD in modern biocontainment facilities, especially at the BSL3 or BSL3Ag levels, presents unique challenges and requires complex housing specifications as well as care and handling practices unavailable at many institutions. As described in the following paragraphs, research on M. bovis infection in WTD at NADC serves to illustrate many of the unique challenges posed by long-term housing of WTD under high-biocontainment conditions. All research at NADC was conducted humanely according to protocols approved by the NADC Care and Use Committee and in accordance with the Guide for Care and Use of Laboratory Animals43 and the Guide for the Care and Use of Agricultural Animals in Research and Teaching.18
Animal Behavior Considerations
Like other ruminants, deer have a central area of binocular vision with peripheral monocular vision thus creating a very wide visual field (approximately 300°). Their depth perception, ability to detect movement, and vision under low-light conditions are excellent.58,61 Their hearing and directional capabilities for sound detection are remarkable also.61 As such, under almost all conditions their ability to detect humans initiates a sense of alertness and sometimes flight behavior. The raised tail of a WTD is a visual signal of danger—if one animal runs, others will follow.61 This following behavior can be used to an animal caretaker's advantage when moving deer from one room to another.
Rooms for housing WTD are both heated and air-conditioned. Temperature is maintained between 17 and 19 °C, with relative humidity at 21% to 40%. Rooms are under negative pressure, with an airflow rate of 10 to 11 air changes hourly, and on a 12:12-h light:dark photocycle.
WTD are particularly nervous and flighty, often making sudden movements when startled. Due to this flight response, it is not uncommon for deer to run, jump, and attempt to escape when personnel enter the animal room. Consequently, gating and penning must be of sufficient height to prevent deer from escaping. One study showed WTD would jump a fence 2.1 m high but not one 2.4 m tall.113 Still, some suggest that a height of at least 3 m should be considered.49 When deer are startled, there is a high risk of slips or falls that can result in contusions, lacerations, or fractures. Therefore, the surface character of the flooring should reduce slipping and falling but be easily sanitized. Depending on flooring type, hooves may require frequent trimming, which generally involves manual or chemical restraint. Flooring at a biocontainment facility at Colorado State University where deer are housed is described as a mixture of sand and epoxy, which results in relatively normal hoof wear.59
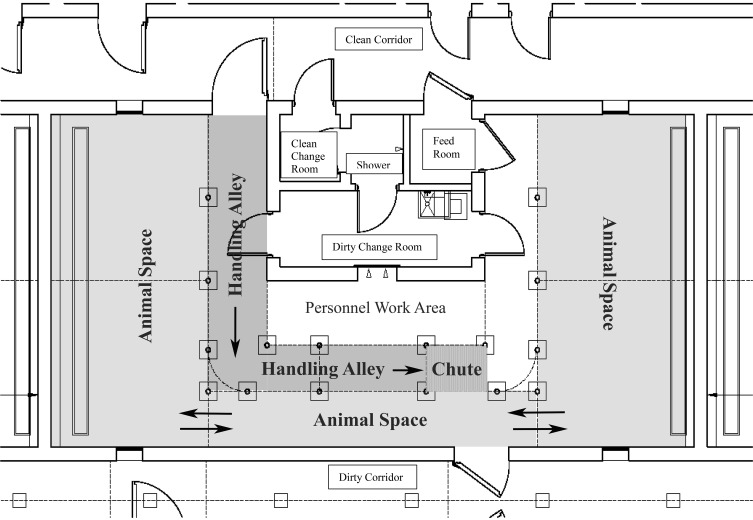
Due to the inherent impulse of deer to flee when approached, it is useful to have a perceived ‘safe’ location to which deer can relocate when personnel enter the animal room. One design that has proven effective in the BSL3Ag facility at NADC is a room that contains a U-shaped animal space (Figure 4). As personnel enter one side of the room for cleaning and feeding, deer move to the other side, out of sight of the caretaker. After one half of the room is cleaned, deer are allowed to move back to the recently cleaned area, allowing cleaning of the opposite side of the room. An additional advantage to the U-shape design is that handling equipment can be placed parallel to the alleyway connecting the 2 sides of the room. When handling is required, deer can be moved to one side of the room, handled through the chute, and exited into the opposite side of the room.
Figure 4.
Schematic of BSL3 animal room used for housing WTD at the National Animal Disease Center (Ames, IA). As personnel enter one side of the animal space for cleaning and feeding, deer move to the opposite side, out of sight of the caretaker. After one half of the room is cleaned, deer are allowed to move back to the recently cleaned area, allowing cleaning of the opposite side. Handling equipment is placed in an alleyway, parallel to the animal space alleyway connecting the 2 sides of the room. When handling is required, deer can be moved to one side of the animal space, handled through the chute, and exited into the opposite side of the animal space.
As with many animal species kept in research settings, barbering—both self-directed and partner-directed—is common in WTD,94 particularly after movement from outside facilities into containment housing. In WTD, it is unclear whether barbering is a result of boredom, anxiety, distress, crowding, social hierarchy, or a combination of factors. As is common in other species, WTD ingest the pulled hair; as such, trichobezoars in the rumen or reticulum are often found at necropsy. At NADC, enrichment devices have empirically decreased barbering such as hanging puzzle feeders containing cracked corn; the height of which is altered periodically for variety. In addition, treats in the form of peanut butter or jelly applied to hanging bucket lids are also used as enrichment.
Penning, Gating, and Animal Handling
For use with WTD, gates, latches, feeders, and watering devices must be designed without sharp corners or angles. Restraint devices specific for handling deer are most effective. For WTD, ‘drop-floor’ chutes (for example, the Deerhandler [Delclayna, Alberta, Canada]) are preferable to traditional livestock squeeze chutes. Devices designed specifically for use with WTD minimize jumping yet allow considerable access to the animal for examination and treatment. Manual restraint in such a chute is suitable for blood collection, foot trimming, artificial insemination, and the administration of drugs or vaccines by oral, intramuscular, and subcutaneous routes.
Moving deer from outside facilities into containment rooms can be challenging. In addition to the potential for physical trauma, diet transition can present a considerable hurdle. At NADC, we have observed periods of anorexia that last 24 to 48 h after movement and often accompanied by temporary hematochezia, which is self-limiting. Beginning 2 wk prior to animal movement, feed is transitioned to alfalfa cubes (rather than long-stem hay) and deer pellets with 18% protein content (for example, Trophy Image Pellets [Kent Nutrition Group, Muscatine, IA]). After WTD have been moved, Hydration Hay (Purina Animal Nutrition LLC, Shoreview, MN) is added to the alfalfa cubes and deer-pellet diet to encourage eating and maintain hydration. Long-stem hay is avoided inside biocontainment housing, because of possible obstruction of drains and plumbing.
Occupational Hazards
The safety of personnel is important when working with WTD inside biocontainment facilities. Knowledge of deer behavior, common maladies of deer, animal restraint, and appropriate training are critical for both animal and personnel safety.95 Hand-raising fawns provides considerable time for animal caretakers to observe normal behavior and recognize signs associated with illness or aggression. The animal's tendency to flee may place personnel at risk of collisions with deer attempting escape from confinement. Veterinarians should be familiar with diseases of deer, particularly those associated with nutrition, stress, and trauma. While hand-raising fawns, veterinarians face many of the common illnesses of neonatal ruminants, such as enteritis, pneumonia, and dehydration.
In terms of decreased stress and risk of injury to both animals and personnel, the benefits of using hand-raised fawns, which have been acclimated to humans and indoor housing, cannot be overstated. Bottle-feeding and hand-raising deer fawns have a profound effect on their suitability as research subjects in general and inside biocontainment housing in particular. Hand-raising is most effective when done inside a building or space reflective of containment housing. A reliable source of fawns is crucial, because hand-raising should begin within 24 h of birth. At NADC, we maintain a breeding herd of WTD as a source of healthy and acclimated deer with a known medical history. The exception to the advantage of hand-raised deer is the breeding buck. Hand-raised deer have a greatly reduced flight distance and will tolerate close physical associations with humans. During the mating season, an intact buck becomes highly aggressive and territorial, and an aggressive buck acclimated to humans may charge animal caretakers, creating a dangerous situation.
Extralabel Drug Usage and Judicious Use of Antimicrobials
Farmed deer are considered a ‘minor species’ by the US Food and Drug Administration. According to the Minor Use Animal Drug Program, the only drugs approved for use in WTD as of January 28, 2017, are xylazine (anesthetic agent) and yohimbine (anesthesia reversal).66 As such, the use of all other drugs is extralabel, which is defined as use in a species not listed in the labeling. Any drug should be used only under the direction of a licensed veterinarian and in the context of a valid veterinarian–client–patient relationship.
Even with good management, appropriate nutrition, appropriate parasite control, and effective vaccination strategies, there will be a need for antimicrobials to treat disease and reduce pain and suffering. Prudent use of antimicrobials will help minimize antimicrobial resistance in bacteria. The use of antimicrobials prophylactically is controversial and must be carefully examined—as should the stress and risk of injury associated with catching, restraining, and treating sick deer. The use of prophylactic antibiotics at NADC is the result of years of empirical evidence coupled with recommendations from veterinarians experienced in the health management of farmed deer herds. The vaccine regimens, parasite control, and antibiotic usage we describe here have proved successful under our conditions, but we in no way imply that alternative practices are inferior. For all products, the dosages we use are those listed on product labels for cattle.
Breeding Herd Management
In the WTD breeding herd at NADC, we maintain a buck:doe ratio of 1:10 to 1:15. As such, our herd consists of 30 to 40 does and 2 or 3 bucks. Except during the breeding season, bucks are housed apart from does on approximately 1.5 acres of pasture, whereas the does are housed on approximately 4 acres of mixed-grass pasture. Both types of pasture contain windbreaks and feeders. Bucks are introduced into the herd for breeding in mid-November and are removed in late January. With a gestation of 195 to 205 d, fawning typically begins in early June, peaks in mid- to late June, and ends by early August.
In April and again in October, all WTD are processed through a drop-floor chute (Figure 5) for fecal and blood collection, deworming with ivermectin, and treatment with a single dose of oxytetracycline as a prophylactic measure for handling-related injuries. In addition, in April, WTD receive a cattle foot-rot vaccine (Fusogard, Elanco Animal Health, Greenfield, IN) to prevent digital dermatitis, and Bovi Sera (Colorado Serum, Denver, CO) as an aid in the prevention and treatment of enteric and respiratory conditions caused by Trueperella pyogenes, E. coli, Mannheimia haemolytica, Pasteurella multocida, and Salmonella typhimurium. In October, deer receive a multivalent Clostridium spp. toxoid (Ultrabac 8, Zoetis, Parsippany, NJ) and a combination bovine respiratory and leptospirosis vaccine (Triangle 10, Boehringer Ingelheim Vetmedica, St Joseph, MO). Also in October, for personnel safety and to decrease severe injuries resulting from interanimal aggression, antlers (in the hard-antler stage) are removed by using an obstetric wire saw. It is important to remove the antler above the pedicle to prevent pedicle damage (Figure 6). Damage to the antler pedicle can result in misshapen antlers in all subsequent seasons. Although antler velvet is highly vascular and innervated, thus requiring anesthesia and analgesia for removal, hard antler is avascular, lacks nerve supply, and is considered dead bone;1,119,120 as such, anesthesia and analgesia are not necessary for the removal of hard antler.
Figure 5.

A WTD restrained in a drop-floor chute; its feet are off the ground, preventing jumping. A caretaker applies firm downward pressure over the deer's thoracic or lumbar spinal region to ensure that the animal remains restrained in the V-shaped region of the chute.
Figure 6.

To prevent serious injury to other animals or personnel, antler is removed by using an obstetric wire saw placed above the antler pedicle to avoid damage to the pedicle. Antler is removed only in the hard-antler stage once all the velvet has been rubbed off.
Deer are browsers or selective grazers, preferring high-quality forages. Breeding animals require 2.5% of their body weight in dry matter and 10% to 12% crude protein for maintenance. Rations for breeding deer should be 14% to 19% protein, whereas growing rations should contain 16% to 20% crude protein. Because our breeding herd often contains younger, growing animals as well as mature deer, we feed a single commercial pelleted feed containing 18% crude protein (Trophy Image Pellets, Kent Nutrition Group) year-round. The feeding rate is dependent on the quality of the hay or pasture and the body condition score and metabolic status of the animals. Trace mineral salt blocks and good-quality hay are provided unrestricted year-round; however, hay consumption varies with season and pasture condition.
Fawn Rearing
Animal care personnel at NADC care for multiple species of livestock. Accordingly, to prevent the introduction of disease (for example, malignant catarrhal fever, paratuberculosis, cryptosporidiosis) from other animals, personnel wear clothing and footwear dedicated for use in the deer pastures. Prior to fawning, the pasture is mowed, leaving 2 strips (approximately 2 to 3 feet wide) of tall pasture grass that run the length of the pasture. These strips of pasture grass provide a place for newborn fawns to hide. Beginning in late May, personnel search the pasture twice daily for newborn fawns, which are often located in the tall strips of grass. To further decrease the potential for spreading disease when handling fawns, personnel wear exam gloves, changing them between fawns. Within 24 h of birth, all fawns are removed from the pasture and transported to a fawn-rearing facility. Medium-sized portable dog crates are used to transport fawns and are sanitized with an appropriate disinfectant (Virkon S, duPont Animal Health Solutions, Wilmington, DE) after each use.
The weight, sex, and body temperature of each fawn is recorded in an individual animal record; when known, the identity of the dam is recorded also. On admission to the fawn-rearing facility, each fawn receives a combination product containing Clostridium perfringens type C antitoxin and a therapeutic antibody specific to E. coli (Ecolizer +C20, Novartis Animal Health, Larchwood, IA), a bovine probiotic (Probios, Vets Plus, Menomonie, WI), and a single dose of florfenicol (Nuflor, Merck Animal Health, Madison, NJ). The navel is treated with 7% iodine solution. Infections at the site of ear-tag placement, sometimes resulting in severe inflammation, have been noted historically; therefore, at admission, the left ear of each fawn is punched for tag placement, which occurs 4 d later. In the intervening 4 d, the punch site is treated with antibiotic ointment (Animax Fougera Pharmaceuticals, Melville, NY). For easier identification, ear tag numbers begin with the year that the fawn is born; female tags are yellow, and male tags are white. For further identification, microchips (EZid, EZidAvid, Greeley, CO) are placed subcutaneously, either to the right of the anus or on the ventral surface of the tail.
Fawns are housed individually in pens bedded with clean straw for as long as 2 wk. Milk consumption, defecation, and urination are recorded daily. After 2 wk of age, fawns are grouped by age and sex and housed together (4 or 5 fawns per group) in pens bedded with clean oat straw. At approximately 30 d of age, fawns are allowed access to larger, outdoor pens. Soiled bedding is removed and replaced with clean fresh straw daily. Animal care personnel don barn-dedicated clothing and boots upon entering the fawn-rearing facility. Boot baths containing an appropriate disinfectant (Wexcide, Wexford Labs, Kirkwood, MO) are placed strategically at building entry and exit points and entry points to pen and crate areas.
Feeding begins with the youngest fawns. Ill fawns are fed last; signage is posted on crates or pens to alert personnel regarding the presence of an ill fawn. Fawns are fed twice daily (approximately 0700 and 1400) with warmed goat colostrum for the first 4 feedings (Figure 7), followed by a 50:50 mixture of colostrum and doe milk replacer (Zoologic Doe Milk Replacer, PetAg, Hampshire, IL), and eventually milk replacer only, with a daily intake goal of 10% to 20% of the fawn's body weight. During this dietary transition period, nutritional scours may occur and are treated, as described elsewhere,59 with a combination of oral probiotic (Probios, Vets Plus), kaolin pectin (Kaolin–Pectin, Durvet, Blue Sprints, MO), and subcutaneous fluids. Caution should be taken to avoid overfeeding, which can increase fawn morbidity and mortality through conditions such as abomasal bloat. Milk replacer is prepared fresh for each feeding, and exam gloves are changed between fawns or groups of fawns. Unused and unconsumed milk is discarded. Bottles are washed in hot soapy water and rinsed thoroughly after each use. Beginning at 4 d of age, the fawns are offered fresh black dirt, which is changed every 3 d; fresh dirt is believed to aid establishment of a healthy intestinal microbiota and serve as a source of micronutrients.51 To further aid the establishment of a healthy intestinal microbiota, bovine probiotic (Probios, Vets Plus) pastes or powders are added to the milk replacer daily. Fawns are offered fresh water without restriction, along with small amounts of alfalfa and a mix comprising 75% deer pellets of 18% protein content (Trophy Image Pellets, Kent Nutrition Group) and 25% cracked corn.
Figure 7.
Sample feeding schedule for WTD fawns. aZoologic Doe Milk Replacer is available from PetAg (Hampshire, IL).
While feeding, fawns are stimulated to urinate and defecate by gentle rubbing of the perineal area by using an unscented baby wipe, which is then discarded. Stimulation is continued until fawns demonstrate the ability to urinate and defecate unaided (typically 3 wk of age). Urination and defecation are recorded, and the physical consistency of feces is characterized (that is, normal, paste-like, pudding-like, or watery). Coccidiosis can occur, resulting in diarrhea (which may or may not contain blood) but can be treated as in bovine calves by using amprolium (CoRid, Merial, Duluth, GA).
Treats (for example, apples) can be offered to fawns beginning at 4 wk of age. Offering treats encourages socialization with animal care personnel. Animal care staff are encouraged to spend time with the fawns to acclimate them to handling and physical examination. Weaning begins when the youngest fawn in a group reaches 60 d of age, but only when all fawns in the group are consistently eating alfalfa and pelleted feed. The duration of the weaning process is typically 2 wk but may be shorter for large, vigorous fawns.
Male fawns are surgically castrated at approximately 75 d of age. An intramuscular combination of xylazine (1 mg/kg; Rompun, Bayer, Leverskusen, Germany) and ketamine (5 mg/kg; Ketalar, Par Pharmaceutical, Spring Valley, NY) typically is used for anesthesia and is followed by subcutaneous tolazoline (4 mg/kg; Tolazine, Lloyd Laboratories, Shenandoah, IA) for reversal, but other anesthetic regimens are available.50,71 At the time of castration, each fawn receives a dose of oxytetracycline to prevent castration-related infections. The timing of castration is important. Antler development is under hormonal control, primarily testosterone. If a fawn is castrated before the antler pedicle (the specialized generative tissue on the dorsum of the frontal bone) is formed, no generative tissue forms, and no antler develops.22 If the pedicle has formed, castration results in a small, velvet-covered antler, which is never cast.
Conclusions
Using WTD in any type of research setting is challenging, but it is especially difficult inside the confines of a BSL3 facility. Nevertheless, in our experience, the use of healthy, hand-raised fawns, which have a known medical background, coupled with acclimation to indoor housing, human presence, and physical handling make for superior research subjects compared with animals unaccustomed to such environments. The use of hand-raised, acclimated WTD increases confidence in research results and facilitates animal care and handling. In addition, and more importantly, hand-raising improves animal wellbeing, decreases animal stress, and avoids painful injuries.
As human populations and their livestock encroach on traditional wildlife habitat, new diseases will emerge and known diseases will arise in previously unaffected species.7 As such, the demand to conduct infectious disease research on wildlife will increase, as will the need for facilities in which to safely conduct such research. Principles and practices that have proved effective for safe, humane research in laboratory animals and livestock will need to be applied to wildlife species. Many issues associated with the use of wildlife are common to research in all animal species, including overall animal wellbeing, enrichment to reduce stress and boredom, prevention of disease introduction, diagnosis and treatment of common maladies, appropriate nutrition, and safe and humane animal handling. In the case of WTD, there is a long history of infectious disease research conducted at ever-increasing levels of sophisticated biosecurity, demonstrating that although challenging, this type of research can be conducted, and valuable insights can be gained.
Acknowledgments
We thank the many animal caretakers, veterinarians, and technicians who have provided excellent care and assistance over many years of WTD research at NADC. USDA is an equal-opportunity provider and employer. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. Research funds were provided by the US Department of Agriculture, Agricultural Research Service, and Animal and Plant Health Inspection Service.
References
- 1.Chapman DI. 1975. Antlers—bones of contention. Mamm Rev 5:121–172. [Google Scholar]
- 2.Cheng C, Nair ADS, Indukuri VV, Gong S, Felsheim RF, Jaworski D, Munderloh UG, Ganta RR. 2013. Targeted and random mutagenesis of Ehrlichia chaffeensis for the identification of genes required for in vivo infection. PLoS Pathog 9:e1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng C, Nair ADS, Indukuri VV, Gong S, Felsheim RF, Jaworski D, Munderloh UG, Ganta RR. 2013. Correction. Targeted and random mutagenesis of Ehrlichia chaffeensis for the identification of genes required for in vivo infection. Last row of Table 2 is missing. PLoS Pathog 9:doi: 10.1371/annotation/322c8bba-8dae-4196-b85d-5de315e44d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Code of Federal Regulations CFR. 2008. 9 CFR Animals and animal products. Washington (DC): US Government Printing Office. [Google Scholar]
- 5.Connell N. 2011. Biologic agents in the laboratory—the regulatory issues. FAS Public Interest Rep 64:13–17. [Google Scholar]
- 6.Dardiri AH, Logan LL, Mebus CA. 1987. Susceptibility of white-tailed deer to experimental heartwater infections. J Wildl Dis 23:215–219. [DOI] [PubMed] [Google Scholar]
- 7.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443–449. [DOI] [PubMed] [Google Scholar]
- 8.Davidson WR, Lockhart JM, Stallknecht DE, Howerth EW, Dawson JE, Rechav Y. 2001. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J Wildl Dis 37:538–544. [DOI] [PubMed] [Google Scholar]
- 9.Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht D, Shaddock J, Bouseman J, Hofmeister E, Olson JG. 1994. White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis 30:162–168. [DOI] [PubMed] [Google Scholar]
- 10.Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, Lockhart JM, Nettles VF, Olson JG, Childs JE. 1994. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol 32:2725–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker DJ, Evensen DT, Siemer WF, Leong KM, Riley SJ, Wild MA, Castle KT, Higgins CL. 2010. Understanding risk perceptions to enhance communication about human–wildlife interactions and the impacts of zoonotic disease. ILAR J 51:255–261. [DOI] [PubMed] [Google Scholar]
- 12.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, Hoover EA. 2012. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drolet BS, Reister LM, Rigg TD, Nol P, Podell BK, Mecham JO, VerCauteren KC, van Rijn PA, Wilson WC, Bowen RA. 2013. Experimental infection of white-tailed deer (Odocoileus virginianus) with Northern European bluetongue virus serotype 8. Vet Microbiol 166:347–355. [DOI] [PubMed] [Google Scholar]
- 14.Duffy MS, Greaves TA, Burt MD. 2004. Establishment of adult Parelaphostrongylus tenuis, patent infections, and acquired immunity after experimental infection of white-tailed deer (Odocoileus virginianus) and red deer (Cervus elaphus elaphus). J Parasitol 90:245–254. [DOI] [PubMed] [Google Scholar]
- 15.Duffy MS, MacAfee N, Burt MD, Appleton JA. 2002. An aspartyl protease inhibitor orthologue expressed by Parelaphostrongylus tenuis is immunogenic in an atypical host. Clin Diagn Lab Immunol 9:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder AM, Henderson DM, Nalls AV, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. 2015. Immediate and ongoing detection of prions in the blood of hamsters and deer following oral, nasal, or blood inoculations. J Virol 89:7421–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. 2013. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federation of Animal Science Societies. 2010. Guide for the care and use of agricultural animals in research and teaching, 3rd ed Champaign (IL): Federation of Animal Science Societies. [Google Scholar]
- 19.Fischer JR, Zhao T, Doyle MP, Goldberg MR, Brown CA, Sewell CT, Kavanaugh DM, Bauman CD. 2001. Experimental and field studies of Escherichia coli O157:H7 in white-tailed deer. Appl Environ Microbiol 67:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletch AL, Karstad LH. 1971. Studies on the pathogenesis of experimental epizootic hemorrhagic disease of white-tailed deer. Can J Comp Med 35:224–229. [PMC free article] [PubMed] [Google Scholar]
- 21.Foreyt WJ, Todd AC. 1979. Selected clinicopathologic changes associated with experimentally induced Fascioloides magna infection in white-tailed deer. J Wildl Dis 15:83–89. [DOI] [PubMed] [Google Scholar]
- 22.Fowler MA. 1986. Horns and antlers, p 489–493. In: Fowler MA. Zoo and wild animal medicine. Current therapy 3. Philadelphia (PA): W B Saunders. [Google Scholar]
- 23.Gaydos JK, Allison AB, Hanson BA, Yellin AS. 2002. Oral and fecal shedding of epizootic hemorrhagic disease virus, serotype 1 from experimentally infected white-tailed deer. J Wildl Dis 38:166–168. [DOI] [PubMed] [Google Scholar]
- 24.Gaydos JK, Davidson WR, Elvinger F, Howerth EW, Murphy M, Stallknecht DE. 2002. Cross-protection between epizootic hemorrhagic disease virus serotypes 1 and 2 in white-tailed deer. J Wildl Dis 38:720–728. [DOI] [PubMed] [Google Scholar]
- 25.Gaydos JK, Davidson WR, Elvinger F, Mead DG, Howerth EW, Stallknecht DE. 2002. Innate resistance to epizootic hemorrhagic disease in white-tailed deer. J Wildl Dis 38:713–719. [DOI] [PubMed] [Google Scholar]
- 26.Gaydos JK, Stallknecht DE, Kavanaugh D, Olson RJ, Fuchs ER. 2002. Dynamics of maternal antibodies to hemorrhagic disease viruses (Reoviridae: Orbivirus) in white-tailed deer. J Wildl Dis 38:253–257. [DOI] [PubMed] [Google Scholar]
- 27.Gnat S, Troscianczyk A, Nowakiewicz A, Majer-Dziedzic B, Ziolkowska G, Dziedzic R, Zieba P, Teodorowski O. 2015. Experimental studies of microbial populations and incidence of zoonotic pathogens in the faeces of red deer (Cervus elaphus). Lett Appl Microbiol 61:446–452. [DOI] [PubMed] [Google Scholar]
- 28.Goñi F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, Peyser D, Estevez V, Denkers N, Xu J, Osborn DA, Miller KV, Warren RJ, Brown DR, Chabalgoity JA, Hoover EA, Wisniewski T. 2015. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine 33:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenlee JJ, Smith JD, Kunkle RA. 2011. White-tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation. Vet Res 42:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haley NJ, Mathiason CK, Carver S, Telling GC, Zabel MD, Hoover EA. 2012. Sensitivity of protein misfolding cyclic amplification versus immunohistochemistry in antemortem detection of chronic wasting disease. J Gen Virol 93:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haley NJ, Siepker C, Walter WD, Thomsen BV, Greenlee JJ, Lehmkuhl AD, Richt JA. 2016. Antemortem detection of chronic wasting disease prions in nasal brush collections and rectal biopsy specimens from white-tailed deer by real-time quaking-induced conversion. J Clin Microbiol 54:1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, Mathiason C, Hoover E. 2013. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS One 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamdy FM, Dardiri AH. 1976. Response of white-tailed deer to infection with peste des petits ruminants virus. J Wildl Dis 12:516–522. [DOI] [PubMed] [Google Scholar]
- 34.Hamdy FM, Dardiri AH, Ferris DH, Breese SS., Jr 1975. Experimental infection of white-tailed deer with rinderpest virus. J Wildl Dis 11:508–515. [DOI] [PubMed] [Google Scholar]
- 35.Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, Hall M. 2011. Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: final report. Can J Vet Res 75:152–156. [PMC free article] [PubMed] [Google Scholar]
- 36.Hamir AN, Kunkle RA, Miller JM, Hall SM. 2006. Abnormal prion protein in ectopic lymphoid tissue in a kidney of an asymptomatic white-tailed deer experimentally inoculated with the agent of chronic wasting disease. Vet Pathol 43:367–369. [DOI] [PubMed] [Google Scholar]
- 37.Hamir AN, Richt JA, Miller JM, Kunkle RA, Hall SM, Nicholson EM, O'Rourke KI, Greenlee JJ, Williams ES. 2008. Experimental transmission of chronic wasting disease (CWD) of elk (Cervus elaphus nelsoni), white-tailed deer (Odocoileus virginianus), and mule deer (Odocoileus hemionus hemionus) to white-tailed deer by intracerebral route. Vet Pathol 45:297–306. [DOI] [PubMed] [Google Scholar]
- 38.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoff GL, Trainer DO. 1972. Experimental infection of Venezuelan equine encephalomyelitis virus in white-tailed deer. Am J Epidemiol 96:379–382. [DOI] [PubMed] [Google Scholar]
- 41.Howerth EW, Greene CE, Prestwood AK. 1988. Experimentally induced bluetongue virus infection in white-tailed deer: coagulation, clinical pathologic, and gross pathologic changes. Am J Vet Res 49:1906–1913. [PubMed] [Google Scholar]
- 42.Howerth EW, Tyler DE. 1988. Experimentally induced bluetongue virus infection in white-tailed deer: ultrastructural findings. Am J Vet Res 49:1914–1922. [PubMed] [Google Scholar]
- 43.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 44.Jaworski DC, Bowen CJ, Wasala NB. 2013. A white-tailed deer–lone star tick model for studying transmission of Ehrlichia chaffeensis. Vector Borne Zoonotic Dis 13:193–195. [DOI] [PubMed] [Google Scholar]
- 45.John TR, Schatzl HM, Gilch S. 2013. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion 7:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CJ, Aiken JM, McKenzie D, Samuel MD, Pedersen JA. 2012. Highly efficient amplification of chronic wasting disease agent by protein misfolding cyclic amplification with beads (PMCAb). PLoS One 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, McKenzie D. 2011. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451:990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirkpatrick RL, Scanlon PF. Care of captive whitetails, p 687–696, Chapter 41. In: Halls LK. White-tailed deer ecology and management. Harrisburg (PA): Stackpole Books. [Google Scholar]
- 50.Kreeger TJ. Drug doses—white-tailed deer, p 144. Handbook of wildlife chemical immobilization. Fort Collins (CO): Wildlife Pharmaceuticals. [Google Scholar]
- 51.Kreulen DA. 1985. Lick use by large herbivores: a review of benefits and banes of soil consumption. Mammal Rev 15:107–123. [Google Scholar]
- 52.Kruse RH, Barbeito MS. 1998. A history of the American Biologic Safety Association, part III: Safety Conferences 1978 to 1987. Appl Biosaf 3:11–25. [Google Scholar]
- 53.Kuttler KL, Graham OH, Johnson SR. 1971. Apparent failure of Boophilus annulatus to transmit anaplasmosis to white-tailed deer (Odocoileus virginianus). J Parasitol 57:657–659. [PubMed] [Google Scholar]
- 54.Luttrell MP, Nakagaki K, Howerth EW, Stallknecht DE, Lee KA. 1994. Experimental infection of Borrelia burgdorferi in white-tailed deer. J Wildl Dis 30:146–154. [DOI] [PubMed] [Google Scholar]
- 55.Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA. 2010. B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J Virol 84:5097–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in preclinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. [DOI] [PubMed] [Google Scholar]
- 58.Matthews LR. 2000. Deer handling and transport, p 331–362. In: Grandin T. Livestock handling and transport. Boston (MA): CAB International. [Google Scholar]
- 59.McNulty E, Selariu AI, Anderson K, Hayes-Klug J, Nalls AV, Powers JG, Hoover EA, Mathiason CK. 2016. Aspects of the husbandry and management of captive cervids. Lab Anim (NY) 45:140–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller MW, Wolfe LL, Sirochman TM, Sirochman MA, Jewell JE, Williams ES. 2012. Survival patterns in white-tailed and mule deer after oral inoculation with a standardized, conspecific prion dose. J Wildl Dis 48:526–529. [DOI] [PubMed] [Google Scholar]
- 61.Moen AN. 1982Senses, communications and the use of space, p 9–105. Chapter 3.The biology and management of wild ruminants. Lansing (NY): CornerBrook Press. [Google Scholar]
- 62.Moniwa M, Embury-Hyatt C, Zhang Z, Hole K, Clavijo A, Copps J, Alexandersen S. 2012. Experimental foot-and-mouth disease virus infection in white-tailed deer. J Comp Pathol 147:330–342. [DOI] [PubMed] [Google Scholar]
- 63.Moyer PL, Varela AS, Luttrell MP, Moore VA, 4th, Stallknecht DE, Little SE. 2006. White-tailed deer (Odocoileus virginianus) develop spirochetemia following experimental infection with Borrelia lonestari. Vet Microbiol 115:229–236. [DOI] [PubMed] [Google Scholar]
- 64.Mulcahy DM. 2003. Does the Animal Welfare Act apply to free-ranging animals? ILAR J 44:252–258. [DOI] [PubMed] [Google Scholar]
- 65.Munderloh UG, Tate CM, Lynch MJ, Howerth EW, Kurtti TJ, Davidson WR. 2003. Isolation of an Anaplasma sp. organism from white-tailed deer by tick cell culture. J Clin Microbiol 41:4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Research Support Program No. 7. [Internet]. 2017. Minor use animal drug program. [Cited 28 February 2017]. Available at: http://www.nrsp-7.org/mumsrx/.
- 67.Nair AD, Cheng C, Jaworski DC, Ganta S, Sanderson MW, Ganta RR. 2015. Attenuated mutants of Ehrlichia chaffeensis induce protection against wild-type infection challenge in the reservoir host and in an incidental host. Infect Immun 83:2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nair AD, Cheng C, Jaworski DC, Willard LH, Sanderson MW, Ganta RR. 2014. Ehrlichia chaffeensis infection in the reservoir host (white-tailed deer) and in an incidental host (dog) is impacted by its prior growth in macrophage and tick cell environments. PLoS One 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negrón ME, Pogranichniy RM, Van Alstine W, Hilton WM, Levy M, Raizman EA. 2012. Evaluation of horizontal transmission of bovine viral diarrhea virus type 1a from experimentally infected white-tailed deer fawns (Odocoileus virginianus) to colostrum-deprived calves. Am J Vet Res 73:257–262. [DOI] [PubMed] [Google Scholar]
- 70.Nichols TA, Spraker TR, Rigg TD, Meyerett-Reid C, Hoover C, Michel B, Bian J, Hoover E, Gidlewski T, Balachandran A, O'Rourke K, Telling GC, Bowen R, Zabel MD, VerCauteren KC. 2013. Intranasal inoculation of white-tailed deer (Odocoileus virginianus) with lyophilized chronic wasting disease prion particulate complexed to montmorillonite clay. PLoS One 8:e62455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nielsen L. 1999. Species specific recommendations, p. 259. In: Chemical immobilization of wild and exotic animals. Ames (IA): Iowa State University Press. [Google Scholar]
- 72.Nol P, Palmer MV, Waters WR, Aldwell FE, Buddle BM, Triantis JM, Linke LM, Phillips GE, Thacker TC, Rhyan JC, Dunbar MR, Salman MD. 2008. Efficacy of oral and parenteral routes of Mycobacterium bovis Bacille Calmette–Guerin vaccination against experimental bovine tuberculosis in white-tailed deer (Odocoileus virginianus): a feasibility study. J Wildl Dis 44:247–259. [DOI] [PubMed] [Google Scholar]
- 73.Oliver JH, Jr, Stallknecht D, Chandler FW, James AM, McGuire BS, Howerth E. 1992. Detection of Borrelia burgdorferi in laboratory-reared Ixodes dammini (Acari: Ixodidae) fed on experimentally inoculated white-tailed deer. J Med Entomol 29:980–984. [DOI] [PubMed] [Google Scholar]
- 74.Olsen SC. 2010. Brucellosis in the United States: role and significance of wildlife reservoirs. Vaccine 28 Suppl 5:F73–F76. [DOI] [PubMed] [Google Scholar]
- 75.Osorio JE, Godsey MS, Defoliart GR, Yuill TM. 1996. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am J Trop Med Hyg 54:338–342. [DOI] [PubMed] [Google Scholar]
- 76.Palmer MV. 2013. Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transbound Emerg Dis 60 Suppl 1:1–13. [DOI] [PubMed] [Google Scholar]
- 77.Palmer MV, Stabel JR, Waters WR, Bannantine JP, Miller JM. 2007. Experimental infection of white-tailed deer (Odocoileus virginianus) with Mycobacterium avium subsp. paratuberculosis. J Wildl Dis 43:597–608. [DOI] [PubMed] [Google Scholar]
- 78.Palmer MV, Thacker TC, Waters WR, Robbe-Austerman S. 2014. Oral vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis Bacillus Calmette–Guerin (BCG). PLoS One 9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer MV, Waters WR, Whipple DL. 2002. Lesion development in white-tailed deer (Odocoileus virginianus) experimentally infected with Mycobacterium bovis. Vet Pathol 39:334–340. [DOI] [PubMed] [Google Scholar]
- 80.Palmer MV, Waters WR, Whipple DL. 2003. Aerosol exposure of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis. J Wildl Dis 39:817–823. [DOI] [PubMed] [Google Scholar]
- 81.Palmer MV, Waters WR, Whipple DL. 2004. Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. J Wildl Dis 40:87–91. [DOI] [PubMed] [Google Scholar]
- 82.Palmer MV, Waters WR, Whipple DL, Slaughter RE, Jones SL. 2004. Evaluation of an in vitro blood-based assay to detect production of interferon γ by Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus). J Vet Diagn Invest 16:17–21. [DOI] [PubMed] [Google Scholar]
- 83.Palmer MV, Whipple DL, Olsen SC. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J Wildl Dis 35:450–457. [DOI] [PubMed] [Google Scholar]
- 84.Passler T, Walz HL, Ditchkoff SS, van Santen E, Brock KV, Walz PH. 2012. Distribution of bovine viral diarrhoea virus antigen in persistently infected white-tailed deer (Odocoileus virginianus). J Comp Pathol 147:533–541. [DOI] [PubMed] [Google Scholar]
- 85.Passler T, Walz PH, Ditchkoff SS, Givens MD, Maxwell HS, Brock KV. 2007. Experimental persistent infection with bovine viral diarrhea virus in white-tailed deer. Vet Microbiol 122:350–356. [DOI] [PubMed] [Google Scholar]
- 86.Presidente PJ, McCraw BM, Lumsden JH. 1974. Early pathologic changes associated with Fasciola hepatica infections in white-tailed deer. Can J Comp Med 38:271–279. [PMC free article] [PubMed] [Google Scholar]
- 87.Presidente PJ, McCraw BM, Lumsden JH. 1975. Experimentally induced Fasciola hepatica infection in white-tailed deer. II. Pathological features. Can J Comp Med 39:166–177. [PMC free article] [PubMed] [Google Scholar]
- 88.Quist CF, Howerth EW, Bounous DI, Stallknecht DE. 1997. Cell-mediated immune response and IL2 production in white-tailed deer experimentally infected with hemorrhagic disease viruses. Vet Immunol Immunopathol 56:283–297. [DOI] [PubMed] [Google Scholar]
- 89.Quist CF, Howerth EW, Stallknecht DE, Brown J, Pisell T, Nettles VF. 1997. Host defense responses associated with experimental hemorrhagic disease in white-tailed deer. J Wildl Dis 33: 584–599. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi T, Stittmatter J, Turner K, Davis DS. 1999. Experimental infection of white-tailed deer with rangiferine brucellosis. J Wildl Dis 35:388–391. [DOI] [PubMed] [Google Scholar]
- 91.Raizman EA, Pogranichniy R, Levy M, Negron M, Langohr I, Van Alstine W. 2009. Experimental infection of white-tailed deer fawns (Odocoileus virginianus) with bovine viral diarrhea virus type 1 isolated from free-ranging white-tailed deer. J Wildl Dis 45:653–660. [DOI] [PubMed] [Google Scholar]
- 92.Raizman EA, Pogranichniy RM, Levy M, Negron M, Van Alstine W. 2011. Experimental infection of colostrum-deprived calves with bovine viral diarrhea virus type 1a isolated from free-ranging white-tailed deer (Odocoileus virginianus). Can J Vet Res 75:65–68. [PMC free article] [PubMed] [Google Scholar]
- 93.Reilly JR, Muraschi TF, Dean DJ. 1962. Experimental Leptospira pomona infection in white-tailed deer Odocoileus virginianus, and in cattle. J Am Vet Med Assoc 140:53–57. [PubMed] [Google Scholar]
- 94.Reinhardt V. 2005. Hair pulling: a review. Lab Anim 39:361–369. [DOI] [PubMed] [Google Scholar]
- 95.Richmond JY, Hill RH, Weyant RS, Nesby-O'Dell SL, Vinson PE. 2003. What's hot in animal biosafety? ILAR J 44:20–27. [DOI] [PubMed] [Google Scholar]
- 96.Ridpath JF, Driskell EA, Chase CC, Neill JD, Palmer MV, Brodersen BW. 2008. Reproductive tract disease associated with inoculation of pregnant white-tailed deer with bovine viral diarrhea virus. Am J Vet Res 69:1630–1636. [DOI] [PubMed] [Google Scholar]
- 97.Ridpath JF, Neill JD, Chase CC. 2012. Impact of BVDV infection of white-tailed deer during 2nd and 3rd trimesters of pregnancy. J Wildl Dis 48:758–762. [DOI] [PubMed] [Google Scholar]
- 98.Robinson RM, Hidalgo RJ, Daniel WS, Rideout DW, Marburger RG. 1970. Salmonellosis in white-tailed deer fawns. J Wildl Dis 6:389–396. [DOI] [PubMed] [Google Scholar]
- 99.Rubenstein R, Chang B, Gray P, Piltch M, Bulgin MS, Sorensen-Melson S, Miller MW. 2011. Prion disease detection, PMCA kinetics, and IgG in urine from sheep naturally–experimentally infected with scrapie and deer with preclinical–clinical chronic wasting disease. J Virol 85:9031–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruder MG, Allison AB, Stallknecht DE, Mead DG, McGraw SM, Carter DL, Kubiski SV, Batten CA, Klement E, Howerth EW. 2012. Susceptibility of white-tailed deer (Odocoileus virginianus) to experimental infection with epizootic hemorrhagic disease virus serotype 7. J Wildl Dis 48:676–685. [DOI] [PubMed] [Google Scholar]
- 101.Ruder MG, Howerth EW, Stallknecht DE, Allison AB, Carter DL, Drolet BS, Klement E, Mead DG. 2012. Vector competence of Culicoides sonorensis (Diptera: Ceratopogonidae) to epizootic hemorrhagic disease virus serotype 7. Parasit Vectors 5:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shope RE, Macnamara LG, Mangold R. 1960. A virus-induced epizootic hemorrhagic disease of the Virginia white-tailed deer (Odocoileus virginianus). J Exp Med 111:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith KE, Stallknecht DE, Nettles VF. 1996. Experimental infection of Culicoides lahillei (Diptera: Ceratopogonidae) with epizootic hemorrhagic disease virus serotype 2 (Orbivirus: Reoviridae). J Med Entomol 33:117–122. [DOI] [PubMed] [Google Scholar]
- 104.Smith KE, Stallknecht DE, Sewell CT, Rollor EA, Mullen GR, Anderson RR. 1996. Monitoring of Culicoides spp. at a site enzootic for hemorrhagic disease in white-tailed deer in Georgia, USA. J Wildl Dis 32:627–642. [DOI] [PubMed] [Google Scholar]
- 105.Stahl RS, Ellis CK, Nol P, Waters WR, Palmer M, VerCauteren KC. 2015. Fecal volatile organic Ccompound profiles from white-tailed deer (Odocoileus virginianus) as indicators of Mycobacterium bovis exposure or Mycobacterium bovis Bacille Calmette–Guerin (BCG) vaccination. PLoS One 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stallknecht DE, Howerth EW, Kellogg ML, Quist CF, Pisell T. 1997. In vitro replication of epizootic hemorrhagic disease and bluetongue viruses in white-tailed deer peripheral blood mononuclear cells and virus-cell association during in vivo infections. J Wildl Dis 33:574–583. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi K, Kitajima N, Abe N, Mishiro S. 2004. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and 4 patients who ate the deer. Virology 330:501–505. [DOI] [PubMed] [Google Scholar]
- 108.Tate CM, Howerth EW, Mead DG, Dugan VG, Luttrell MP, Sahora AI, Munderloh UG, Davidson WR, Yabsley MJ. 2013. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick Borne Dis 4:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tate CM, Mead DG, Luttrell MP, Howerth EW, Dugan VG, Munderloh UG, Davidson WR. 2005. Experimental infection of white-tailed deer with Anaplasma phagocytophilum, etiologic agent of human granulocytic anaplasmosis. J Clin Microbiol 43:3595–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ueti MW, Olafson PU, Freeman JM, Johnson WC, Scoles GA. 2015. A virulent Babesia bovis strain failed to infect white-tailed deer (Odocoileus virginianus). PLoS One 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Van Campen H, Williams ES, Edwards J, Cook W, Stout G. 1997. Experimental infection of deer with bovine viral diarrhea virus. J Wildl Dis 33:567–573. [DOI] [PubMed] [Google Scholar]
- 112.Varela AS, Stallknecht DE, Yabsley MJ, Moore VA, 4th, Howerth EW, Davidson WR, Little SE. 2005. Primary and secondary infection with Ehrlichia chaffeensis in white-tailed deer (Odocoileus virginianus). Vector Borne Zoonotic Dis 5:48–57. [DOI] [PubMed] [Google Scholar]
- 113.Vercauteren KC, Vandeelen TR, Lavelle MJ, Hall WH. 2010. Assessment of abilities of white-tailed deer to jump fences. J Wildl Manage 74:1378–1381. [Google Scholar]
- 114.Vosdingh RA, Trainer DO, Easterday BC. 1968. Experimental bluetongue disease in white-tailed deer. Can J Comp Med 32:382–387. [PMC free article] [PubMed] [Google Scholar]
- 115.Watts DM, Tammariello RF, Dalrymple JM, Eldridge BF, Russell PK, Top FH., Jr 1979. Experimental infection of vertebrates of the Pocomoke Cypress Swamp, Maryland, with Keystone and Jamestown Canyon viruses. Am J Trop Med Hyg 28:344–350. [DOI] [PubMed] [Google Scholar]
- 116.Whitenack DL, Castro AE, Kocan AA. 1981. Experimental malignant catarrhal fever (African form) in white-tailed deer. J Wildl Dis 17:443–451. [DOI] [PubMed] [Google Scholar]
- 117.Williams ES, Snyder SP, Martin KL. 1983. Experimental infection of some North American wild ruminants and domestic sheep with Mycobacterium paratuberculosis: clinical and bacteriological findings. J Wildl Dis 19:185–191. [DOI] [PubMed] [Google Scholar]
- 118.Wilson DE, Chosewood LC. Risk criteria for establishing ascending levels of containment, p 4–5. In: Wilson D, Chosewood L. Biosafety in microbiological and biomedical laboratories. Washington (DC): US Department of Health and Human Services. [Google Scholar]
- 119.Wilson PR, Thomas DG, Stafford KJ, Mellor DJ. 1999. Routes and doses of lignocaine hydrochloride for analgesia of the velvet antler of stags. N Z Vet J 47:167–174. [DOI] [PubMed] [Google Scholar]
- 120.Woodbury MR, Haigh JC. 1996. Innervation and anesthesia of the antler pedicle in wapiti and fallow deer. Can Vet J 37:486–489. [PMC free article] [PubMed] [Google Scholar]
- 121.Woods LW, Lehmkuhl HD, Swift PK, Chiu PH, Hanley RS, Nordhausen RW, Stillian MH, Drew ML. 2001. Experimental adenovirus hemorrhagic disease in white-tailed deer fawns. J Wildl Dis 37:153–158. [DOI] [PubMed] [Google Scholar]
- 122.Yabsley MJ, Loftis AD, Little SE. 2008. Natural and experimental infection of white-tailed deer (Odocoileus virginianus) from the United States with an Ehrlichia sp. closely related to Ehrlichia ruminantium. J Wildl Dis 44:381–387. [DOI] [PubMed] [Google Scholar]
- 123.Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. 2002. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg Infect Dis 8:668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]