Abstract
Safety pharmacology studies in dogs often integrate behavioral assessments made using video recording with physiologic measurements collected by telemetry. However, whether merely wearing the telemetry vest affects canine behavior and other parameters has not been evaluated. This pilot study assessed the effect of a telemetry vest on behavioral and physiologic responses to an environmental stressor, the sounds of a thunderstorm, in Labrador retrievers. Dogs were assigned to one of 2 experimental groups (Vest and No-Vest, n = 8 dogs per group) by using a matched pairs design, with a previously determined, sound-associated anxiety score as the blocking variable. Dogs were individually retested with the same standardized sound stimulus (thunderstorm) in an open-field arena, and their behavioral responses were video recorded. Video analysis of locomotor activity and anxiety-related behavior and manual determination of heart rate and body temperature were performed; results were compared between groups. Vest wearing did not affect total locomotor activity or rectal body temperature but significantly decreased heart rate by 8% and overall mean anxiety score by 34% during open-field test sessions. Our results suggest that the use of telemetry vests in dogs influences the measurement of physiologic parameters and behaviors that are assessed in safety pharmacology studies.
Abbreviations: MAS, mean anxiety score; OFT, open-field test
An important objective of safety pharmacology studies is to ensure that approved drugs do not adversely affect human physiologic function.4 The development of safety pharmacology as a scientific discipline was accelerated in 2000 when the International Conference on Harmonization published its guideline (ICH S7A) for safety pharmacology studies for human pharmaceuticals.4 This guideline requires that test substances be assessed for potential adverse effects on the major vital organ systems including the cardiovascular system, central nervous system, and respiratory system.10,21 ICH S7A does not specify a test species; however, several reviews suggest that dogs and monkeys have the greatest value for predicting human cardiovascular and neurologic toxicity.16,20,32 A second guideline (ICH S7B)22 addresses the evaluation of drug-induced delays in ventricular repolarization. This guideline advocates against the use of mice and rats for in vivo cardiac electrophysiology studies because their ionic mechanisms of repolarization differ from humans, dogs, pigs, and other larger mammalian species.22 A recent survey of industrial toxicity testing laboratories that perform ICH S7 studies indicated that domesticated dogs were the animals used most frequently for the assessment of new chemical entities.3
Safety pharmacology studies in dogs often rely on telemetry and other noninvasive approaches to monitor electrocardiography, respiration, temperature, and locomotor activity.3,15,18 In telemetry studies, a device continuously transmits physiologic data to a remote receiver using radio frequency communication, and allows evaluation of unrestrained experimental animals. The 2 types of telemetry systems that are most commonly used are noninvasive vest (jacketed or external) systems and invasive implanted (or internal) systems. Noninvasive collection of physiologic data by using a jacketed system has the advantage of avoiding surgical device implantation and eliminating the influences of anesthetic and restraint-induced stress.33,40 These factors may alter the sensitivity of the models to detect drug-induced effects.24 Validation studies have compared the effectiveness of using noninvasive telemetry with implanted and other forms of invasive telemetry methods in dogs.6,12,33,40 These studies show good correlation between invasive and noninvasive telemetry methods for several endpoints including blood pressure, respiratory rate, heart rate, and other parameters of interest to safety pharmacologists.
Research application of these systems is widespread. Indeed, the US Food and Drug Administration requires the submission of telemetric measurements for cardiovascular parameters with an Investigational New Drug application,34 and telemetry studies of cardiovascular function in dogs are commonly completed to meet this regulatory requirement.2,3,9,31,38 Some dog telemetry studies also include simultaneous assessment of neurologic function by using videorecording of behavior and cardiovascular and respiratory parameters by telemetry.30,37 This approach is an ethical and cost-effective way to reduce animal use by replacing the need for additional rodent neurotoxicity studies.29,35 Other investigators have shown that evaluation of multiple endpoints (for example, physiology, pharmacokinetics, clinical chemistry) in the same animal results in more robust experimental data and concurrently reduces animal use.5
Despite the advantages of telemetry, the effects of telemetry vest wearing on physiologic and behavioral outcome measures have not been described. Previous studies have shown that pressure wraps (for example, the Anxiety Wrap [The Company of Animals, Davenport, FL], ThunderShirt [ThunderWorks, Durham, NC]) reduce a dog's response to thunderstorms and other anxiety-causing events.7,8,23 The similarity in design between these pressure wraps and telemetry vests suggests that telemetry vest-wearing in dogs, as may occur during safety pharmacology studies, may also influence dog behavior.
This pilot study was designed to assess whether spontaneous locomotor activity, physiologic variables, and behavioral response to an environmental stressor in dogs are influenced by the wearing of a commercially available jacketed telemetry system (vest).40 Our experiment took advantage of an open-field test (OFT)1 that was adapted by our laboratory,17 in which behavioral responses of individual dogs are observed and quantified after exposure to an anxiety-producing loud sound. Our hypothesis was that dogs wearing telemetry vests would exhibit lower heart rate and anxiety measures than dogs without vests.
Materials and Methods
Animals.
This experiment used 16 adult field-trial–bred Labrador retrievers acquired by a military working dog training facility (K2 Solutions, Southern Pines, NC). Dogs were processed and quarantined at the K2 Canine Facility prior to shipment to the North Carolina State University College of Veterinary Medicine. At the time of study, dogs were 2.50 to 4.25 y old. There were 8 intact males, 5 intact females, and 3 spayed females; 10 dogs had black coat color, and 6 were yellow. Dogs were used in several studies prior to this experiment to assess their emotional resilience17,36 and visual and olfactory discrimination capacities.25,26 All dogs had a previous exposure to the OFT approximately 6 mo prior to the conduct of this study and a preliminary study using the telemetry system approximately 4 mo before the current experiment. Dogs were not acclimated to the vests prior to the conduct of either study.
Dogs were individually housed in an environmentally controlled, cinder-block building containing 18 solid-floor pens (1.5 × 2.4 m), each with a raised resting surface. The temperature set point was 22 °C, and relative humidity was maintained between 30% to 70%. Dogs were provided continuous access to water in stainless steel buckets or bowls and were fed twice daily in amounts sufficient to maintain appropriate body condition (Iams Mini Chunks, P and G Pet Care, Cincinnati, OH). Several dogs developed minor skin or gastrointestinal problems during their stay at the facility and received an alternate diet (Purina Performance [Purina Mills International, St Louis, MO] and Iams Kangaroo and Oat [P and G Pet Care]); at the time of this study, there were no gastrointestinal signs, and a minor skin problem in only one dog. During daily cleaning of the facility, dogs were turned out on a concrete slab for exercise and were hand-walked twice weekly for 15 min (in addition to walking to adjacent buildings for testing). They received hard rubber or plastic toys in their runs during the day (Kong Company, Golden, CO; Jolly Pets, Streetsboro, OH). Dogs received a physical examination on arrival at the facility and were observed daily by trained animal care technicians. They received monthly heartworm preventative (oral ivermectin or topical selamectin [Revolution, Zoestis, Parsippany, NJ]).
Experimental protocols were reviewed and approved by the North Carolina State University IACUC and the US Army Medical Research and Materiel Command Animal Care and Use Review Office. The North Carolina State University College of Veterinary Medicine is AAALAC-accredited.
Experimental design and animal assignment.
The 2 experimental groups (Vest and No-Vest, n = 8 dogs per group) were assigned by using a randomized matched-pairs design, with previously determined OFT sound-associated anxiety scores used as the blocking variable.17 Global anxiety scores17 were used to rank the dogs from lowest to highest anxiety rating. The first of each pair of dogs was randomly assigned (using a coin toss) to either Vest or No-Vest groups; there was no significant difference (t test; P = 0.654) in global anxiety scores for the 2 experimental groups (Table 1). The order of the dogs was initially randomized, after which dogs were tested in the same order each day; dogs in the Vest or No-Vest groups were intentionally alternated during test sessions. Testing was conducted at the same time each day for 3 d (starting at 1100), with half of the dogs at a time; the other half were tested the following week. A single size of vest and undershirt was used for all dogs in this study. Variations in dog size were accommodated by the fabric hook-and-loop fasteners closure systems for both the spandex undershirt and vest, which allowed each to be fitted snugly; the vest was tightened to allow 2 fingers to be placed under the vest. For the purposes of this study, the Vest group refers to the dogs that wore the undershirt and telemetry vest.
Table 1.
Demographic information and results of prestudy behavioral evaluations used to form experimental groups
| Dog no. | Sex | Color | Age (y) | Global Anxiety Score |
| Vest | ||||
| 2 | M | black | 2.7 | 0.125 |
| 3 | F | black | 2.9 | 0.250 |
| 6 | M | yellow | 3.1 | 0.750 |
| 7 | FS | yellow | 2.8 | 0.875 |
| 9 | F | yellow | 2.6 | 1.125 |
| 14 | FS | black | 3.4 | 1.625 |
| 12 | F | black | 2.6 | 1.875 |
| 15 | F | yellow | 2.8 | 2.625 |
| Mean | 2.9 ± 0.1 | 1.16 ± 0.30 | ||
| No vest | ||||
| 1 | FS | black | 3.3 | 0.250 |
| 8 | M | black | 3.8 | 0.500 |
| 5 | M | black | 2.9 | 0.750 |
| 4 | M | black | 2.7 | 0.875 |
| 10 | M | yellow | 4.2 | 1.625 |
| 11 | M | black | 2.7 | 1.875 |
| 13 | M | black | 3.8 | 2.000 |
| 16 | F | yellow | 2.8 | 3.000 |
| Mean | 3.3 ± 0.2 | 1.36 ± 0.33 |
F, female; FS, female spayed; M, male
Previously determined global anxiety scores from reference 17 were used as the blocking variable in the present study. Overall data (mean ± SEM) are shown for group age and global anxiety score.
Dogs in the No-Vest group were fitted with the telemetry undershirt and vest prior to the OFT to simulate handling procedures used in the Vest group. The undershirt and vest were left on only long enough to obtain manual heart rate and rectal temperature (approximately 2 min) and were removed immediately prior to the start of the OFT. Results collected during the 3-d OFT were compared between dogs that wore a telemetry vest and those that did not.
OFT and measurement of spontaneous locomotor activity.
Details of the OFT arena, testing protocol, and data collection have been reported previously.1,17 Briefly, the OFT arena consisted of a room approximately 2.9 × 2.7 m, located in a dedicated free-standing building that minimized noise and other disturbances. The arena was equipped with a 61 × 76 × 91-cm open-floored hide. A camera was mounted in the center, above ceiling level, and a second horizontally mounted camera located 0.6 m above the OFT floor recorded the dog's behavior while in the hide. (Because the time spent in the hide was so short, these data were not analyzed in the present study.) Each dog was placed in the open field for 9 min on 3 consecutive test days, and each test session was recorded digitally by using a dedicated behavioral analysis program (EthoVision XT software, Noldus Information Technology, Leesburg, VA). The 9-min period was divided into three 3-min test phases. The first and last test phase on each day had no auditory stimuli (quiet), whereas the middle test phase was either quiet (days 1 and 3) or (day 2) included an audio recording of a thunderstorm (CanCog Technologies, Toronto, Ontario, Canada). The mean thunderstorm sound level was 88.8 dB SPL; the peak level was 104 to 105 dB; the A-weighted sound exposure level was 110.9 dBA.
Video recordings from the overhead camera were analyzed for distance traveled per time period by using EthoVision XT 7.1 (Noldus Information Technology). The Ethovision system also provided an estimate of the total time that a dog spent moving within the OFT arena. The analysis parameters included 10 samples per second and maximum smoothing applied posttracking. The thresholds for determining time not moving were set to 0.10 and 0.07 m/s for start and stop velocities, respectively.
Anxiety scores.
Open-field sessions for each dog were coded and scored in random order by a single trained observer, as validated previously in our lab.17 The videos were watched and assessed without sound so the observer remained blind to treatment day (that is, thunderstorm during middle phase on day 2). Anxiety behavior in the OFT was assessed and scored for each 3-min test phase in the open field, as previously reported.17 Anxiety scores are subjective measures based on duration and intensity of anxiety behaviors observed over a given period of time. Scores were based on a scale of 1 through 6, where a score of 1 reflects no expression of anxiety behaviors, increasing stepwise by half points, to 6 for severe anxiety behavior exhibited most of the time. Anxiety scores were assessed using the following scoring rubric: 1, no anxiety for activity; 2, mild anxiety seen occasionally; 3, either mild anxiety demonstrated some of the time and mild or moderate anxiety seen occasionally; 4, either mild anxiety seen most of the time or moderate anxiety seen some of the time, or severe anxiety seen occasionally; 5, severe anxiety seen some of the time or moderate anxiety seen most of the time; and 6, severe anxiety seen most of the time. Behaviors were considered to occur ‘occasionally’ when they were seen once or twice; ‘some of the time’ indicates they were present approximately 25% of the test-phase time, and ‘most of the time’ when they were present during 50% or more of a 3-min test phase.17
Scores were initially assessed in 3 categories of anxiety behaviors for each 3-min test phase: negative (passive), positive (active), and mean global (subjective intermediary of negative and positive scores). Negative anxiety behaviors included decreased activity, such as freezing, hiding, position against wall or at door; lowered body postures, such as crouching, tail tucking and ears back; and conflict behaviors, such as panting, shaking, salivating, yawning, lip licking, or elimination. Positive anxiety behaviors included startling, bolting, vigilance, scanning, and active responses, such as pacing, aimless activity, stereotypic circling, retreat or escape attempts, digging, or climbing. Negative and positive anxiety scores were averaged to obtain a mean (calculated) anxiety score (MAS). Therefore, each dog had 4 scores for each 3-min test phase (3 assigned by the observer and one calculated mean), for a total of 12 anxiety scores for each 9-min test session. For the present study, we analyzed only the MAS, which we considered less subjective and less subject to bias than individual scores because we could not blind the observer. In addition, the MAS and global anxiety scores showed a strong positive linear correlation for scores obtained before (r2 = 0.989, P < 0.0001), during (r2 = 0.994, P < 0.0001), and after (r2 = 0.995, P < 0.0001) the sound stimulation.
Manual physiology data collection.
Heart rate (femoral pulse) and rectal temperature were collected by a veterinarian (RF) in the anteroom immediately after fitting the undershirt and vest and immediately after each 9 min OFT session.
Remote physiology data collection.
Physiologic data and accelerometer activity were monitored in dogs in the Vest group by using the emkaPACK noninvasive telemetry system paired with IOX 2.8.0.11 collection software and ecgAUTO version 3.10.12 analysis software (emka Technologies, Falls Church, VA). The system was fully deployed to simulate an actual telemetry session, and data were collected (and are presented) to illustrate the output from this system. All dogs’ hair was clipped along both sides of the chest 1 to 2 wk before testing. Dogs were prepared for telemetry recording in an anteroom so that the quality of recording could be verified prior to the test session. On the day of testing, the skin of Vest group dogs was cleaned with an alcohol wipe and allowed to dry. Single-lead ECG recordings were made by attaching repositionable monitoring electrodes (RedDot Ag/AgCl, 3M, St Paul, MN) to the chest. The positive lead was placed at the costochondral junction of the sixth rib on the right side, and the negative lead was placed in the same location on the left side of the chest. A neutral lead was placed over the last rib on the right side. A skin-temperature probe (Mesurex, Malaga, Spain) was placed in the axillary region of the left side. The electrodes were held in place with adhesive elastic bandage wrapped around the dog. A Lomir undershirt and vest (Lomir Biomedical, Quebec, Canada) were worn over the electrodes and held an emkaPack transmitter. An emka XactTrace elastic belt fit in the undershirt and fastened around the lower chest to record respiration by electrical impedance changes. The emkaPack transmitter contained an accelerometer activity monitor. Continuous recordings of the ECG, respiration, skin temperature, and activity were made while the dog was in the open-field arena. A mean value for heart rate, respiratory rate, skin temperature, and activity was calculated for each 30-second interval (that is, 18 steps for each 9-min test session). These values were used to subsequently calculate an overall mean for the 3 phases of the OFT test (that is, Pre, During, and Post).
Statistical Analyses.
For manual measures of heart rate and body temperature, changes in these values were calculated by subtracting postsession values taken immediately after the end of the OFT session from presession values obtained just prior to the start of the OFT session. Similarly, the change in MAS was calculated by subtracting the score obtained during the middle 3-min test phase (that is, quiet or thunderstorm sound) from that of the initial 3-min test phase. Manual physiologic data, total distance traveled, time spent active during the 9-min OFT, and change in MAS were analyzed using a full factorial repeated-measures analysis, with vest-wearing and sex as between-subject factors and test day as a within-subject factor (MANOVA). When a factor was identified as not statistically significant, the data were pooled appropriately. Significant MANOVA were followed by ANOVA, including tests of data homogeneity (Levene test) and pairwise comparisons with one-tailed Student t tests, reflecting our directional hypothesis that vest wearing would decrease heart rate and MAS. Mean telemetry values for individual dogs were calculated for each OFT day and test phase and analyzed by using the Levene test, ANOVA, and Tukey test. Data were analyzed by using JMP Pro version 11.0 (SAS Institute, Cary, NC). The results were considered statistically significant when the P value was 0.05 or less. Results are shown for all dogs (n = 8 per group) and are reported as means ± SEM unless otherwise indicated.
Results
Spontaneous locomotor activity.
Total daily distance traveled in the OFT was assessed using Ethovision system analysis of the recorded videos. There were no significant differences in total distance traveled seen for either sex (F = 0.342, P = 0.56) or treatment day (F = 0.006, P = 0.994). There was a significant interaction between vest-wearing and sex (F = 5.03, P < 0.0001), but no other interactions (for example, vest and treatment day) were significant. When the data for male and female dogs were pooled across days there was no overall effect of vest wearing on total distance traveled (F = 0.029, P = 0.87). The mean distance traveled by vest-wearing dogs on days 1, 2, and 3 was 65.9 ± 38.1, 59.8 ± 38.7, and 61.6 ± 47.9 m, respectively, and did not differ among days (F = 0.010, P = 0.99). The mean distance traveled by nonvest-wearing dogs on days 1, 2, and 3 was 67.5 ± 31.1, 72.6 ± 38.0, and 62.4 ± 31.1 m, respectively. Mean time spent active was 2.1 ± 0.6, 1.9 ± 0.6, and 1.7 ± 0.6 min during session days 1, 2, and 3, respectively. Time spent active during the OFT was not affected by session day, sex, or vest-wearing (data not shown).
Anxiety scores.
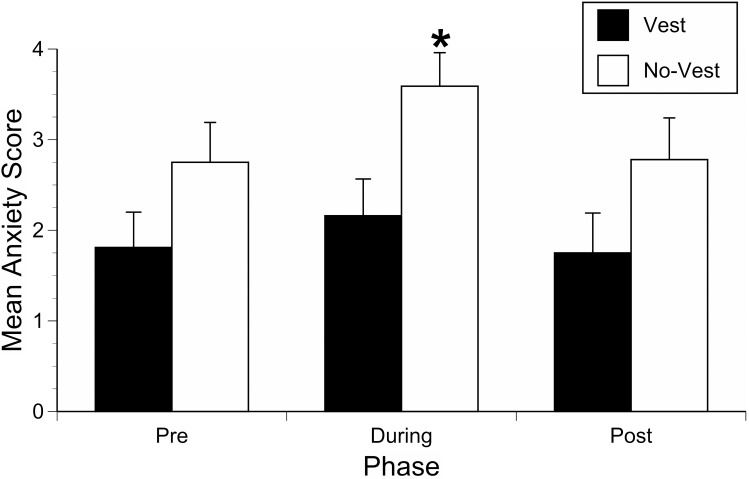
There were no differences in MAS for either sex (F = 0.016, P = 0.90) or treatment day (F = 0.13, P = 0.88). There was a significant interaction between vest-wearing and sex (F = 5.775, P = 0.0215), but no other interactions (for example, vest and treatment day) were significant. When the data for male and female dogs were pooled across days, there was an overall significant effect of vest wearing on MAS (F = 0.241.3, P = 0.0031). The overall MAS for vest-wearing dogs was 1.9 ± 0.4, a 34% decrease compared with the nonvest-wearing dogs (2.9 ± 0.4; t = –1.80, P = 0.0471). In addition, the overall mean change in MAS between the During and Pre periods was lower in vest-wearing dogs compared with nonvest wearing dogs (-0.08 ± 0.1 compared with 0.25 ± 0.1; t = –2.23, P = 0.0213), and vest-wearing was associated with a 40% lower MAS during the thunderstorm sound stimulus (phase 2 on the second OFT session day) when compared with nonvest-wearing dogs (Figure 1).
Figure 1.
Anxiety scores (mean ± SEM) during the first (Pre), second (During), and last (Post) 3-min test phase on session day 2. A statistically significant decrease in mean anxiety score was seen in vest-wearing dogs during the middle test phase when the auditory stimulus was presented. *, P < 0.05.
Manually collected physiology data.
Heart rates and rectal temperatures were within normal physiologic ranges in all dogs (Table 2). Heart rates were similar between male (99.4 ± 1.7 bpm) and female (100.0 ± 2.0 bpm) dogs and did not differ between sexes (F = 2.17, P = 0.14) or treatment days (F = 1.79, P = 0.17). There was a significant interaction between vest-wearing and sex (F = 8.56, P = 0.0044), but no other interactions (for example, vest and treatment day) were significant. When the data for male and female dogs were pooled across days, there was a significant effect of vest wearing on heart rate (F = 11.7, P = 0.0009), with vest-wearing dogs having an 8% lower heart rate compared with nonvest-wearing dogs (95.5 ± 1.5 compared with 103.9 ± 2.0 bpm). There was no significant difference in heart rate change between Pre and Post periods due to either vest-wearing (F = 0.58, P = 0.460) or sex (F = 3.24, P = 0.097) or for the interaction between vest-wearing and sex (F = 0.161, P = 0.695; data not shown).
Table 2.
Manually collected rectal temperatures and heart rates before (Pre) and after (Post) each daily OFT session
| Day 1 |
Day 2 |
Day 3 |
|||||
| Pre | Post | Pre | Post | Pre | Post | ||
| Rectal temperature (°C) | Vest | 39.3 ± 0.2 | 39.1 ± 0.1 | 39.1 ± 0.2 | 38.9 ± 0.1 | 39.2 ± 0.1 | 39.0 ± 0.1 |
| No vest | 39.2 ± 0.3 | 39.0 ± 0.2 | 39.0 ± 0.2 | 38.9 ± 0.2 | 39.0 ± 0.1 | 38.9 ± 0.1 | |
| Heart rate (bpm) | Vest | 97.3 ± 4.0 | 98.0 ± 2.8 | 96.8 ± 3.5 | 93.3 ± 4.4 | 95.5 ± 3.7 | 92.0 ± 3.9 |
| No vest | 108.0 ± 6.6 | 106.5 ± 6.3 | 99.0 ± 4.0 | 100.0 ± 4.0 | 107.0 ± 4.1 | 103.0 ± 4.0 | |
Data are given as means ± SEM.
Vest-wearing had an overall effect on heart rate (see Results).
Rectal temperature did not differ between treatment days (F = 1.34, P = 0.27). Effects of sex (F = 10.2, P = 0.002) and the interaction between vest-wearing and sex (F = 14.3, P = 0.0003) were significant, but no effect of sex was seen when nonsignificant factors were removed from the model (male, 38.9 ± 0.1 °C; female, 39.2 ± 0.1 °C; F = 3.82, P = 0.07). Likewise, mean rectal body temperature did not differ between vest-wearing and nonvest-wearing dogs (39.1 ± 0.1 °C compared with 39.0 ± 0.2 °C; F = 0.283, P = 0.603). For the change in rectal temperature between Post and Pre periods, no significant main effects were seen for either vest-wearing (F = 0.099, P = 0.758) or sex (F = 0.0.099, P = 0.758), or for the interaction between vest-wearing and sex (F = 1.86, P = 0.198; data not shown).
Telemetry data.
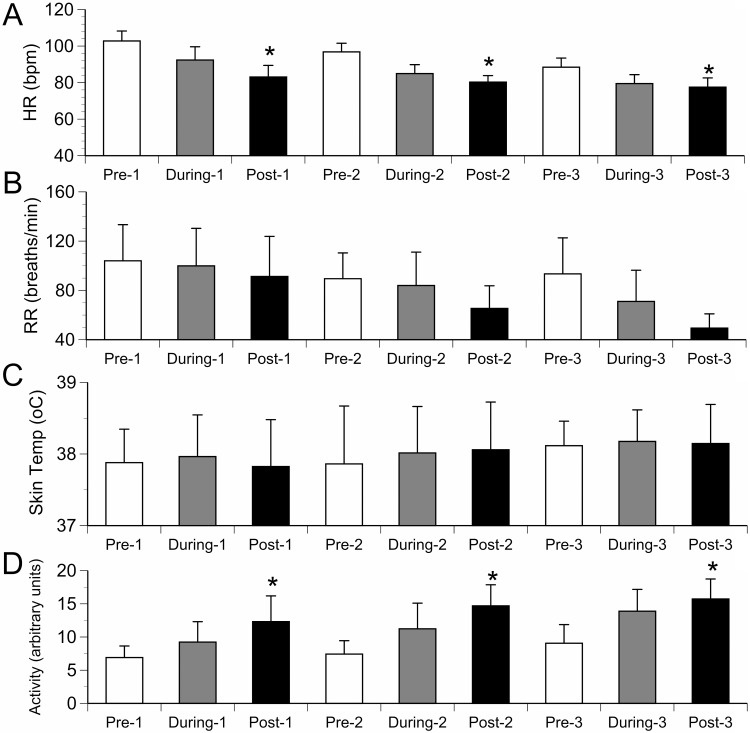
An advantage of telemetry is that physiologic data can be collected during an OFT session. To illustrate this advantage, we examined heart rate, respiratory rate, skin temperature, and accelerometer activity for OFT session day and test phase (Figure 2). Because this analysis was a secondary objective, and to reduce possible sex-related effects and small group size, the 2 males assigned to the vest-wearing group were excluded from these analyses. A significant effect of OFT session day on these parameters was not seen. Mean heart rate showed a statistically significant decrease during the daily OFT sessions. Overall mean heart rates seen in the last 3-min phase (80.3 ± 2.8 bpm) were lower than those seen during the first 3-min phase (96.0 ± 3.1 bpm; P = 0.0027). Overall mean activity levels seen in the last 3-min phase (14.2 ± 1.9 arbitrary units) were higher than those seen during the first 3-min phase (7.8 ± 1.2 arbitrary units; P = 0.036). Thunderstorm noise had no effect on heart or respiratory rates, skin temperature, or locomotor activity (Figure 2).
Figure 2.
(A) Heart rate (HR), (B) respiratory rate (RR), (C) skin temperature (Temp), and (D) activity (mean ± SEM) as measured by using telemetry in 6 female dogs during 3 consecutive (Pre, During, and Post) daily (1, 2, and 3) 9-min OFT sessions. Dogs were exposed to thunderstorm noise during the second test phase on day 2. Overall mean heart rates seen in the last 3-min (Post) phase were lower than those seen during the first 3-min (Pre) phase. Overall mean activity levels seen in the last 3-min (Post) phase were higher than those seen during the first 3-min phase. *, P < 0.05.
Discussion
We are unaware of previously published studies that examined whether dogs wearing jacketed telemetry systems (undershirt, vest, and associated equipment) have altered physiologic or behavioral responses when compared with unjacketed dogs. In the present study, we examined the effect of vest-wearing on 2 physiologic measures (heart rate and rectal temperature) in dogs. These measures were manually determined and were collected immediately before and after each 9-min OFT session. Vest-wearing had no effect on rectal temperature, but dogs that wore a telemetry vest had an 8% lower heart rate than dogs that did not wear a vest. This observation is similar to that seen in dogs with an anxiety disorder treated with a commercially available wrap (ThunderShirt), and heart rates monitored by using a Polar FT40 computer and Polar Wearlink transmitter.23 In that study, groups of anxious dogs were monitored during a 15-min session while left alone in a kennel. Dogs that wore the shirt snugly (according to the manufacturer's recommendations) showed less of an increase in heart rate above baseline than did dogs that didn't wear a shirt or wore the shirt loosely. An antianxiolytic effect of wearing tight-fitting materials has also been seen in human medicine. For example, swaddling of human infants is associated with small, but statistically significant, decreases in heart rate,13 and an overall “calming” effect has been associated with swaddling or use of a weighted vest in children.11,39 In human infants, swaddling has an effect on brain stem arousal patterns.14
We also sought to determine whether wearing a telemetry vest would affect a dog's behavior in OFT. For this study, we focused on 2 behavioral measures, that is, spontaneous locomotor activity and anxiety-associated behaviors. We used a matched-cohort experimental design based on the dogs’ behavioral response during our earlier OFT experiment.17 The present study showed that the wearing of a telemetry vest did not affect the distance that dogs traveled in the OFT arena but was associated with a 34% reduction in overall MAS during the course of the 3-d study and a 40% lower MAS on the second OFT session day (when a thunderstorm sound stimulus was provided during phase 2). Animals wearing a telemetry vest had occasional evidence of mild anxiety, whereas dogs that did not wear a telemetry vest demonstrated anxiety-associated behaviors more frequently or had an increase in the severity of their anxiety-associated behaviors (to moderate severity). Our results regarding telemetry vest-wearing are similar to those seen in dogs with thunderstorm phobia that wore a commercially available product (Anxiety Wrap). For example, one study7 found reductions in owner-reported anxiety scores in dogs with thunderstorm phobia wearing an Anxiety Wrap. Likewise, other authors23 reported a near-significant decrease in certain anxiety-associated behaviors in dogs wearing a ThunderShirt. Each of these products is marketed as a pressure wrap and, like the telemetry vest, must be worn snugly to be effective.
Monitoring physiologic parameters in freely moving animals with radiotelemetry devices is widely used in pharmacology and other biomedical sciences. Radiotelemetry has numerous advantages in eliminating the influences of anesthetic and restraint-induced stress, because these factors may alter the sensitivity of the models to detect drug-induced effects.24 Accordingly, the use of conscious unrestrained animals has been recommended for safety pharmacology studies, and the recording and analysis of cardiovascular parameters by telemetry is commonly used and recognized worldwide as an appropriate assessment of preclinical cardiovascular changes following the administration of new drugs.21,22 Noninvasive collection of physiologic data by using a jacketed system has the advantage of avoiding surgical device implantation.33,40 Validation studies have compared the effectiveness of using noninvasive telemetry with implanted and other forms of invasive telemetry methods in dogs.6,12,33,40 These studies show good correlation between invasive and noninvasive telemetry methods for a number of endpoints including blood pressure, respiratory rate, heart rate, and other parameters of interest to safety pharmacologists.
Our study was not designed to provide a detailed analysis of all possible physiologic parameters that could be evaluated using a jacketed telemetry system but rather to demonstrate how telemetry could be used to assess changes during and among OFT sessions. Heart rates decreased over time during OFT sessions, suggesting a physiologic habituation effect during the 9-min OFT, when human contact did not occur. Habituation in an open-field model is an adaptive CNS response that follows a continuous or repeated stimulus over time. In general, the activity of dogs and other animals in an open-field is decreased both within a session and between sessions as the environment loses its novelty.19,27,28 Unlike our initial studies with this cohort of dogs,17 we did not see a decrease in activity (that is, habituation) either during the OFT sessions or from day 1 to 3.
This pilot study has several limitations. One inherent limitation in our study design was that the research staff could not be blinded to the treatment condition (vest compared with no vest) during their assessment of the video-recorded behaviors. This potential bias was reduced by randomizing the sessions and phases, such that the observer was unaware of which phases involved exposure to thunderstorm sound. A second limitation of the study was that allocation of dogs to the vest or no-vest treatment group resulted in an unbalanced design with regard to the sex of the dog. The initial studies with these dogs17 found no effects of sex on any of the physiologic or behavioral variables that were measured, so we infer that this imbalance of sex and treatment (vest wearing) did not affect the overall interpretation of the results. We chose this method of group allocation, rather than a random allocation and cross-over design, because of the difficulty in interpreting repeated behavioral assessments when habituation is expected. A third limitation is that significant treatment effects were associated with short-term vest-wearing and may not persist when the telemetry vest is worn continuously for days to weeks. Finally, the breed used in the current study (Labrador retrievers) is infrequently used in safety pharmacology, and breed-dependent responses to the wearing of telemetry vests remain unknown.
In conclusion, our results highlight several key features associated with the use of telemetry vests in dogs. First, unlike hand collection of data, telemetry vests afford an investigator the ability to continuously monitor physiologic data during the course of a study. Second, vest wearing has a significant effect on heart rate in dogs. Toxicologists and other scientists should consider whether this difference in heart rate may affect the use of a jacketed telemetry system when designing a safety pharmacology study (for example, to what extent might vest-wearing prevent detection of a pharmacologic increase in heart rate). Third, dogs wearing a telemetry vest have less anxiety in an open-field test environment. Thus, the wearing of a telemetry vest may complicate the interpretation of certain efficacy studies, especially those involving anxiolytic drugs and other CNS-active materials. Finally, a decrease in anxious behaviors with vest-wearing suggests a potential animal welfare benefit in the laboratory animal environment, either for particularly anxious dogs or in situations where an increased level of anxiety is expected (for example, arrival in a new facility or during transport).
Acknowledgments
This work was funded through a contract to K2 Solutions from the United States Office of Naval Research. We thank Beth Case for technical assistance, the NCSU Laboratory Animal Resources staff for excellent animal care, and K2 Solutions personnel for facilitating this study. Wilant van Giessen (Noldus Information Technology) advised on technical matters. Dr Margaret E Gruen received support from the NIH Ruth L Kirschstein National Research Service Award T32OD011130.
References
- 1.Araujo JA, de Rivera C, Landsberg GM, Adams PE, Milgram NW. 2013. Development and validation of a novel laboratory model of sound-induced fear and anxiety in beagle dogs. J Vet Behav 8:204–212. [Google Scholar]
- 2.Authier S, Legaspi M, Gauvin D, Chaurand F, Fournier S, Troncy E. 2008. Validation of respiratory safety pharmacology models: conscious and anesthetized beagle dogs. J Pharmacol Toxicol Methods 57:52–60. [DOI] [PubMed] [Google Scholar]
- 3.Authier S, Vargas HM, Curtis MJ, Holbrook M, Pugsley MK. 2013. Safety pharmacology investigations in toxicology studies: an industry survey. J Pharmacol Toxicol Methods 68:44–51. [DOI] [PubMed] [Google Scholar]
- 4.Cavero I. 2009. Exploratory safety pharmacology: a new safety paradigm to de-risk drug candidates prior to selection for regulatory science investigations. Expert Opin Drug Saf 8:627–647. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Brott D, Luo W, Gangl E, Kamendi H, Barthlow H, Lengel D, Fikes J, Kinter L, Valentin JP, Bialecki R. 2013. Assessment of cisplatin-induced kidney injury using an integrated rodent platform. Toxicol Appl Pharmacol 268:352–361. [DOI] [PubMed] [Google Scholar]
- 6.Chui RW, Fosdick A, Conner R, Jiang J, Bruenner BA, Vargas HM. 2009. Assessment of 2 external telemetry systems (PhysioJacket and JET) in beagle dogs with telemetry implants. J Pharmacol Toxicol Methods 60:58–68. [DOI] [PubMed] [Google Scholar]
- 7.Cottam N, Dodman NH, Ha JC. 2013. The effectiveness of the Anxiety Wrap in the treatment of canine thunderstorm phobia: an open-label trial. J Vet Behav 8:154–161. [Google Scholar]
- 8.Dodman N., [Internet] 2011. Thunderstorm phobia in dogs—an update. [Cited 2 June 2016]. Available at: http://www.veterinarypracticenews.com/August-2011/Thunderstorm-Phobia-In-Dogs-An-Update/.
- 9.Dürmüller N, Guillaume P, Lacroix P, Porsolt RD, Moser P. 2007. The use of the dog electroencephalogram (EEG) in safety pharmacology to evaluate proconvulsant risk. J Pharmacol Toxicol Methods 56:234–238. [DOI] [PubMed] [Google Scholar]
- 10.Ewart L, Milne A, Adkins D, Benjamin A, Bialecki R, Chen Y, Ericsson AC, Gardner S, Grant C, Lengel D, Lindgren S, Lowing S, Marks L, Moors J, Oldman K, Pietras M, Prior H, Punton J, Redfern WS, Salmond R, Skinner M, Some M, Stanton A, Swedberg M, Finch J, Valentin JP. 2013. A multi-site comparison of in vivo safety pharmacology studies conducted to support ICH S7A and B regulatory submissions. J Pharmacol Toxicol Methods 68:30–43. [DOI] [PubMed] [Google Scholar]
- 11.Fertel-Daly D, Bedell G, Hinojosa J. 2001. Effects of a weighted vest on attention to task and self-stimulatory behaviors in preschoolers with pervasive developmental disorders. Am J Occup Ther 55:629–640. [DOI] [PubMed] [Google Scholar]
- 12.Gelzer AR, Ball HA. 1997. Validation of a telemetry system for measurement of blood pressure, electrocardiogram and locomotor activity in beagle dogs. Clin Exp Hypertens 19:1135–1160. [DOI] [PubMed] [Google Scholar]
- 13.Gerard CM, Harris KA, Thach BT. 2002. Physiologic studies on swaddling: an ancient child care practice, which may promote the supine position for infant sleep. J Pediatr 141:398–404. [DOI] [PubMed] [Google Scholar]
- 14.Gerard CM, Harris KA, Thach BT. 2002. Spontaneous arousals in supine infants while swaddled and unswaddled during rapid eye movement and quiet sleep. Pediatrics 110:e70. [DOI] [PubMed] [Google Scholar]
- 15.Goineau S, Lemaire M, Froget G. 2001. Overview of safety pharmacology. Unit 10.1. In: Current protocols in pharmacology. Hoboken (NJ): John Wiley and Sons. [DOI] [PubMed] [Google Scholar]
- 16.Greaves P. 1998. Patterns of drug-induced cardiovascular pathology in the beagle dog: relevance for humans. Exp Toxicol Pathol 50:283–293. [DOI] [PubMed] [Google Scholar]
- 17.Gruen ME, Case BC, Foster ML, Lazarowski L, Fish RE, Landsberg G, DePuy V, Dorman DC, Sherman BL. 2015. The use of an open field model to assess sound-induced fear and anxiety-associated behaviors in Labrador retrievers. J Vet Behav 10:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdam J, Sethu S, Smith T, Alfirevic A, Alhaidari M, Atkinson J, Ayala M, Box H, Cross M, Delaunois A, Dermody A, Govindappa K, Guillon JM, Jenkins R, Kenna G, Lemmer B, Meecham K, Olayanju A, Pestel S, Rothfuss A, Sidaway J, Sison-Young R, Smith E, Stebbings R, Tingle Y, Valentin JP, Williams A, Williams D, Park K, Goldring C. 2013. Safety pharmacology—current and emerging concepts. Toxicol Appl Pharmacol 273:229–241. [DOI] [PubMed] [Google Scholar]
- 19.Head E, Callahan H, Cummings BJ, Cotman CW, Ruehl WW, Muggenberg BA, Milgram NW. 1997. Open field activity and human interaction as a function of age and breed in dogs. Physiol Behav 62:963–971. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Nakane S, Kitagawa T. 1995. Predictability of clinical adverse reactions of drugs by general pharmacology studies. J Toxicol Sci 20:77–92. [DOI] [PubMed] [Google Scholar]
- 21.International Conference on Harmonization (ICH). [Internet] 2000. S7A: safety pharmacology studies for human pharmaceuticals. [Cited 2 June 2016]. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7A/Step4/S7A_Guideline.pdf). [PubMed]
- 22.International Conference on Harmonization (ICH). [Internet] 2005. ICH S7B: the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. [Cited 2 June 2016]. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf. [PubMed]
- 23.King CA, Buffington L, Smith TJ, Grandin T. 2014. The effect of a pressure wrap (ThunderShirt) on heart rate and behavior in canines diagnosed with anxiety disorder. J Vet Behav 9: 215–221. [Google Scholar]
- 24.Kramer K, Kinter LB. 2003. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics 13:197–205. [DOI] [PubMed] [Google Scholar]
- 25.Lazarowski L, Foster ML, Gruen ME, Sherman BL, Case BC, Fish RE, Milgram NW, Dorman DC. 2013. Acquisition of a visual discrimination and reversal learning task by Labrador retrievers. Anim Cogn 17:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarowski L, Foster ML, Gruen ME, Sherman BL, Fish RE, Milgram NW, Dorman DC. 2015. Olfactory discrimination and generalization of ammonium nitrate and structurally related odorants in Labrador retrievers. Anim Cogn 18:1255–1265. [DOI] [PubMed] [Google Scholar]
- 27.Leussis MP, Bolivar VJ. 2006. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev 30:1045–1064. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga W, Watanabe E. 2010. Habituation of medaka (Oryzias latipes) demonstrated by open-field testing. Behav Processes 85:142–150. [DOI] [PubMed] [Google Scholar]
- 29.Morton DB, Hawkins P, Bevan R, Heath K, Kirkwood J, Pearce P, Scott L, Whelan G, Webb A, British Veterinary Association Animal Welfare Foundation. Fund for Replacement of Animals in Medical Experiments. Royal Society for the Prevention of Cruelty to Animals. Universities Federation for Animal Welfare 2003. Refinements in telemetry procedures. Seventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement, Part A. Lab Anim 37:261–299. [DOI] [PubMed] [Google Scholar]
- 30.Moscardo E, Fasdelli N, Giarola A, Tontodonati M, Dorigatti R. 2009. An optimised neurobehavioural observation battery integrated with the assessment of cardiovascular function in the beagle dog. J Pharmacol Toxicol Methods 60:198–209. [DOI] [PubMed] [Google Scholar]
- 31.Ollerstam A, Visser SA, Duker G, Forsberg T, Persson AH, Nilsson LB, Björkman JA, Gabrielsson J, Al-Saffar A. 2007. Comparison of the QT interval response during sinus and paced rhythm in conscious and anesthetized beagle dogs. J Pharmacol Toxicol Methods 56:131–144. [DOI] [PubMed] [Google Scholar]
- 32.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. 2000. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32:56–67. [DOI] [PubMed] [Google Scholar]
- 33.Prior H, McMahon N, Schofield J, Valentin JP. 2009. Noninvasive telemetric electrocardiogram assessment in conscious beagle dogs. J Pharmacol Toxicol Methods 60:167–173. [DOI] [PubMed] [Google Scholar]
- 34.Pugsley MK, Authier S, Curtis MJ. 2008. Principles of safety pharmacology. Br J Pharmacol 154:1382–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samson N, Dumont S, Specq ML, Praud JP. 2011. Radiotelemetry devices to monitor breathing in nonsedated animals. Respir Physiol Neurobiol 179:111–118. [DOI] [PubMed] [Google Scholar]
- 36.Sherman BL, Gruen ME, Case BC, Foster ML, Fish RE, Lazarowski L, DePuy V, Dorman DC. 2015. A test for the evaluation of emotional reactivity in Labrador retrievers used for explosives detection. J Vet Behav 10:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tontodonati M, Fasdelli N, Moscardo E, Giarola A, Dorigatti R. 2007. A canine model used to simultaneously assess potential neurobehavioural and cardiovascular effects of candidate drugs. J Pharmacol Toxicol Methods 56:265–275. [DOI] [PubMed] [Google Scholar]
- 38.Toyoshima S, Kanno A, Kitayama T, Sekiya K, Nakai K, Haruna M, Mino T, Miyazaki H, Yano K, Yamamoto K. 2005. QT PRODACT: in vivo QT assay in the conscious dog for assessing the potential for QT interval prolongation by human pharmaceuticals. J Pharmacol Sci 99:459–471. [DOI] [PubMed] [Google Scholar]
- 39.van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, Kuis W, Schulpen TW, L'Hoir MP. 2007. Swaddling: a systematic review. Pediatrics 120:e1097–e1106. [DOI] [PubMed] [Google Scholar]
- 40.Ward G, Milliken P, Patel B, McMahon N. 2012. Comparison of noninvasive and implanted telemetric measurement of blood pressure and electrocardiogram in conscious beagle dogs. J Pharmacol Toxicol Methods 66:106–113. [DOI] [PubMed] [Google Scholar]