Abstract
Rhesus macaques (Macaca mulatta) are the most commonly used NHP biomedical model and experience both research and clinical procedures requiring analgesia. Opioids are a mainstay of analgesic therapy. A novel, transdermal fentanyl solution (TFS) has been developed as a long-acting, single-administration topical opioid and was reported to provide at least 4 d of effective plasma concentrations in beagles (Canis familiaris). To evaluate the pharmacokinetic profile of TFS in healthy adult rhesus macaques, we used a 2-period, 2-treatment crossover study of a single topical administration of 1.3 (25) and 2.6 mg/kg (50 μL/kg) TFS. TFS was applied to the clipped dorsal skin of adult rhesus macaques (n = 6; 3 male, 3 female) under ketamine sedation (10 mg/kg IM). We hypothesized that TFS in rhesus macaques would provide at least 4 d of effective plasma concentrations (assumed to be ≥ 0.2 ng/mL, based on human studies). Plasma fentanyl concentrations were determined by liquid chromatography–tandem mass spectrometry before drug administration and at 0, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 168, 240, 336, 408, and 504 h afterward. Noncompartmental pharmacokinetic analysis was performed. For each dose (1.3 and 2.6 mg/kg), respectively, the maximal plasma concentration was 1.95 ± 0.40 and 4.19 ± 0.69 ng/mL, occurring at 21.3 ± 4.1 and 30.7 ± 8.7 h; the AUC was 227.3 ± 31.7 and 447.0 ± 49.1 h/ng/mL, and the terminal elimination half-life was 93.7 ± 7.1 and 98.8 ± 5.4 h. No adverse effects were noted after drug administration at either dose. Macaques maintained plasma fentanyl concentrations of 0.2 ng/mL or greater for at least 7 d after 1.3 mg/kg and at least 10 d after 2.6 mg/kg topical administration of TFS. A single TFS dose may provide efficacious analgesia to rhesus macaques and reduce stress, discomfort, and risk to animals and personnel.
Abbreviations: Cmax, maximal concentration; MEC, minimal effective concentration, TFS, transdermal fentanyl solution, tmax, time at maximal concentration
Nonhuman primates are important preclinical research models. They bridge the gap between basic and clinical investigations; they are necessary for safety and efficacy testing of biologics such as antibodies, receptors, and cytokines, because these compounds may fail to bind in nonphysiologically similar animals; they are the best translational models of disease for understanding pathogenic mechanisms that readily translate into therapy development; and they can be taught to perform sophisticated sensorimotor tasks that test the tactile sensation and fine motor control of the digits.1,13 Model limitations are diminished with Old World species because they are physiologically similar to humans,64 and rhesus and long-tailed macaques are the most commonly studied NHP species.15,19,27 However, because of the noted physiologic, neuroanatomical, reproductive, developmental, cognitive, and social similarities that NHP share with humans, their use in biomedical research must be balanced with their welfare.58
Innovation in pain management has burgeoned in the last decade. Now more than ever, providing adequate postoperative analgesia to research animals is an ethical and clinical imperative and is now mandated by federal regulations, such as the Animal Welfare Act and Regulations, the Public Health Service policy, and the Guide for the Care and Use of Laboratory Animals.2,3,37,52 Given the tremendous advancement in pain management, animal welfare is greatly enhanced by refining and providing cutting-edge treatment regimens to NHP used in biomedical research.
Identifying novel or improved forms of administration, especially in the form of long-acting, single-dose formulations, are a refinement of significant clinical interest in the care of NHP. These analgesic formulations may have the benefit of providing adequate analgesia while limiting the required handling, and thus stress, secondary to repeated-dose administrations. Transdermal drug delivery, as with the transdermal fentanyl patch, is an appealing alternative to hypodermic injection and oral administration, and transdermal delivery systems are generally noninvasive and inexpensive. In addition, they decrease the generation of hazardous medical waste, and they allow for the sustained release of drug, which inherently improves patient compliance.
Transdermal delivery of drugs can be counterintuitive, given that one of the main roles of the skin is to shield the body from toxins. The skin is fundamentally impermeable to the entrance of foreign molecules. The stratum corneum, the most superficial layer of the epidermis, acts as a major barrier to drug absorption; therefore, to penetrate the skin, a drug should possess 3 major characteristics: a molecular mass less than 500 Da, a high lipophilicity, and a low required daily dosage.47 Opioids, such as fentanyl, are a mainstay of balanced analgesia and anesthesia both in human and veterinary medicine, being regarded as the standard of care for postoperative analgesia.7,21,24,25,30 Fentanyl, a highly selective and potent opioid agonist that mainly acts at the μ-opioid receptor with some activity at the δ- and κ-opioid receptors, is generally limited to the perioperative and immediate postoperative period in the form of parenteral injections or constant-rate infusions due to poor bioavailability and rapid clearance.24,25,30 However, fentanyl possesses all the characteristics needed to penetrate the skin: a molecular weight of 286 Da, an aqueous solubility of 1:30 to 100 µg/mL, and a low daily dosage.40
Transdermal delivery systems of fentanyl in the form of skin patches have been approved by the FDA since 1990.53 This modality is commonly used in human and veterinary medicine to provide sustained analgesia to patients. In rhesus macaques, the use of fentanyl patches has posed some challenges. Maintaining adequate adhesion of the fentanyl transdermal patch on rhesus macaques is problematic even with the help of a bandage or jacket. Rhesus macaques are highly dexterous, inquisitive, and active and are usually housed in pairs or groups to enhance their social wellbeing. All of these attributes may affect the constant adhesion of the transdermal delivery system.
Compared with the standard transdermal delivery system (that, is fentanyl patch), a novel, long-acting transdermal fentanyl solution (TFS; Recuvyra, Elanco, Greenfield, IN) has been shown to provide adequate analgesia for a minimum of 4 d with a single topical administration at 2.6 mg/kg (50 μL/kg) between the scapulae in dogs.24,25,48 This new formulation of a commercial transdermal fentanyl solution is an attractive alternative for the control of pain in NHP. This formulation was also recently investigated for use and compared with transdermal fentanyl patches for efficacy in cynomolgus macaques.7
The primary objective of the present study was to establish the pharmacokinetic profile of 2 topical doses of the transdermal fentanyl solution (Recuvyra, Elanco) in rhesus macaques; the secondary objective was then to compare these data with each other and with those previously published in beagles. We hypothesized that TFS at either 1.3 or 2.6 mg/kg (25 and 50 μL/kg) topically would provide effective plasma concentrations as established in beagles for at least 4 d in rhesus macaques. We chose to set the minimum for comparison at 4 d of effective plasma concentration to ensure time above the minimal effective concentration (MEC) for rhesus macaques was at least comparable to the available TFS data as established in canines. Canine data was used for comparison because the US Food and Drug Administration has approved this compound for use in dogs and because no TFS data existed for any NHP species at the time of study design. The 2 doses were chosen because they had been established in dogs as safe and effective, with minimal adverse effects noted.24,25 Because of the variable range of minimal effective fentanyl concentrations reported in the literature in dogs,24,25,28,32,62 humans,29,33,69 and in particular, NHP,7,46,54,68 a minimal threshold of 0.2 ng/mL was chosen as the absolute lowest effective concentration for fentanyl, as determined in humans undergoing surgical procedures,29,33,69 and the effects of these levels in other species are likely similar to those in humans;16,28,42 therefore, the human MEC range of 0.2 to 1.2 ng/mL is presented here for completeness.
Materials and Methods
Animals.
Six healthy, adult rhesus macaques (3 male, 3 female), housed at the California National Primate Research Center (Davis, CA) were used in this pharmacokinetic study. A clinical history, physical examination, CBC, and serum biochemical profile were performed on all animals prior to, during the 10-wk washout period, and at the conclusion of the study to ensure their health and wellbeing prior to and throughout the duration of study. At the start of each treatment period, weights were collected for accurate dose calculations, and weights and ages are presented herein. At the start of the first treatment period, body weight (mean ± 1 SD) was 13.5 ± 2.7 kg with a median of 12.7 kg and a range of 9.8 to 17.7 kg; at the start of the second treatment period, body weight was 13.2 ± 3.2 kg with a median of 12.5 kg and range of 8.6 to 18.0 kg. At the start of the first treatment period, age was 10.9 ± 3.6 y with a median of 11.0 y and range of 6.7 to 16.7 y; at the start of the second treatment period, age was 11.1 ± 3.6 y with a median of 11.2 y and range of 6.9 to 16.9 y. At the start of the study, body condition scores (mean ± 1 SD) assigned by using a validated system11 were 3.9 ± 0.6 with a median of 4.0 and range of 3.5 to 4.5.
All animals were singly housed for approximately 1 wk after dosing to accommodate daily observation for any potential adverse reactions and then were subsequently pair-housed intermittently (paired approximately 0700 to 1500). Animals were provided with species-appropriate environmental enrichment, fed chow twice daily (LabDiet Monkey Diet 5047, Purina Laboratory, St Louis, MO), offered water without restriction through automatic watering devices, and supplemented with fruits and vegetables biweekly. Each animal underwent acclimation and behavioral conditioning for approximately 3 wk prior to the start of the study and throughout the study period to present for conscious, cooperative, cageside cephalic venipuncture by trained personnel.
The current study was approved by the IACUC of the University of California, Davis. Animals were maintained in accordance with the USDA Animal Welfare Act and regulations and the Guide for the Care and Use of Laboratory Animals.2,3,37 The animal care and use program of the University of California, Davis is fully accredited by AAALAC, is USDA-registered, and maintains a Public Health Services Assurance.52
Study design and sample collection.
A 2-period, 2-treatment crossover protocol was used to assess the pharmacokinetic parameters of 2 (1.3 and 2.6 mg/kg [25 and 50 μL/kg]) topical doses of TFS (Recuvyra, Elanco). This clear, colorless to light yellow solution contains 5% w/v (50 mg/mL) fentanyl base, the skin penetration enhancer octyl salicylate, and the volatile solvent isopropanol.17,24,25 Prior to dosing, animals were sedated with ketamine (10 mg/kg IM; 100 mg/mL, MWI/VetOne, Boise, ID) to facilitate real-life clinical use of TFS in rhesus macaques. Before dosing, each macaque was weighed and its dorsum shaved midscapulae to ensure accurate dose calculation and administration.
In the first treatment period, half of the animals (n = 3) randomly received the 1.3-mg/kg topical dose of TFS, with the remaining 3 macaques receiving the 2.6-mg/kg dose. After a 10-wk washout period, all animals were crossed over to the other dose for the second treatment period. As instructed by the manufacturer, the TFS was allowed to air dry for 5 min as care was taken to ensure correct dose absorption and to avoid inadvertent absorption by handlers or conspecifics.17 All dosages were administered topically to the dorsal midscapulae by using the manufacturer's syringe, as previously illustrated.7 Initial blood samples were collected while macaques recovered from sedation, with remaining samples collected through conscious, cageside venipuncture from the cephalic vein. Samples were obtained at 0, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 168, 240, 336, 408, and 504 h after administration of TFS, placed in 10-mL collection tubes containing sodium heparin (Becton Dickenson, Franklin Lakes, NJ), and immediately stored on ice until processed. All blood volumes collected during this study were in compliance with IACUC and institutional standard operating procedures for blood collection. Blood samples were centrifuged at 3000 × g at 4 °C for 10 min; plasma was harvested and stored at –80 °C in 5-mL aliquots in microcentrifuge tubes (Thermo Fisher Scientific, Waltham, MA) until assayed.
Selection of dose and site of administration.
Because an appropriate dose for rhesus macaques has not yet been established, the TFS dose and site of administration were based on those established in dogs;24,25 these data were used for all comparisons. In addition, the dorsal midscapulae was chosen as the site of administration given the ease of administration in sedated rhesus macaques prior to a surgical procedure and to reduce the likelihood of inadvertent ingestion.
Daily observation.
Adverse effects of opioids, specifically fentanyl, are generally dose-dependent sedation; respiratory, CNS, and circulatory depression; and, in the case of transdermal fentanyl patches, rashes at the site of administration, dysphoria, constipation, and urinary retention.59 Dedicated, trained personnel performed daily observations for 1 wk after administration while macaques were singly housed to specifically document any potential adverse reactions, most notably in the form of sedation or respiratory depression. In addition, stool quality was assessed by using objective fecal scoring for 1 wk after administration.6 These observations were complemented by routine daily health monitoring throughout the treatment and washout periods for subjective assessment of appetite, hydration, and stool quality.
Determination of fentanyl concentration.
Plasma samples were assayed for fentanyl concentration by using a previously described liquid chromatography–tandem mass spectrometry method after solid phase extraction.66 A partial validation was performed by using rhesus macaque plasma as the matrix. Plasma calibrators were prepared by diluting the fentanyl working standard solutions into drug-free plasma collected from rhesus macaques to obtain concentrations ranging from 0.01 to 20 ng/mL. Calibration curves were prepared fresh for each quantitative assay. In addition, quality-control samples (at 3 concentrations within the standard curve) were included with each sample set as an additional check of accuracy.
The response for fentanyl was linear and gave correlation coefficients (R2) of 0.99 or better. The intraday, interday, analyst-to-analyst precision and accuracy of the assay were determined by evaluating fentanyl concentration in quality-control samples in replicates (n = 6). Accuracy was reported as percentage nominal concentration, and precision was reported as percentage relative standard deviation. For fentanyl, accuracy was 113%, 111%, and 103% for 0.03, 2.0, and 15.0 ng/mL, respectively. Precision was 4.0%, 5.0%, and 3.0% for 0.03, 2.0, and 15 ng/mL, respectively. All accuracy and precision data met the Food and Drug Administration's requirements for bioanalytical method validation.67 The technique was optimized to provide a limit of quantitation of 0.01 ng/mL and a limit of detection of approximately 0.005 ng/mL for fentanyl.
Pharmacokinetic calculations.
Pharmacokinetic evaluation of plasma fentanyl concentrations was performed by using noncompartmental analysis and a commercially available software program (Phoenix WinNonlin version 6.4, Pharsight, Cary, NC). The maximum measured plasma concentration (Cmax) and time to maximal plasma concentration (tmax) were obtained directly from the plasma concentration data. The terminal first-order rate constant was calculated by determination of the slope of the terminal portion of the plasma concentration versus time curve and the terminal half-life by using the formula (ln 2) / the terminal first-order rate constant. Areas under the curve from time 0 to the last time point collected (AUC0-last) and from 0 h to infinity (AUC0-∞) were calculated by using the log-linear trapezoidal method. The AUC 0-∞ percentage extrapolated was calculated by using the formula [(AUC0-∞ – AUC0-last) / AUC0-∞] × 100%. Pharmacokinetic parameters and plasma concentrations for fentanyl are reported as individual, mean (± 1 SD), and median values.
Statistical analysis.
Pharmacokinetic parameters from the 1.3- and 2.6-mg/kg topical TFS doses administered to rhesus macaques were compared (Stata 13, StataCorp, College Station, TX) with those reported for canines25 at the same doses by using one-sample t tests. The canine pharmacokinetic parameters were used as the basis of comparison because these parameters have been shown to exceed the MEC of fentanyl as established in humans undergoing abdominal surgery.29
Fentanyl plasma concentrations for the 1.3- and 2.6-mg/kg topical doses were compared (Stata 13, StataCorp) at the measured time points (0, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 168, 240, 336, 408, and 504 h after administration) by using multilevel mixed-effects linear regression, with time point and dose as fixed effects, and animal ID as a random effect. Fentanyl concentration at time 0 was below the limit of detection and was assigned the value 0 ng/mL for data analysis.
For each measured time point, fentanyl plasma concentrations for the 1.3- and 2.6-mg/kg topical doses were compared with the reported range of effective concentrations of 0.2, 0.6, and 1.2 ng/mL by using one-sample t tests to test the one-sided hypothesis that the mean concentration exceeded each reported effective concentration.
Fentanyl plasma concentrations for the 1.3- and 2.6-mg/kg topical doses were compared (Stata 13, StataCorp) between male and female macaques by using repeated-measures ANOVA, with sex, dose, and time and a dose×time interaction as fixed effects and animal ID as the repeated-measures term.
Results
All macaques remained healthy throughout the study, and no adverse effects were noted after TFS administration at either dose. Physical examination and clinical laboratory tests showed no abnormalities before or after concluding the study periods. No adverse effects, generally or specifically in the form of over-sedation, respiratory depression, gastrointestinal disturbances, or administration site reactions, were noted in any animal, at any dose, throughout the observation periods.
Pharmacokinetic parameters in rhesus macaques for both doses are shown in Table 1. In macaques, t1/2 was significantly longer than in canines at both dose levels (P < 0.01, Table 2). In addition, tmax and AUC0-∞ differed significantly between macaques and canines at one dose level but not at both dose levels (P = 0.02, Table 2).
Table 1.
Pharmacokinetic parameters for fentanyl administered via a single topical application of 1.3 or 2.6 mg/kg in rhesus macaques (n= 6)
| 1.3 mg/kg |
2.6 mg/kg |
|||
| Mean ± 1 SD | Median (range) | Mean ± 1SD | Median (Range) | |
| tmax (h) | 21.3 ± 10.0 | 24.0 (8.0–36) | 30.7 ± 21.3 | 30.0 (8.0–60.0) |
| Cmax (µg∕mL) | 1.95 ± 0.99 | 1.57 (1.21–3.83) | 4.19 ± 1.69 | 3.78 (2.34–6.96) |
| λz (1∕h) | 0.007 ± 0.002 | 0.007 (0.005–0.010) | 0.007 ± 0.001 | 0.007 (0.006–0.008) |
| t1/2 λz (h)a | 90.9 ± 17.3 | 93.5 (66.9–121) | 97.4 ± 13.2 | 93.9 (87.8–122) |
| AUC0–∞ (h/µg/mL) | 227.0 ± 77.6 | 213 (144–337) | 447 ± 120 | 399 (328–644) |
| AUC0–∞ extrapolated (%) | 2.69 ± 1.25 | 2.48 (1.10–4.48) | 3.12 ± 1.68 | 2.59 (1.51–5.35) |
| AUC0–504 (h/µg/mL) | 221.0 ± 76.3 | 205 (140–333) | 433 ± 114 | 383 (321–612) |
| Vz/F (L/kg) | 864 ± 334 | 930 (373–1192) | 870 ± 218 | 894 (620–1169) |
| Cl/F (mL/kg×h) | 6297 ± 2082 | 6117 (3863–9019) | 6136 ± 1447 | 6513 (4036–7924) |
λz, terminal slope; AUC0–∞, area under the plasma concentration time–curve extrapolated to infinity; AUC0–504, area under the plasma concentration time curve from 0 to 504 h (21 d); Cmax, observed maximum plasma concentration; Cl/F, clearance expressed as a function of bioavailability; t1/2, plasma half-life; tmax, time to observed maximum plasma concentration; Vz/F, volume of distribution based on the terminal phase expressed as a function of bioavailability
Harmonic mean
Table 2.
Comparison of pharmacokinetic parameters (mean ± SE) for fentanyl administered through a single topical application of 1.3 or 2.6 mg/kg in rhesus macaques (n= 6), with pharmacokinetic parameters for a single topical application at the same dosages in canines as previously reported25
| Dose (mg/kg) | NHP | Canines | P | |
| Cmax (ng/mL) | 1.3 | 1.95 ± 0.40 | 2.28 ± 0.36 | 0.45 |
| 2.6 | 4.19 ± 0.69 | 2.67 ± 0.48 | 0.08 | |
| tmax (h) | 1.3 | 21.33 ± 4.09 | 58.0 ± 10.9 | <0.01 |
| 2.6 | 30.67 ± 8.68 | 52.0 ± 11.5 | 0.06 | |
| AUC0-∞ (ng×h/mL) | 1.3 | 227.25 ± 31.67 | 160.0 ± 15.7 | 0.09 |
| 2.6 | 446.97 ± 49.08 | 275.0 ± 38.5 | 0.02 | |
| AUC0-∞ extrapolated (%) | 1.3 | 2.69 ± 0.51 | 2.75 ± 0.89 | 0.91 |
| 2.6 | 3.12 ± 0.69 | 3.48 ± 1.74 | 0.62 | |
| t1/2 (h) | 1.3 | 93.65 ± 7.06 | 53.7 ± 8.48 | <0.01 |
| 2.6 | 98.75 ± 5.40 | 69.6 (± 9.66) | <0.01 |
AUC0–∞, area under the plasma concentration time–curve extrapolated to infinity; Cmax, observed maximum plasma concentration; t1/2, plasma half-life; tmax, time to observed maximum plasma concentration;
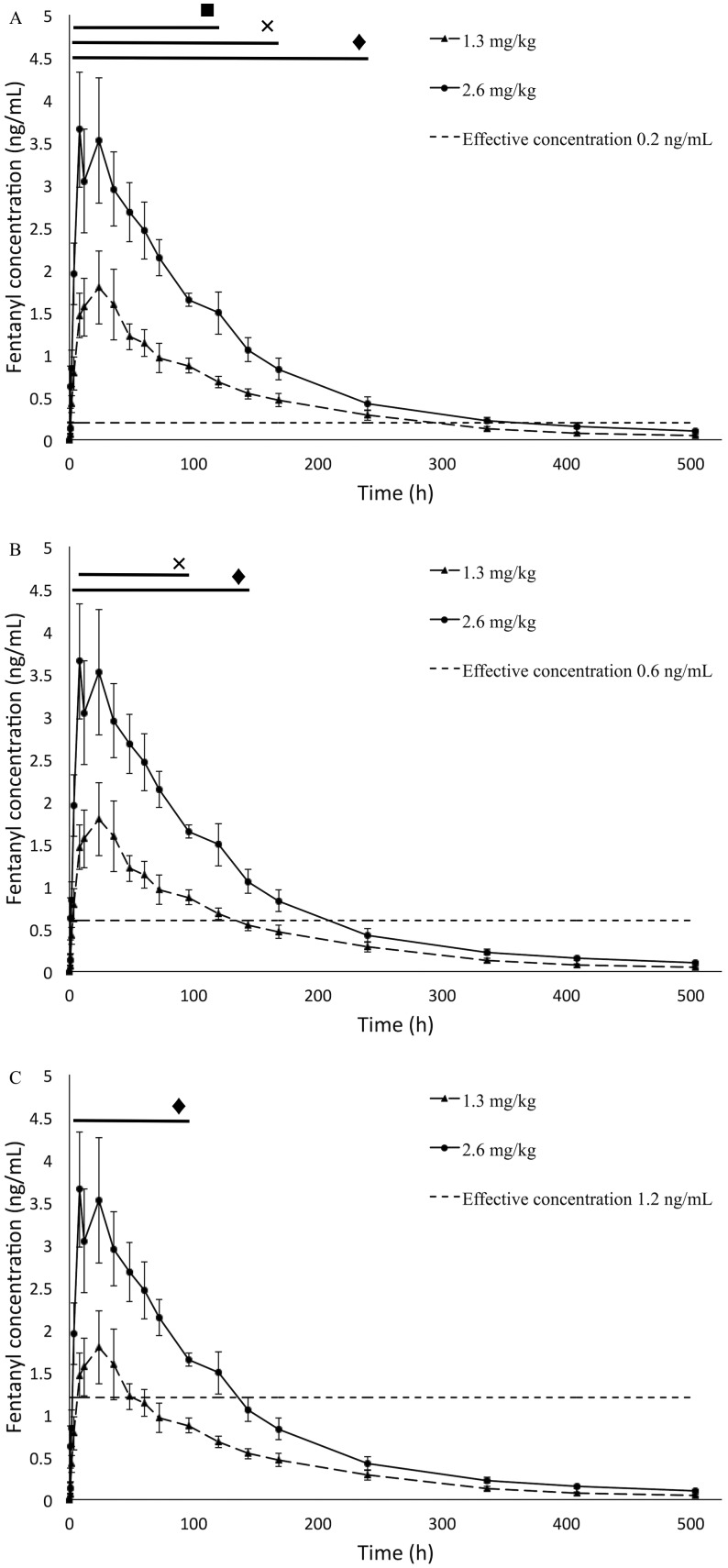
At both dosages, mean plasma concentrations of fentanyl exceeded the lower bound of the MEC (0.2 ng/mL) starting at 2 h after administration (P = 0.05, Figure 1 A). Mean plasma fentanyl concentrations remained above the lower bound of the MEC for 7 d at the 1.3-mg/kg dose and for 10 d at the 2.6-mg/kg dose (P = 0.05, Figure 1 A).
Figure 1.
Mean plasma fentanyl concentrations (ng/mL) over 504 h (21 d) in rhesus macaques administered TFS at a dose of either 1.3 or 2.6 mg/kg by means of a single topical administration as compared with the range of minimum effective concentrations (MEC) in humans undergoing abdominal laparotomy. (A) MEC = 0.2 ng/mL. (B) MEC = 0.6 mg/mL. (C) MEC of 1.2 ng/mL. Error bars represent 1 SEM above and below the data point; ×, time points at which fentanyl concentrations for the 1.3-mg/kg dose were significantly (P < 0.05) above the respective MEC; ♦, time points at which fentanyl concentrations for the 2.6-mg/kg were significantly (P < 0.05) above the respective MEC; ▪, time points at which fentanyl concentrations differed significantly between doses (panel A only).
At the 1.3-mg/kg dose, the mean plasma concentration of fentanyl was above the average reported effective concentration (0.6 ng/mL) from 8 h through 4 d after administration, whereas at the 2.6-mg/kg dose, the mean plasma concentration of fentanyl was above the reported average MEC from 4 h through 6 d after administration (P = 0.05, Figure 1 B).
At the 2.6-mg/kg dose, the mean plasma concentration of fentanyl was above the upper bound of the reported MEC (1.2 ng/mL) starting at 4 h after administration and remaining there through 4 d after administration; the 1.3-mg/kg dose never exceeded the reported MEC upper bound of 1.2 ng/mL (P = 0.05, Figure 1 C).
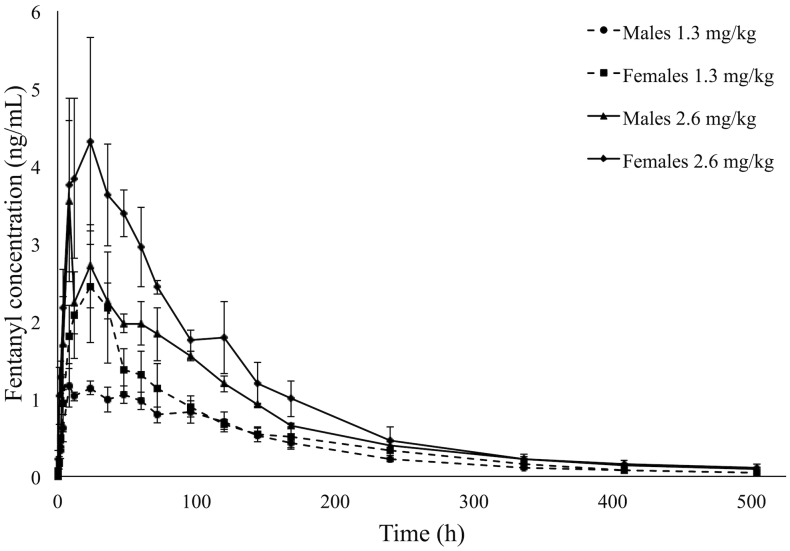
Fentanyl plasma concentrations differed significantly between the 1.3- and 2.6-mg/kg doses from 4 h to 5 d after administration (P < 0.01, Figure 1 A). Plasma concentrations differed significantly between male and female macaques, with fentanyl concentrations lower in male macaques than in female macaques (P < 0.01, Figure 2).
Figure 2.
Mean plasma fentanyl concentrations (ng/mL) over 504 h (21 d) in rhesus macaques by sex (3 male, 3 female) administered TFS at a dose of either 1.3 or 2.6 mg/kg by means of a single topical administration. Error bars represent 1 SE above and below the data point.
Discussion
The current study investigated the use of a novel TFS in rhesus macaques. This fentanyl formulation represents a potential analgesic refinement for the care and wellbeing of NHP in biomedical research; it also addresses the regulatory requirement of providing analgesia in laboratory animal species undergoing potentially painful procedures. This formulation has been approved by the FDA for use in dogs,17 and the current rhesus data described herein is compared with the previously reported canine data.24,25
Fentanyl has been described for use in several species, including domestic,9,26,28,32,50,59,71 exotic,34,35,38,45,60,73 and laboratory animal species,8,12,21,22,36,49,72 and although fentanyl is cited for use8 and has been studied in NHP,7,20,35,46,54,68,70 little exists in the literature regarding the pharmacokinetics of fentanyl in rhesus macaques. Fentanyl is commonly dosed through the intravenous route, either as a bolus or constant-rate infusion, or by means of a transdermal delivery system, such as a patch.7,8,21,24,25,32,59 In addition, fentanyl is commercially available in a neuroleptanalgesic combination with droperidol (Innovar-vet) for use as a sedative or preanesthetic drug for minor surgical procedures.8,21 There are also reports of effective transmucosal administration of fentanyl to great apes for sedation and immobilization in the form of a lollipop treat, given that this route of administration is approved by the FDA for use in humans.35 The use of transdermal fentanyl patches in NHP has been studied but is not a standardly used formulation, due to the risk of respiratory depression and death after ingestion of the patches.14 For this reason, we chose the dorsal midscapulae as site of the administration in the current study. In addition, manufacturer dosing recommendations and prior studies describe dorsal midscapular dosing in beagles.17,24,25 Daily observation was performed to ensure the absence of adverse effects, in the form of administration-site reactions, oversedation, or more serious events, such as respiratory depression or death. No adverse reactions were noted in any macaque at either dose.
The 2 doses (1.3 and 2.6 mg/kg topically) evaluated in the current study were selected based on those previously studied in beagles.25 In rhesus macaques, both doses reached minimal effective plasma concentrations, defined herein as 0.2 ng/mL as discussed previously. The lower dose (1.3 mg/kg) reached this level within 2 h after administration, whereas the 2.6-mg/kg topical dose reached minimal effective plasma levels within 1 h afterward. As expected, the maximal plasma concentration was approximately 2 times higher with the 2.6-mg/kg dose as compared with the 1.3-mg/kg dose. Plasma concentrations remained above the minimal effective plasma level of 0.2 ng/mL longer after the high dose compared with the low dose. In addition, AUC values for the high dose were approximately 2 times greater than those of the low dose. The terminal half-lives of both doses were comparable to each other and are both longer than that previously established (approximately 3 h) for fentanyl after intravenous administration of fentanyl citrate (Sublimaze, Janssen Pharmaceutica, Titusville, NJ) in rhesus macaques.21,24,68 This value is in line with prior observations of absorption-dependent (that is, flip-flip) kinetics, a phenomenon where the rate of drug absorption is slower than elimination, resulting in a terminal half-life that is reflective of absorption instead of elimination.23,25,41 This phenomenon is likely a result of the TFS formulation, in that the volatile solvent, isopropanol, allows for rapid air-drying and dermal absorption within approximately 5 min after administration and that the compound octyl salicylate penetrates the skin and sequesters the fentanyl in the stratum corneum for slow, steady absorption into the blood stream.25,41
With regard to rhesus macaques, an apparent sex-associated difference was noted, in that males had significantly lower plasma concentrations than females when dose and time points were accounted for as fixed effects (P < 0.01, Figure 2). Other studies investigating the effects of sex on opioid, specifically fentanyl, pharmacokinetics and efficacy have likewise noted sex-associated differences, although little agreement exists regarding the cause of these differences. In rats given subcutaneous fentanyl, females had significantly lower Cmax and AUC 0-∞ values and a larger volume of distribution (Vd/F) compared with males, suggesting a sex-specific difference related to distribution to fat.5,56 A follow-up study investigating drug distribution after intravenous fentanyl in male and female rats to elucidate sex-specific differences in distribution did not reveal a significant sex-associated difference in any pharmacokinetic parameter.55 In studies investigating the efficacy of different classes of opioids, fentanyl antinociception also showed no apparent sex-associated differences, although if present, these might be opioid receptor-, assay-, dose-, or time-dependent.4,39 Although the exact mechanism remains elusive, these potential sex-associated differences might be directly or indirectly related to differences in body composition; cytochrome P450 concentrations and drug metabolism; sex-steroid effects, specifically based on reproductive status or throughout the menstrual cycle; drug binding; health status or social habits of the individual; or the sex-related pharmacokinetics affecting bioavailability.10,63 The apparent sex differences noted herein could be related to any of the suggested hypotheses, but it is noteworthy that our data show sex-associated effects that are opposite to those previously noted in the literature. To further elucidate potential sex-associated differences in rhesus macaques and how they compare with those in other species, future research using a larger sample size is likely indicated. In addition, although determining the pharmacodynamics of TFS was beyond the scope of the current study, future research may elucidate further understanding of these potential fentanyl sex-associated effects.
Both doses evaluated here likely represent appropriate doses for this potentially long-acting, potent, topical opioid for use in rhesus macaques to provide adequate and effective analgesia and to limit or eliminate potential stressors secondary to providing analgesia. Assuming an absolute minimal effective plasma concentration of 0.2 ng/mL, the low dose of 1.3 mg/kg topical TFS would provide effective plasma concentrations within 2 h of topical administration and would maintain effective levels for at least 7 d in rhesus macaques (Figure 1 A). Compared with the same dose in beagles, the Cmax and AUC in macaques were not significantly different, suggesting a similar pharmacokinetic profile at this dose; however, the time to maximal concentration (tmax) and terminal half-life of fentanyl in rhesus macaques were significantly different from those established in beagles. In rhesus macaques, the time to maximal concentration was significantly shorter (approximately 3-fold faster), whereas the terminal half-life was significantly longer than those in dogs (P < 0.01, Table 2). Given the range of effective plasma concentrations noted in humans and assumed in dogs, the 1.3-mg/kg dose may not reach the range of effective plasma levels (that is, 0.6 or 1.2 ng/mL, Figure 1 B and C, respectively). Therefore, care must be taken to ensure that adequate analgesia is achieved if the 1.3-mg/kg dose is used.
In rhesus macaques, the high dose of TFS (2.6 mg/kg topically) provided plasma concentrations >0.2 ng/mL for at least 10 d and achieved these levels by 1 to 2 h after administration (Figure 1 A). The maximal plasma concentration after 2.6 mg/kg topical TFS in rhesus macaques was approximately 1.5-fold higher than that reported in beagles, and the AUC in macaques was greater than in beagles at the same dose (P < 0.02, Table 2). The longer terminal half-life may be a result of species-specific differences in absorption related to physiochemical properties of the skin and subcutaneous tissues, such as the thickness of the stratum corneum, densities of hair follicles and apocrine sweat glands, vascularity, and potentially a major fentanyl reservoir within the stratum corneum that contributes to the longer terminal half-life, as suggested in other species.50,61 The high dose is likely to provide effective plasma levels of greater than 1.2 ng/mL for as long as 4 d after administration (Figure 1 C).
The MEC is defined as the minimal drug concentration in serum required to produce a desirable therapeutic effect; this level can vary as much as 6-fold in humans, depending on individual responsiveness, as shown by the human fentanyl MEC range of 0.2 to 1.2 ng/mL.25,29 In the case of opioids, the MEC is a concept that suggests that continuous analgesia should be achieved when the serum concentration of a particular opioid is maintained in excess of its MEC value and that the analgesic effect will abate when the serum opioid concentration decreases below the MEC value.29 Unfortunately, MEC values for fentanyl are difficult to establish and fluctuate between species and individual patients. In addition, reported MEC values for fentanyl rely on the study design, intensity of surgical stimulus, the postoperative period, previous opioid treatment and tolerance of the subjects, the characterization and monitoring of analgesia, and the activity level of the patients (at rest or in movement).16,28,29,42,44,54,69 Because limited information exists in the literature regarding the pharmacokinetics and efficacy of fentanyl in rhesus macaques, we considered both 0.2 and 1.2 ng/mL as limits of a minimally effective range in plasma on the basis of the range in humans.
Although establishing an MEC of fentanyl in rhesus macaques was beyond the scope of this pharmacokinetic study, discussing the MEC established in other species is helpful. Fentanyl MEC varies between species and individuals; studies in dogs suggest an MEC ranging from 0.4 to 1.3 ng/mL,25,32,43,62 whereas other studies using fentanyl patches in dogs advocate a mean MEC of 0.6 ng/mL62 to 1.2 ng/mL.43 In cats, fentanyl MEC have been estimated at 1.6 to 1.7 ng/mL.32 In humans, fentanyl MEC varies from 0.6 to 3 ng/mL;57 however, most studies that included a surgical stimulus reported MEC values between 0.9 to 2.0 ng/mL,33,69 with a 6-fold difference in MEC between patients. In human studies in which the patient titrates the delivered dose of fentanyl, patients required a larger dose during the first 6 h after surgery; this higher drug requirement is believed to be necessary to establish a steady-state drug concentration, which depends on the drug's half-life.29 In addition, even minor movements, such as deep breathing, and ambulation, were associated with a higher fentanyl demand or higher visual analog scale score (a visual pain scoring system used in human medicine to evaluate adequate analgesia).29,44
In addition to the variability that exists regarding species- specific and individual characteristics, different pharmacokinetic profiles of fentanyl in rhesus macaques have been reported. One study investigating increasing doses of intravenous boluses of fentanyl and measuring tail latency responses in rhesus macaques suggested a serum MEC of 2.97 ng/mL as measured by radioimmunoassay,54 whereas in the present study we measured plasma fentanyl concentrations by using liquid chromatography–mass spectrometry as described recently.7,24,25 In one study65 comparing fentanyl plasma concentrations in horses as measured by both radioimmunoassay and liquid chromatography–mass spectrometry, radioimmunoassay was less accurate and overestimated concentrations near the lower limit of quantitation. This finding suggests that the assay techniques by which fentanyl concentrations were measured (that is, radioimmunoassay compared with liquid chromatography–mass spectrometry) and the medium from which the drug is measured (that is, plasma compared with serum) may contribute to discrepancy among reports,65 such that results from different studies may not be directly comparable.
A limitation of this study was the need for ketamine sedation prior to topical administration of the fentanyl dose in the study subjects. Both ketamine and fentanyl are metabolized in the human liver through the cytochrome P450 pathway, specifically via the enzyme CYP3A4;18,21,31,51 therefore, competition during metabolism may alter the clearance of one or both drugs. To the best of our knowledge, the enzymes responsible for the metabolism of fentanyl and ketamine in rhesus macaques have not yet been elucidated. If similar to the situation in humans, the same enzyme is responsible for metabolism of both compounds in macaques, thus potentially affecting the calculated pharmacokinetic parameters we reported here. However, because sedation is recommended for effective administration of TFS, the use of ketamine in the current study is representative of the clinical situation.
Given the variability that exists between species, individuals within the same species, and study designs investigating the MEC and efficacy of fentanyl analgesia, future research is needed to more accurately determine the MEC of fentanyl for clinical use in rhesus macaques. The current study shows that a single 1.3-mg/kg topical dose of TFS in rhesus macaques may provide 7 d of effective plasma levels when assuming the reported lower bound MEC of 0.2 ng/mL as established in beagles; this dose achieves these plasma levels within 2 h of administration. In comparison, a single 2.6-mg/kg topical dose of TFS in rhesus macaques provided 10 d of effective plasma levels (0.2 ng/mL) within 1 h of administration. TFS should be dosed preoperatively to ensure adequate plasma concentrations prior to any painful stimuli being elicited. In addition, future investigation of this compound for use in other NHP and laboratory animal species is likely indicated as well.
In conclusion, the present study established the pharmacokinetic profiles for the 1.3- and 2.6-mg/kg topical doses of TFS in rhesus macaques and compared those parameters with each other and with those previously reported in beagles at the same doses. Our findings confirmed that both doses of TFS in rhesus macaques have similar pharmacokinetic profiles, with significantly longer half-lives than in dogs, and remain above the minimal effective plasma concentration (0.2 ng/mL) for 7 and 10 d, respectively. Therefore, the use of this novel, long-acting, single-dose, TFS represents an important refinement for the provision of sustained analgesia, reduction of stress from repeated dosing of analgesics and interactions with personnel, and, ultimately, improvement of animal welfare of NHP used in biomedical research.
Acknowledgments
The project described was supported by grants from the Animal Welfare Institute and by the Office of the Director of NIH (P51 OD011107-54). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the Director of the NIH. We thank Ross Allen and John Hyde of the CNPRC and Dan Mckemie and Sandy Yim of the KL Maddy Equine Analytical Chemistry Laboratory for their respective expertise and technical support.
References
- 1.’t Hart BA, Abbott DH, Nakamura K, Fuchs E. 2012. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov Today 17:1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended 2013 7 USC §2131–2159. [Google Scholar]
- 3.Animal Welfare Regulations 2013 9 CFR § 3.129. [Google Scholar]
- 4.Bartok RE, Craft RM. 1997. Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–778. [PubMed] [Google Scholar]
- 5.Björkman S, Stanski DR, Verotta D, Harashima H. 1990. Comparative tissue concentration profiles of fentanyl and alfentanil in humans predicted from tissue–blood partition data obtained in rats. Anesthesiology 72:865–873. [DOI] [PubMed] [Google Scholar]
- 6.Blackwood RS, Tarara RP, Christe KL, Spinner A, Lerche NW. 2008. Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comp Med 58:81–87. [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson AM, Kelly Iii R, Fetterer DP, Rico PJ, Bailey EJ. 2016. Pharmacokinetics of 2 formulations of transdermal fentanyl in cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 55:436–442. [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter JW. 2012. Exotic animal formulary, 3rd ed. St. Louis (MO): Elsevier Health Sciences. [Google Scholar]
- 9.Carroll GL, Hooper RN, Boothe DM, Hartsfield SM, Randoll LA. 1999. Pharmacokinetics of fentanyl after intravenous and transdermal administration in goats. Am J Vet Res 60:986–991. [PubMed] [Google Scholar]
- 10.Ciccone GK, Holdcroft A. 1999. Drugs and sex differences: a review of drugs relating to anaesthesia. Br J Anaesth 82:255–265. [DOI] [PubMed] [Google Scholar]
- 11.Clingerman KJ, Summers L. 2012. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): inter- and intrarater variability. J Am Assoc Lab Anim Sci 51:31–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Clowry GJ, Flecknell PA. 2000. The successful use of fentanyl–fluanisone (‘Hypnorm’) as an anaesthetic for intracranial surgery in neonatal rats. Lab Anim 34:260–264. [DOI] [PubMed] [Google Scholar]
- 13.Darian-Smith C. 2007. Monkey models of recovery of voluntary hand movement after spinal cord and dorsal root injury. ILAR J 48:396–410. [DOI] [PubMed] [Google Scholar]
- 14.Deschamps JY, Gaulier JM, Podevin G, Cherel Y, Ferry N, Roux FA. 2012. Fatal overdose after ingestion of a transdermal fentanyl patch in 2 nonhuman primates. Vet Anaesth Analg 39:653–656. [DOI] [PubMed] [Google Scholar]
- 15.Ebeling M, Kung E, See A, Broger C, Steiner G, Berrera M, Heckel T, Iniguez L, Albert T, Schmucki R, Biller H, Singer T, Certa U. 2011. Genome-based analysis of the nonhuman primate Macaca fascicularis as a model for drug safety assessment. Genome Res 21:1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger CM, Duke T, Archer J, Cribb PH. 1998. Comparison of plasma fentanyl concentrations by using 3 transdermal fentanyl patch sizes in dogs. Vet Surg 27:159–166. [DOI] [PubMed] [Google Scholar]
- 17.Elanco Animal Health 2014. Recuvyra (fentanyl) transdermal solution [technical detailer]. Indianapolis (IA): Elanco. [Google Scholar]
- 18.Feierman DE, Lasker JM. 1996. Metabolism of fentanyl, a synthetic opioid analgesic, by human liver microsomes. Role of CYP3A4. Drug Metab Dispos 24:932–939. [PubMed] [Google Scholar]
- 19.Ferguson B, Street SL, Wright H, Pearson C, Jia Y, Thompson SL, Allibone P, Dubay CJ, Spindel E, Norgren RB., Jr 2007. Single-nucleotide polymorphisms (SNPs) distinguish Indian-origin and Chinese-origin rhesus macaques (Macaca mulatta). BMC Genomics 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field WE, Yelnosky J, Mundy J, Mitchell J. 1966. Use of droperidol and fentanyl for analgesia and sedation in primates. J Am Vet Med Assoc 149:896–901. [PubMed] [Google Scholar]
- 21.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals, 2nd ed. San Francisco (CA): Elsevier Science. [Google Scholar]
- 22.Foley PL, Henderson AL, Bissonette EA, Wimer GR, Feldman SH. 2001. Evaluation of fentanyl transdermal patches in rabbits: blood concentrations and physiologic response. Comp Med 51:239–244. [PubMed] [Google Scholar]
- 23.Freise KJ, Linton DD, Newbound GC, Tudan C, Clark TP. 2012. Population pharmacokinetics of transdermal fentanyl solution following a single dose administered prior to soft tissue and orthopedic surgery in dogs. J Vet Pharmacol Ther 35 Suppl 2:65–72. [DOI] [PubMed] [Google Scholar]
- 24.Freise KJ, Newbound GC, Tudan C, Clark TP. 2012. Pharmacokinetics and the effect of application site on a novel, long-acting transdermal fentanyl solution in healthy laboratory beagles. J Vet Pharmacol Ther 35 Suppl 2:27–33. [DOI] [PubMed] [Google Scholar]
- 25.Freise KJ, Savides MC, Riggs KL, Owens JG, Newbound GC, Clark TP. 2012. Pharmacokinetics and dose selection of a novel, long-acting transdermal fentanyl solution in healthy laboratory beagles. J Vet Pharmacol Ther 35 Suppl 2:21–26. [DOI] [PubMed] [Google Scholar]
- 26.Gauntlett IS, Fisher DM, Hertzka RE, Kuhls E, Spellman MJ, Rudolph C. 1988. Pharmacokinetics of fentanyl in neonatal humans and lambs: effects of age. Anesthesiology 69:683–687. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu Y-S, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers Y-H, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang S-P, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csürös M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX-Z, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit A FA, Ullmer B, Wang H, Xing J, Burhan R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas L J, Han S-G, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani Ka, Kehrer-Sawatzki H, Kolb J, Patil S, Pu L-L, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'Brien WE, Prüfer K, Stenson PD, Wallace JC, Ke H, Liu X-M, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert DB, Motzel SL, Das SR. 2003. Postoperative pain management using fentanyl patches in dogs. Contemp Top Lab Anim Sci 42:21–26. [PubMed] [Google Scholar]
- 29.Gourlay GK, Kowalski SR, Plummer JL, Cousins MJ, Armstrong PJ. 1988. Fentanyl blood concentration–analgesic response relationship in the treatment of postoperative pain. Anesth Analg 67:329–337. [PubMed] [Google Scholar]
- 30.Grape S, Schug SA, Lauer S, Schug BS. 2010. Formulations of fentanyl for the management of pain. Drugs 70:57–72. [DOI] [PubMed] [Google Scholar]
- 31.Hijazi Y, Boulieu R. 2002. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30:853–858. [DOI] [PubMed] [Google Scholar]
- 32.Hofmeister EH, Egger CM. 2004. Transdermal fentanyl patches in small animals. J Am Anim Hosp Assoc 40:468–478. [DOI] [PubMed] [Google Scholar]
- 33.Holley FO, van Steennis C. 1988. Postoperative analgesia with fentanyl—pharmacokinetics and pharmacodynamics of constant-rate iv and transdermal delivery. Br J Anaesth 60:608–613. [DOI] [PubMed] [Google Scholar]
- 34.Hoppes S, Flammer K, Hoersch K, Papich M, Paul-Murphy J. 2003. Disposition and analgesic cockatoos effects of fentanyl in white cockatoos (Cacatua alba). J Avian Med Surg 17:124–130. [Google Scholar]
- 35.Hunter RP, Isaza R, Carpenter JW, Koch DE. 2004. Clinical effects and plasma concentrations of fentanyl after transmucosal administration in 3 species of great ape. J Zoo Wildl Med 35:162–166. [DOI] [PubMed] [Google Scholar]
- 36.Husby P, Heltne JK, Koller ME, Birkeland S, Westby J, Fosse R, Lund T. 1998. Midazolam–fentanyl–isoflurane anaesthesia is suitable for haemodynamic and fluid balance studies in pigs. Lab Anim 32:316–323. [DOI] [PubMed] [Google Scholar]
- 37.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 38.Jensen JM. 1982. Fentanyl citrate immobilization of zoo ungulates. The Journal of zoo animal medicine 13:101–103. [Google Scholar]
- 39.Kest B, Sarton E, Dahan A. 2000. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology 93:539–547. [DOI] [PubMed] [Google Scholar]
- 40.Kornick CA, Santiago-Palma J, Moryl N, Payne R, Obbens EA. 2003. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf 26:951–973. [DOI] [PubMed] [Google Scholar]
- 41.Kukanich B, Clark TP. 2012. The history and pharmacology of fentanyl: relevance to a novel, long-acting transdermal fentanyl solution newly approved for use in dogs. J Vet Pharmacol Ther 35 Suppl 2:3–19. [DOI] [PubMed] [Google Scholar]
- 42.Kyles AE. 1998. Transdermal fentanyl. Compendium 20:721–726. [Google Scholar]
- 43.Kyles AE, Hardie EM, Hansen BD, Papich MG. 1998. Comparison of transdermal fentanyl and intramuscular oxymorphone on postoperative behaviour after ovariohysterectomy in dogs. Res Vet Sci 65:245–251. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann LJ, DeSio JM, Radvany T, Bikhazi GB. 1997. Transdermal fentanyl in postoperative pain. Reg Anesth 22:24–28. [DOI] [PubMed] [Google Scholar]
- 45.Love JA. 1970. Use of fentanyl and droperidol in guinea pigs, lemmings, ground squirrels, and cats. J Am Vet Med Assoc 157:675–677. [PubMed] [Google Scholar]
- 46.Mama KR, Valverde CR, Steffey EP, Kollias-Baker C. 2000. Effect of fentanyl on minimum alveolar concentration of isoflurane in rhesus monkeys. Vet Anaesth Analg 27:58. [DOI] [PubMed] [Google Scholar]
- 47.Margetts L, Sawyer R. 2007. Transdermal drug delivery: principles and opioid therapy. Continuiing education in anaesthesia, critical care & pain 7:171–176. [Google Scholar]
- 48.Martinez SA, Wilson MG, Linton DD, Newbound GC, Freise KJ, Lin TL, Clark TP. 2013. The safety and effectiveness of a long-acting transdermal fentanyl solution compared with oxymorphone for the control of postoperative pain in dogs: a randomized, multicentered clinical study. J Vet Pharmacol Ther 37:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392. [DOI] [PubMed] [Google Scholar]
- 50.Maxwell LK, Thomasy SM, Slovis N, Kollias-Baker C. 2003. Pharmacokinetics of fentanyl following intravenous and transdermal administration in horses. Equine Vet J 35:484–490. [DOI] [PubMed] [Google Scholar]
- 51.Meyer MR, Maurer HH. 2011. Absorption, distribution, metabolism, and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics 12:215–233. [DOI] [PubMed] [Google Scholar]
- 52.National Institutes of Health 2002. Public Health Service policy on humane care and use of laboratory animals. Bethesda (MD): Office of Laboratory Animal Welfare. [Google Scholar]
- 53.Nelson L, Schwaner R. 2009. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol 5:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nussmeier NA, Benthuysen JL, Steffey EP, Anderson JH, Carstens EE, Eisele JH, Jr, Stanley TH. 1991. Cardiovascular, respiratory, and analgesic effects of fentanyl in unanesthetized rhesus monkeys. Anesth Analg 72:221–226. [DOI] [PubMed] [Google Scholar]
- 55.Ohtsuka H, Fujita K, Kobayashi H. 2007. Pharmacokinetics of fentanyl in male and female rats after intravenous administration. Arzneimittelforschung 57:260–263. [DOI] [PubMed] [Google Scholar]
- 56.Ohtsuka H, Kobayashi H. 2001. Pharmacokinetics of fentanyl after intravenous and subcutaneous administration to rats and dogs. Japanese Pharmacology and Therapeutics 29:855–864. [Google Scholar]
- 57.Peng PWH, Sandler AN. 1999. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90:576–599. [DOI] [PubMed] [Google Scholar]
- 58.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. 2014. Why primate models matter. Am J Primatol 76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plumb DC. 2011. Plumb's veterinary drug handbook, 7th ed. Ames (IA): Wiley. [Google Scholar]
- 60.Ramsay EC. 2008. Use of analgesics in exotic felids, p 289–293. In: Fowler ME, Miller RE. Zoo and wild animal medicine, vol 6. St Louis (MO): Saunders Elsevier. [Google Scholar]
- 61.Riviere JE, Papich MG. 2001. Potential and problems of developing transdermal patches for veterinary applications. Adv Drug Deliv Rev 50:175–203. [DOI] [PubMed] [Google Scholar]
- 62.Robinson TM, Kruse-Elliott KT, Markel MD, Pluhar GE, Massa K, Bjorling DE. 1999. A comparison of transdermal fentanyl compared with epidural morphine for analgesia in dogs undergoing major orthopedic surgery. J Am Anim Hosp Assoc 35:95–100. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz JB. 2003. The influence of sex on pharmacokinetics. Clin Pharmacokinet 42:107–121. [DOI] [PubMed] [Google Scholar]
- 64.Shively CA, Clarkson TB. 2009. The unique value of primate models in translational research. Nonhuman primate models of women's health: introduction and overview. Am J Primatol 71:715–721. [DOI] [PubMed] [Google Scholar]
- 65.Thomasy SM, Mama KR, Stanley SD. 2008. Comparison of liquid chromatography–mass spectrometry and radioimmunoassay for measurement of fentanyl and determination of pharmacokinetics in equine plasma. J Anal Toxicol 32:754–759. [DOI] [PubMed] [Google Scholar]
- 66.Thomasy SM, Mama KR, Whitley K, Steffey EP, Stanley SD. 2007. Influence of general anaesthesia on the pharmacokinetics of intravenous fentanyl and its primary metabolite in horses. Equine Vet J 39:54–58. [DOI] [PubMed] [Google Scholar]
- 67.United States Department of Health and Human Services, Food and Drug Administration 2001. Guidance for industry: bioanalytical method validation. Washington (DC): US Department of Health and Human Services. [Google Scholar]
- 68.Valverde CR, Mama KR, Kollias-Baker C, Steffey EP, Baggot JD. 2000. Pharmacokinetics and cardiopulmonary effects of fentanyl in isoflurane-anesthetized rhesus monkeys (Macaca mulatta). Am J Vet Res 61:931–934. [DOI] [PubMed] [Google Scholar]
- 69.Varvel JR, Shafer SL, Hwang SS, Coen PA, Stanski DR. 1989. Absorption characteristics of transdermally administered fentanyl. Anesthesiology 70:928–934. [DOI] [PubMed] [Google Scholar]
- 70.Votava M, Hess L, Schreiberova J, Malek J, Stein K. 2011. Short-term pharmacological immobilization in macaque monkeys. Vet Anaesth Analg 38:490–493. [DOI] [PubMed] [Google Scholar]
- 71.Wegner K, Franklin RP, Long MT, Robertson S. 2002. How to use fentanyl transdermal patches for analgesia in horses, p 291–294. Proceedings of the 48th Annual Convention of the American Association of Equine Practioners, Orlando, Florida. [Google Scholar]
- 72.Wilkinson AC, Thomas ML, 3rd, Morse BC. 2001. Evaluation of a transdermal fentanyl system in Yucatan miniature pigs. Contemp Top Lab Anim Sci 40:12–16. [PubMed] [Google Scholar]
- 73.Williams TD, Williams AL, Siniff DB. 1981. Fentanyl and azaperone produced neuroleptanalgesia in the sea otter (Enhydra lutris). J Wildl Dis 17:337–342. [DOI] [PubMed] [Google Scholar]