Abstract
Objective
There is substantial interest in delineating the course of cognitive functioning in bipolar (BP) youth. However, there are no longitudinal studies aimed at defining subgroups of BP youth based on their distinctive cognitive trajectories and their associated clinical variables.
Method
Cognitive functioning was measured in 135 participants from the Course and Outcome of BP Youth (COBY) study using several subtests of the Cambridge Neuropsychological Test Automated Battery (CANTAB). Youth were prospectively evaluated three times on average every 13.75 months over 2.5 years. Clinical and functional outcomes were assessed using the Longitudinal Interval Follow-Up Evaluation (LIFE).
Results
Latent class growth analysis identified three longitudinal patterns of cognitive functioning based on a general cognitive index: class 1, “persistently high” (N=21; 15.6%); class 2, “persistently moderate” (N=82; 60.74%); and class 3, “persistently low” (N=32; 23.7%). All classes showed normal cognitive functioning when compared with the CANTAB normative data. After adjustment for confounders, youth from class 3 had a significantly greater percentage of time with overall, manic, and depressive syndromal symptoms than youth in the other two classes. Also, after adjustment for confounders, youth from class 3 had significantly poorer global, academic, and social functioning than youth from class 1.
Conclusions
BP youth showed normal overall cognitive functioning that remained stable during the follow-up within each class. However, 24% of BP youth showed relatively poorer cognitive functioning than the other BP youth. This subgroup had poorer mood course and functioning, and may benefit from cognitive remediation and early management with evidence-based pharmacological treatments.
Keywords: cognitive functioning, children, adolescents, bipolar disorder, longitudinal studies
Introduction
There is a need to understand the longitudinal course of cognitive functioning in youth with bipolar disorder (BP)1,2. Specifically, it is unclear whether BP youth exhibit either cognitive decline, stable/unchanged cognitive trajectory, or cognitive improvement as they transit into adulthood3. By elucidating this issue, we could obtain more evidence about what mechanism of cognitive dysfunction might be associated with academic or social dysfunction4,5. Additionally, longitudinal studies of cognitive performance in BP patients may highlight critical windows of cognitive development that may be amenable to novel treatments, such as computer assisted cognitive remediation or cognitive enhancing medications6.
To our knowledge, only three studies have evaluated longitudinal cognitive functioning in youth with BP. Pavuluri and colleagues7 compared 26 youth with BP-I and 17 healthy controls (HC) (mean age: 11 years old) at baseline and 3 years later using the Trail Making Test, the Digit Span Subtest from the Weschler Memory Scale, the California Verbal Learning Test, several subtests from the University of Pennsylvania Computerized Neuropsychological Battery, and the Cogtest. BP youth exhibited deficits in attention, executive functions, working memory, verbal memory, visual memory, and visual-spatial perception at baseline and again 3 years later. At 3 years, BP youth showed less developmental progress in executive functions, and verbal memory than HC youth, even when outcomes controlled for changes in mood state and treatment with stimulants. Whitney and colleagues8 studied 35 youth with BP-I and 25 HC (mean age: 15 years old). At baseline, BP youth exhibited bias away from positive valence stimuli (adjectives such as friendly, helpful, lucky, and nice), which persisted over a 1-year period, independent of mood state, medication exposure, and psychiatric comorbidities. Finally, Lera-Miguel and colleagues9 compared 20 youth with BP (90% BP-I, 10% BP-II) and 20 HC who were assessed at baseline (mean age:16 years old) and 2.5 years later using several neuropsychological tests including, but not limited to, the Wechsler Intelligence Scale, the Wechsler Memory Scale, and the Stroop Test. Adjusting for changes in mood severity over time, BP youth improved more than HC in verbal reasoning, working memory, processing speed, visual-motor skills, and visual memory. However, among BP youth, scores in executive functions did not improve and remained lower than HC performance at follow-up. Psychotic symptoms and treatment with lithium at baseline, but not psychiatric comorbidities, were associated with poorer performance in executive control tasks in BP youth.
While the above mentioned studies are important and included both BP and HC groups, they have the following limitations: 1) small sample sizes; 2) inclusion of youth predominantly diagnosed with BP-I; 3) following the sample only one time; 4) no consideration of the relationship between cognitive functioning and mood symptoms over time; and 5) most studies did not adjust for the effects of time-varying factors such as treatment, symptom severity, mood polarity, comorbid disorders, or Intelligence Quotient (IQ).
Using the Cambridge Neuropsychological Test Automated Battery (CANTAB)10, we previously found that BP youth with BP-I/II (n= 92) participating in the Course and Outcome of BP Youth (COBY) study had specific deficits in cognitive flexibility at intake relative to youth with BP-Not Otherwise Specified (BP-NOS) (n= 28)11. In addition, comparison of the COBY BP I/II with HCs (n= 55) recruited through the Longitudinal Assessment of Manic Symptoms (LAMS) study, showed that across all BP subtypes, BP participants had impairments in sustained attention and information processing for emotionally valenced words.
The goal of this study was to extend COBY’s prior baseline findings by evaluating the longitudinal course of cognitive functioning and its relationship with the mood trajectory and psychosocial functioning in COBY youth using Latent Class Growth Analysis (LCGA). LCGA has not been used to assess the longitudinal cognitive functioning of either adults or youth with BP, but it has been successfully utilized to evaluate cognitive trajectories in patients with schizophrenia, dementia, and major depression12–14. Particularly, LCGA may help us identify potential subgroups at greater risk for cognitive dysfunction and their associated clinical variables. Thus, in this study we aimed to evaluate: 1) whether there are several distinctive cognitive trajectories among BP youth; 2) the baseline and longitudinal factors associated with distinctive cognitive trajectories (e.g., demographic variables, IQ, exposure to medications, BP subtype, age of BP onset, psychiatric comorbidities); and 3) the relationship between these distinctive cognitive trajectories, mood course (percentage of time in euthymia, and sub/syndromal mood symptoms of different polarities), and psychosocial functioning (global, academic, social) over time.
Method
Participants
The methods for the COBY study have been described in detail elsewhere15–17. Briefly, 446 youth aged 7–17 years with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) BP-I or -II or an operationally defined BP-NOS were recruited at Brown University, University of California Los Angeles, and the University of Pittsburgh. Age of BP onset was defined as the onset of a DSM-IV mood episode or an episode fulfilling the COBY’s modified DSM-IV BP-NOS. BP-NOS was defined as a distinct period(s) of abnormally elevated, expansive or irritable mood, plus: (1) at least two DSM-IV manic symptoms (three if the mood is irritable only) that were clearly associated with the onset of abnormal mood; (2) clear change in functioning; (3) mood and symptoms present for a significant part of the day (minimum of 4 hours); and (4) a minimum of four days (not necessarily consecutive) meeting these mood, symptom, duration, and functional change criteria over the participant´s lifetime. Youth diagnosed with these operationalized criteria for BP-NOS in COBY have shown similarly poor functioning, comorbid disorders, risk for suicidality and substance abuse, and family history of mania, as those with BP-I17. Moreover, they are at high risk to convert into BP-I and II16. To date, 29% of the youth with BP-NOS at intake converted into BP-I or II by the time of the last cognitive assessment. BP youth were enrolled independent of current mood state or treatment status. Youth with schizophrenia, mental retardation, autism, and mood disorders secondary to substances, medications, or medical conditions were excluded from the study.
BP youth were recruited from outpatient clinics (84.4%), inpatient units (4.4%), advertisements (6.7%), and referrals from other physicians (4.4%) from October 2000 through July 2006. The CANTAB was administered for the first time to 203 youth, on average 53 months after the initiation of the COBY study. For the current analyses, only participants who had at least one additional administration (N=135) are included. Participants who were included in the current study were not significantly different from those excluded (N=68) in terms of demographic characteristics, age at BP onset, duration of mood illness, BP subtype, number of hospitalizations, exposure to medications, lifetime psychiatric comorbidities, and cognitive functioning at the first CANTAB assessment (p> 0.05).
BP youth included in the current study were evaluated using the CANTAB at three time points (T1, n=135; T2, n=135; and T3, n=82). On average each CANTAB was administered every 13.7±7.1 months over a period of 2.5 years (range 12–90 months).
Demographic and clinical characteristics at T1 are presented in Table 1.
Table 1.
Demographic and clinical characteristics at CANTAB T1 assessment in youth with bipolar disorder (N= 135).
| N (%) | M (SD) | |
|---|---|---|
| Age | 16.18 (3.28) | |
| Sex (male) | 79 (58.5) | |
| Race (white) | 110 (81.5) | |
| SES | 3.27 (1.31) | |
| IQ | 104.84 (15.05) | |
| BP subtype | ||
| BP-I | 77 (57.03) | |
| BP-II | 16 (11.85) | |
| BP-NOS | 42 (31.11) | |
| Age at onset of BP (years) | 8.3 (3.55) | |
| Duration of mood illness (years) | 7.88 (2.76) | |
| First degree family history of depression | 92 (68.8) | |
| First degree family history of (hypo)mania | 45 (33.4) | |
| Second degree family history of depression | 88 (65.7) | |
| Second degree family history of (hypo)mania | 45 (33.9) | |
| Comorbid psychiatric disorders | ||
| PTSD | 7 (5.18) | |
| OCD | 16 (11.85) | |
| ADHD | 84 (62.2) | |
| SUD | 9 (6.66) |
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; BP-NOS, bipolar disorder not otherwise specified; CANTAB, Cambridge Neuropsychological Test Automated Battery; IQ, Intelligence Quotient; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder; SES, socioeconomic status; SUD, substance use disorder
Instruments
Clinical Assessments
At intake psychiatric disorders were evaluated using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL)18.
History of physical and sexual abuse was evaluated at intake using the specific questions from the PTSD section of the KSADS-PL18 (separately to caregivers and children). Also, history of abuse was obtained using a medical history questionnaire.
Parents were also interviewed about their first and second degree psychiatric family history using the Family History Screen (FHS)19.
Socioeconomic status (SES) was ascertained using the 4-factor Hollingshead scale20.
The Petersen Pubertal Developmental Scale (PDS) and their equivalent Tanner stages were used to evaluate and categorize pubertal stages21.
Longitudinal changes in psychiatric symptoms were assessed using the Longitudinal Interval Follow-Up Evaluation (LIFE) and tracked on a week-by-week basis using this instrument’s Psychiatric Status Rating (PSR) scales22,23. These scales use numeric values that have been operationally linked to the DSM-IV criteria. For mood syndromes (depression, mania, hypomania, mixed) scores on the PSR scales range from 1–2 for no symptoms, to 3–4 for varying levels of subsyndromal symptoms and impairment, to 5–6 for meeting full criteria with different degrees of severity or impairment. Comorbid conditions were scored on the PSR scales using a 1 to 3 rating scale (with 3 signifying threshold criteria, 2 signifying subthreshold criteria, and 1 signifying minimal or no symptoms), or on a 1 to 6 point scale (with 5–6 signifying threshold criteria, 3–4 signifying subthreshold criteria, and 1–2 signifying minimal or no symptoms). Comorbid conditions included the presence of psychotic symptoms (hallucinations and/or delusions) and psychiatric comorbidities such as substance use disorders, attention deficit hyperactivity disorder (ADHD), disruptive disorders, any anxiety disorder (panic disorder, separation anxiety disorder, agoraphobia, specific phobia, social phobia, generalized anxiety disorder, anxiety disorder NOS), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD).
Exposure to medications during the entire follow-up period was ascertained using the Psychotropic Treatment Record of the LIFE. In addition, clinicians asked youth about their use of medications and substances 24 hours prior to each CANTAB testing. Youth who endorsed illicit substance use within 24 hours of the cognitive assessment were excluded from participation.
Longitudinal changes in global functioning were assessed through the Children’s Global Assessment Scale (C-GAS) or the Global Assessment of Functioning (GAF) for youth 22 years old or older24,25. In addition, we used the Psychosocial Functioning Interview (PSF) of the LIFE to assess academic and psychosocial relationships (with parents, siblings, partner, and friends). Data were encoded using a 1 to 5 scale (with 1 signifying high level of functioning, and 5 signifying severe impairment).
To test potential effects of mood status on cognitive performance, mood severity two days prior to the CANTAB was assessed using the Mania Rating Scale (MRS)26 and the Depression Rating Scale (DRS) section of the K-SADS-P27. Also, at the time that the CANTAB was administered, general intelligence (IQ) was evaluated using the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scales of Intelligence (WASI)28.
Cognitive Testing
The CANTAB contains a suite of the world’s most validated and sensitive computerized neuropsychological tests of cognition including: memory, executive function, attention, decision making, and social cognition. Since the COBY study did not include a HC group, we only included those cognitive tasks for which CANTAB provided a z-score based on normative data from the CANTAB. The following CANTAB subtests were used:
Intra/Extra-dimensional Set Shift (IDED)
This set-shifting task mirrors the Wisconsin Card Sorting Task (WCST). Stimuli are presented in pairs during 9 stages, each of which requires the participant to successfully complete 6 trials in a maximum of 50 attempts, or else the test is discontinued. Participants use feedback during trial-and error learning to determine which of two stimuli shapes is rewarded—e.g., purple square rather than purple circle. Stage 2 reverses the stimulus/reward association—e.g., purple circle rather than purple square. White line designs are added as distracters during stage 3–9. However, during stages 3–7, reinforcement depends only on shape, with line design being irrelevant. Stage 6 is known as the “intra-dimensional shift” because new line/shapes replace the old, but choice of the correct shape continues to determine reinforcement. Stage 8 is the “extra-dimensional” shift because it is the first stage when the previously irrelevant construct—e.g., white line design—is rewarded. Outcome data include stages completed, errors, and trials for each stage, for all trials before the ED shift (e.g., stages 1–7 “pre-ED shift”), and those at and after the ED shift. Outcome measures are related to executive functions such as abstract reasoning and categorization (e.g., completed stage trials), and cognitive flexibility/reversal learning (e.g., ED-shift errors).
Pattern Recognition Memory (PRM)
Participants first view 12 shapes one at a time, and then, pairs of shapes are presented, one novel and one previously presented. Participants must identify the previously presented, rather than novel, shape within the pair. Outcome data include number and percentage correct and mean latency to correct responses. It assesses visual pattern recognition memory.
Spatial Memory Span (SSP)
This test of working memory is modeled after the Corsi Block Test29. Participants watch squares on the screen change colors one at a time from white to a different color. Participants then touch the squares on the screen in the same sequence in which they changed colors. The number of blocks increases from 2 to 9 across trials. Outcome data include length of memory span, total errors (e.g., number of times the participant selected an incorrect box), and total usage errors (i.e., number of times the participant selected a box not in the sequence being recalled).
Rapid Visual Information Processing (RVP)
A white box appears in the center of the computer screen, inside of which digits from 2 to 9 appear in pseudo-random order at the rate of 100 digits per minute. Participants are asked to press a button whenever they detect a specified target sequence—e.g., “press the button when you see 2–4–6”). Outcome data include A′ [signal detection theory measure of sensitivity to errors, regardless of error tendency—e.g., how good the participant is at detecting target sequence (range 0.00–1.00)], B′ [signal detection measure of strength of trace required to elicit a response—i.e., tendency to respond regardless of whether target sequence is present or not (range −1.00 to 1.00)], and probability (e.g., change of making specific response) of hits, misses, false alarms, and rejections. This subtest is an analogue of the Continuous Performance Test (CPT) and measures sustained attention30.
Procedure
Each university’s Institutional Review Board approved the study, and assent and consent were obtained from youth and parents, respectively. All assessments were completed by research staff trained to reliably administer the above noted interviews, and presented to child psychiatrists/psychologists who confirmed the diagnoses and the longitudinal mood ratings16.
Statistics
First, raw scores on the cognitive outcome measures at each time point were compared with the CANTAB normative data to calculate z-scores. As referred to by other CANTAB reports31, outliers beyond ±3.0 z-scores were curtailed to values of +3.0 or −3.0. The number of outliers for any cognitive measurement did not exceed 6% at any time. Similar to Pavuluri and colleagues6, standardized domain scores were then calculated for executive functions, sustained attention, working memory, and visual memory by combining z-scores within each of the four CANTAB subtests. Internal consistencies (Chronbach α) of scores comprising each cognitive domain were always greater than 0.87. As reported by other studies7, 32–34 a general cognitive index was calculated by averaging the four cognitive domain scores for each subject per time point.
Second, using LCGA youth were clustered into several classes based on the trajectory of their general cognitive index from T1 to T3. Using the SAS Trajectory procedure (TRAJ)35, the number of classes was determined by selecting the model with a minimum value of Bayesian information criterion, a minimum of 20 participants per class, and clinical interpretability of the classes obtained. Scores in MRS/DRS and IQ were not included as covariates in the LCGA because preliminary analyses using linear mixed models did not indicate significant differences between time points in these variables (p> 0.05).
Third, univariate analyses using analyses of (co)variance and chi square tests were performed to compare the clinical variables between LCGA classes. Variables at T1 were: demographics, age at onset of BP, duration of mood illness, history of sexual/physical abuse, first/second degree family history of depression/(hypo)mania. Longitudinal variables were: BP subtype at the last assessment, exposure to each medication class during the follow-up (present or absent), psychiatric comorbidities at T1 and during the follow-up (present or absent), number/duration of hospitalizations at T1 and during the follow-up, percentage of follow-up time with sub/syndromal mood symptomatology in general and for each polarity specifically, and global/academic/social functioning during the follow-up (average scores). Medications were grouped by class (antipsychotics, antidepressants, lithium and anticonvulsants, benzodiazepines, stimulants, and atomoxetine). To avoid type I error, multiple-univariate analyses were adjusted using the false discovery rate (FDR) as described by the Benjamini–Hochberg procedure36,37. Post-hoc pairwise comparisons were adjusted using the Bonferroni correction. P-values were based on 2-tailed tests with α=0.05.
Results
Latent Class Growth Analysis
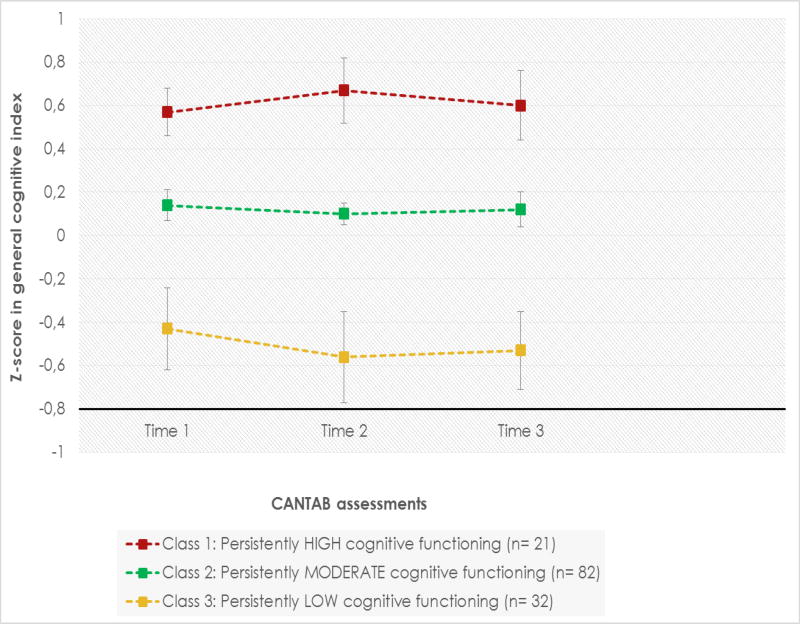
LCGA identified three longitudinal patterns of cognitive functioning based on the general cognitive index: class 1, “persistently high” (N=21; 15.6%); class 2, “persistently moderate” (N=82; 60.74%); and class 3, “persistently low” (N=32; 23.7%). Within these classes, z-scores on the general cognitive index across time points ranged from −0.45/-0.59, 0.10/0.17, and 0.57/0.69, respectively. As illustrated in Figure 1, youth in the three classes showed stable cognitive functioning across time points. There were no between-class differences in the duration of follow-up, number of cognitive assessments, and time elapsed between cognitive assessments (p> 0.05). Analyses of all CANTAB subtests (IDED, PRM, SSP, and RVP) using LCGA showed the same pattern as described for the general cognitive index (persistently high vs. persistently moderate vs. persistently low). A secondary analysis including the whole sample of BP youth (N= 135) also showed stable, normal cognitive functioning on the general cognitive index and each CANTAB subtest.
Figure 1.
Latent class growth analysis based on z-scores in the general cognitive index at three time points for youth with bipolar disorder.
CANTAB, Cambridge Neuropsychological Test Automated Battery
Clinical Characteristics
As shown in Table 2, at T1 there were no significant between-class differences in demographic variables, age at onset of BP, duration of mood illness, history of sexual/physical abuse and first/second degree family history of depression/(hypo)mania (p> 0.05).
Table 2.
Clinical characteristics in latent classes of youth with bipolar disorder.
| Variables | Class 1- Persistently high cognitive functioning |
Class 2- Persistently moderate cognitive functioning |
Class 3- Persistently low cognitive functioning |
df | Statistics | p values | Effect size (partialη2/V) |
|---|---|---|---|---|---|---|---|
| (N = 21) | (N = 82) | (N = 32) | |||||
|
|
|
|
|||||
| M (SD) / % | M (SD) / % | M (SD) / % | |||||
| Clinical data at T1 | |||||||
| Age | 16.95 (2.88) | 16.61 (1.95) | 15.78 (3.63) | 2, 132 | F = 1.53 | 0.49 | 0.05 |
| Sex (male) | 76.21 | 57.14 | 50 | 2 | X2 = 3.70 | 0.15 | 0.16 |
| Race (white) | 85.71 | 78.04 | 87.5 | 2 | X2 = 1.65 | 0.43 | 0.11 |
| SES | 3.47 (0.93) | 3.31 (1.05) | 2.99 (1.26) | 2, 132 | F = 2.71 | 0.09 | 0.03 |
| Age at onset of BP (years) | 8.29 (4.16) | 7.74 (3.32) | 7.19 (3.64) | 2, 132 | F = 3.02 | 0.19 | 0.08 |
| Duration of mood illness (years) | 8.11 (1.81) | 8.28 (2.64) | 8.55 (3.02) | 2, 132 | F = 0.15 | 0.84 | < 0.001 |
| First degree family history of depression | 65.9 | 67.33 | 70.09 | 2 | X2 = 2.64 | 0.24 | 0.13 |
| First degree family history of (hypo)mania | 31.13 | 33.01 | 33.77 | 2 | X2 = 1.59 | 0.41 | 0.11 |
| First degree family history of depression | 64.8 | 64.71 | 66.03 | 2 | X2 = 1.54 | 0.49 | 0.1 |
| First degree family history of (hypo)mania | 33.76 | 35.21 | 34.65 | 2 | X2 = 1.12 | 0.63 | 0.11 |
| History of sexual abuse | 10.9 | 11.7 | 11.5 | 2 | X2 = 1.55 | 0.47 | 0.1 |
| History of physical abuse | 13.12 | 13.9 | 15.25 | 2 | X2 = 1.49 | 0.53 | 0.12 |
| Longitudinal clinical data | |||||||
| Number of hospitalizations | 3.36 (1.54) | 3.43 (1.41) | 3.79 (1.55) | 2, 132 | F = 3.15 | 0.14 | 0.05 |
| Duration of hospitalizations (weeks) | 3.88 (5.19) | 3.82 (6.05) | 3.99 (5.24) | 2, 132 | F = 1.95 | 0.46 | 0.02 |
| BP subtype | |||||||
| BP-I | 61.90 | 57.31 | 71.87 | 4 | X2 = 5.16 | 0.27 | 0.13 |
| BP-II | 23.80 | 24.39 | 6.25 | ||||
| BP-NOS | 14.30 | 18.30 | 21.88 | ||||
| Psychiatric comorbid disorders | |||||||
| ADHD | 52.383 | 65.853 | 78.121,2 | 2 | X2 = 7.01 | 0.01a | 0.24 |
| Disruptive disorders | 47.61 | 54.87 | 53.12 | 2 | X2 = 0.33 | 0.75 | 0.05 |
| OCD | 19.04 | 13.41 | 12.50 | 2 | X2 = 0.85 | 0.56 | 0.06 |
| PTSD | 9.52 | 10.97 | 12.50 | 2 | X2 = 0.99 | 0.58 | 0.07 |
| Any anxiety disorder | 52.38 | 59.75 | 71.87 | 2 | X2 = 4.61 | 0.09 | 0.20 |
| SUD | 14.28 | 7.31 | 12.50 | 2 | X2 = 1.39 | 0.14 | 0.12 |
| Psychotic symptoms | 14.28 | 10.97 | 15.62 | 2 | X2 = 1.63 | 0.26 | 0.13 |
| Medication exposure | |||||||
| Antipsychotics | 75 | 63.41 | 65.62 | 2 | X2 = 3.38 | 0.18 | 0.15 |
| Antidepressants | 33.33 | 19.51 | 28.12 | 2 | X2 = 2.21 | 0.33 | 0.12 |
| Atomoxetine | 9.52 | 8.53 | 12.50 | 2 | X2 = 0.41 | 0.81 | 0.05 |
| Stimulants | 40 | 34.14 | 50 | 2 | X2 = 3.24 | 0.19 | 0.15 |
| Lithium, anticonvulsants | 66.66 | 50 | 46.87 | 2 | X2 = 1.86 | 0.39 | 0.11 |
| Benzodiazepines | 0 | 7.31 | 12.50 | 2 | X2 = 2.19 | 0.23 | 0.14 |
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; BP-NOS, bipolar disorder not otherwise specified; IQ, intelligence quotient, OCD, obsessive-compulsive, disorder; PTSD, posttraumatic stress disorder; SES, socioeconomic status; SUD, substance use disorder; T1/3, time point 1/3
Significant p value after controlling for multiple-univariate analyses (Benjamini–Hochberg FDR)
Significant difference with class 1 after post-hoc Bonferroni correction
Significant difference with class 2 after post-hoc Bonferroni correction
Significant difference with class 3 after post-hoc Bonferroni correction
Regarding the longitudinal data, youth in class 3 had significantly greater prevalence of ADHD than those in class 1 and 2. These differences remained significant after controlling for multiple-univariate analyses. There were no between-class differences in the exposure to any type of psychopharmacological treatment, BP subtype, and number/duration of hospitalizations (p> 0.05).
Percentage of Time with Syndromal and Subsyndromal Mood Symptoms
During the follow-up, youth with persistently low cognitive functioning (class 3) had significantly greater percentage of time with syndromal mood symptoms than youth in class 1 and 2 (see Table 3). Specifically, youth in class 3 had significantly greater percentage of time with manic and depressive syndromal symptoms than those in class 1 and 2. Between-class differences in overall, manic, and depressive syndromal symptoms remained significant after controlling for ADHD comorbidity (p> 0.05). These differences remained significant after controlling for multiple-univariate analyses.
Table 3.
Longitudinal data on the percentage of time with mood symptoms in latent classes of youth with bipolar disorder.
| Variables | Class 1- Persistently high cognitive functioning |
Class 2- Persistently moderate cognitive functioning |
Class 3- Persistently low cognitive functioning |
df | Statistics | p values | Effect size (partialη2) |
|---|---|---|---|---|---|---|---|
| (N = 21) | (N = 82) | (N = 32) | |||||
|
|
|
||||||
| M (SD) | M (SD) | M (SD) | |||||
| Percentage of time euthymic | 51.17 (39.04) | 43.26 (33.21) | 32.95 (36.75) | 2, 132 | F= 1.84 | 0.16 | 0.03 |
| Percentage of time in any syndromal mood | 5.903(10.63) | 10.513(16.91) | 24.461,2(29.98) | 2, 132 | F = 7.13 | <0.001a | 0.10 |
| Mania | 0.073(0.32) | 0.483(2.02) | 4.401,2(13.41) | 2, 132 | F = 4.40 | 0.01a | 0.06 |
| Hypomania | 0.0 (0.0) | 2.79 (8.27) | 4.80 (12.82) | 2, 132 | F = 1.81 | 0.16 | 0.02 |
| Depression | 4.803(10.02) | 6.413(13.01) | 15.021,2(25.47) | 2, 132 | F = 3.67 | 0.01a | 0.06 |
| Mixed states | 1.02 (4.20) | 0.78 (3.96) | 0.22 (0.93) | 2, 132 | F = 0.39 | 0.67 | 0.01 |
| Percentage of time in any subsyndromal mood | 42.93 (37.41) | 46.23 (32.35) | 42.59 (35.07) | 2, 132 | F = 0.19 | 0.82 | 0.01 |
Significant p value after controlling for multiple-univariate analyses (Benjamini–Hochberg FDR)
Significant difference with class 1 after post-hoc Bonferroni correction
Significant difference with class 2 after post-hoc Bonferroni correction
Significant difference with class 3 after post-hoc Bonferroni correction
Global, Academic, and Social Functioning
As noted in Table 4, youth with persistently low cognitive functioning (class 3) had significantly poorer global, academic, and social functioning than youth in class 1 during the follow-up period. Specifically, they showed more impaired relationships with friends than those in class 1. Between-class differences in these variables remained significant after controlling for ADHD, and the percentage of time with overall, manic, and depressive syndromal mood symptoms (p> 0.05). These differences remained significant after controlling for multiple-univariate analyses.
Table 4.
Longitudinal data on global, academic, and social functioning in latent classes of youth with bipolar disorder.
| Variables | Class 1- Persistently high cognitive functioning |
Class 2- Persistently moderate cognitive functioning |
Class 3- Persistently low cognitive functioning |
df | Statistics | p values | Effect size (partialη2) |
|---|---|---|---|---|---|---|---|
| (N = 21) | (N = 82) | (N = 32) | |||||
|
|
|
|
|||||
| M (SD) | M (SD) | M (SD) | |||||
| Global functioning (CGAS) | 60.903(9.45) | 59.60 (13.19) | 511(8.02) | 2, 132 | F = 4.30 | 0.01a | 0.07 |
| Academic functioning (PSF) | 2.633(1.55) | 3.08 (1.35) | 3.491(1.43) | 2, 132 | F = 4.57 | 0.01a | 0.06 |
| Global social adjustment (PSF) | 33(0.70) | 3.11 (0.79) | 3.471(0.71) | 2, 132 | F = 4.41 | 0.01a | 0.06 |
| Relationship with biological parents (PSF) | 3.05 (1.35) | 2.89 (1.45) | 3.38 (1.49) | 2, 132 | F = 1.23 | 0.29 | 0.01 |
| Relationship with step-parents (PSF) | 3 (1.41) | 3.10 (1.33) | 3.50 (1.22) | 2, 132 | F = 0.23 | 0.79 | 0.01 |
| Relationship with siblings (PSF) | 3.11 (1.31) | 3.21 (1.17) | 3.48 (1.18) | 2, 132 | F = 0.75 | 0.47 | 0.01 |
| Relationship with partner (PSF) | 1.80 (0.83) | 2.25 (1.00) | 2.78 (1.09) | 2, 132 | F= 1.63 | 0.21 | 0.10 |
| Relationship with friends (PSF) | 2.273(1.35) | 2.87 (1.11) | 3.031(1.17) | 2, 132 | F= 4.76 | 0.01a | 0.07 |
CGAS, Children-Global Assessment Scale; PSF, Psychosocial Functioning Scale from the Longitudinal Interval Follow-Up Evaluation (LIFE)
Significant p value after controlling for multiple-univariate analyses (Benjamini–Hochberg FDR)
Significant difference with class 1 after post-hoc Bonferroni correction
Significant difference with class 2 after post-hoc Bonferroni correction
Significant difference with class 3 after post-hoc Bonferroni correction
Discussion
To the best of our knowledge, this is the first study aimed at delineating distinctive cognitive trajectories in BP youth and their associated clinical variables. Our main findings were: 1) Three overall cognitive trajectories were identified in BP youth: persistently high, moderate, and low cognitive functioning; 2) In comparison with the CANTAB normative data, all classes had z-scores which are considered above the cutoff levels (z < −1.00/−1.5) for cognitive impairment38. However, BP youth in class 3 had relatively low general cognitive functioning; and 3) After controlling for multiple comparisons and confounders, youth with persistently low cognitive functioning had greater prevalence of ADHD comorbidity and greater percentage of time with total, manic, and depressive syndromal symptoms than youth in the other two classes. Also, they showed poorer global, academic, and social functioning than youth with persistently high cognitive functioning.
We found that the three classes showed stable course of cognitive functioning over time. This finding is in accordance with longitudinal studies including large samples of euthymic and non-euthymic adults with BP3,39 and two longitudinal cognitive studies in BP youth7,8. In contrast, another longitudinal study among BP youth showed improvement in working memory over 2.5 years9. However, this latter study included a small sample (N=20) and, with the exception for the effects of lithium at intake, it did not control for the effects of pharmacological treatment during the follow-up. Overall, our and the above studies suggest that BP youth do not experience a cognitive worsening over time, and preliminarily, do not support the “hypothesis of neuroprogression”39. Notwithstanding, further longitudinal studies with larger samples and longer duration of follow-up are warranted to ensure the lack of cognitive decline in BP.
The current study also showed that the three classes had normal cognitive functioning on the general cognitive index and on each of the specific cognitive domains. These results are in contrast to cross-sectional and longitudinal studies in BP youth. These studies showed cognitive impairments in verbal/visual-spatial memory, processing speed, working memory, or social cognition, even as ascertained by the CANTAB1. Specifically, one longitudinal study in BP youth, which also used similar average cognitive scores as the one used in this study, found stable impairments in all cognitive domains over time7. It is possible that divergences between the results of these studies and the current research may be explained by the fact that we used a normal group derived from the CANTAB normative data rather than a HC group. Also, prior longitudinal studies in BP youth included small sample sizes and they did not adjust for the presence of confounders (e.g., treatment other than stimulants, comorbid disorders other than ADHD)7–9.
Our study also showed that the presence of ADHD comorbidity was associated with poorer overall cognitive functioning over time. This result is in contrast with those findings from other longitudinal8,9 and cross-sectional studies in BP youth1, which reported no effects of ADHD comorbidity on those cognitive domains included in our general cognitive index (e.g., sustained attention, working memory, executive functions). Differences with prior studies may be accounted for by the fact that we used a large sample size of youth fully covering the bipolar spectrum disorders.
We also found that poorer overall cognitive functioning was associated with poorer global, social, and academic adjustment over time. Our finding is similar to other cross-sectional studies in BP youth4,40, but in contrast with one longitudinal study which showed that poorer executive functions were not associated with math difficulties in BP-I youth6. However, unlike our study, this longitudinal research measured math difficulties as a categorical variable (yes/no) reported by parents. Other longitudinal studies among adults with BP are in line with the association between cognition (mainly executive functions) and functioning. Tabares-Seisdedos and colleagues found that a worsening of executive functions in 43 BP-I adults predicted poorer global functioning over one-year follow-up41. Similarly, Bonnin and colleagues showed that a decrease in executive functions among 32 adults with BP-I and BP-II predicted greater functional maladjustment over four-year follow-up42. The central role of executive functions on functional outcomes is supported by the fact that these cognitive domains refer to the capacity to think or change an idea before acting, increasing the ability to deal with new learning, situations, and people. Because of this potential link between cognition and functioning, the use of cognitive remediation therapy may be indicated for BP youth6.
Consistent with another longitudinal study44, we found an association between poorer cognitive functioning and poorer mood course. Also, cross-sectional studies reported that worse cognitive functioning among BP patients with greater number of mood episodes, particularly manic44,45 had worse cognitive functioning. The pernicious relationship between cognitive function and BP illness may be explained by the fact that both clinical variables may imply similar brain regions, particularly in the prefrontal and anterior cingulate cortex and the subgenual region46. Hence, improving cognitive impairments could be an indirect strategy to ameliorate the mood course among BP youth.
Limitations
The results of this study need to be taken in the context of the following limitations. First, although we evaluated several key cognitive domains (e.g., sustained attention, and executive functions), other cognitive domains such as social cognition and verbal memory were not assessed47. Second, the generalizability of the observations to other populations remains uncertain because most participants were Caucasian, and they were recruited primarily from outpatient settings48. Nevertheless, course and morbidity in non-clinically referred adolescents with BP have shown similar results to those found in referred populations49 Finally, the average follow-up between CANTAB administrations was only 2.5 years and 53/135 participants at T2 have not yet complete the CANTAB assessment at T3.
Conclusions
BP youth showed normal overall cognitive functioning, which in each class remained stable during the follow-up. However, about 24% of BP youth showed relatively poorer cognitive functioning that the other BP youth. This subgroup of BP youth had poorer mood course and functioning, and may benefit from cognitive remediation and early management with evidence-based pharmacological treatments.
Acknowledgments
This research was supported by the National Institute of Mental Health grants MH059929 (B.B.), MH059691 (M.B.K./S.Y.), and MH059977 (M.S.). The authors thank the study participants and families for their participation, the COBY research staff and the National Institutes of Mental Health for their support.
Dr. Frías received grants for Short-term Specialization and Research in Psychiatry from the Alicia Koplowitz Foundation (2015). Dr. Strober received support from the Resnick Endowed Chair in Eating Disorders. Dr. B. Goldstein received grant or research support from Brain Canada, the Canadian Institutes of Health Research, the Brain and Behavior Research Foundation (NARSAD), the National Institute of Mental Health, the Ontario Mental Health Foundation, and the Ontario Ministry of Research and Innovation. Dr. Yen receives grant funding from National Institute of Mental Health and the American Foundation for Suicide Prevention, and is a consultant at Janssen Research and Development, LLC. Dr. T. Goldstein receives grant support from NIMH, AFSP, and The Brain and Behavior Foundation, and royalties from Guilford Press. Dr. Ryan received grant or research support from the National Institute of Mental Health. He served on the Scientific Advisory Board of the Child Mind Institute. Dr. Hunt serves as the Senior Editor of the Brown Psychopharm Newsletter published by Wiley Publishers. Dr. Dickstein received grant or research support from the National Institute of Mental Health and an independent investigator grant from the National Alliance for Research on Schizophrenia and Depression: the Brain and Behavior Research Foundation. Dr. Birmaher receives grant support from NIMH and royalties for publications from Random House, Inc., UpToDate, and Lippincott Williams and Wilkins.
Footnotes
Disclosure of Interests
All other authors declare that they have no conflicts of interest.
References
- 1.Frías Á, Palma C, Farriols N. Neurocognitive impairments among youth with pediatric bipolar disorder: a systematic review of neuropsychological research. J Affect Disord. 2014;166:297–306. doi: 10.1016/j.jad.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Haarman BC, Riemersma-Van der Lek RF, Burger H, Drexhage HA, Nolen WA. The dysregulated brain: consequences of spatial and temporal brain complexity for bipolar disorder pathophysiology and diagnosis. Bipolar Disord. 2016;18:696–701. doi: 10.1111/bdi.12454. [DOI] [PubMed] [Google Scholar]
- 3.Samamé C, Martino DJ. Strejilevich SA. Longitudinal course of cognitive deficits in bipolar disorder: a meta-analytic study. J Affect Disord. 2014;164:130–138. doi: 10.1016/j.jad.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Pavuluri MN, O’Connor MM, Harral EM, Moss M, Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: A clinical translation. Biol Psychiatry. 2006;60:951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Schenkel LS, Chamberlain TF, Towne TL. Impaired Theory of Mind and psychosocial functioning among pediatric patients with Type I versus Type II bipolar disorder. Psychiatry Res. 2014;215:740–746. doi: 10.1016/j.psychres.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Dickstein DP, Cushman GK, Kim KL, Weissman AB, Wegbreit E. Cognitive remediation: potential novel brain-based treatment for bipolar disorder in children and adolescents. CNS Spectr. 2015;20:382–390. doi: 10.1017/S109285291500036X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavuluri MN, West A, Hill SK, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youth. J Am Acad Child Adolesc Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitney J, Joormann J, Gotlib IH et al. Information processing in adolescents with bipolar I disorder. J Child Psychol Psychiatry. 2012;53:937–945. doi: 10.1111/j.1469-7610.2012.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lera-Miguel S, Andrés-Perpiñá S, Fatjó-Vilas M, Fañanás L, Lázaro L. Two-year follow-up of treated adolescents with early-onset bipolar disorder: Changes in neurocognition. J Affect Disord. 2014;172:48–54. doi: 10.1016/j.jad.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dement Geriatr Cogn Disord. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein DP, Axelson D, Weissman AB et al. Cognitive flexibility and performance in children and adolescents with threshold and sub-threshold bipolar disorder. Eur Child Adolesc Psychiatry. 2016;25:625–638. doi: 10.1007/s00787-015-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett JH, Croudace TJ, Jaycock S et al. Improvement and decline of cognitive function in schizophrenia over one year: a longitudinal investigation using latent growth modelling. BMC Psychiatry. 2007;7:16. doi: 10.1186/1471-244X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogner HR, Richie MB, de Vries HF, Morales KH. Depression cognition apolipoprotein e genotype: latent class approach to identifying subtype. Am J Geriatr Psychiatry. 2009;17:344–352. doi: 10.1097/JGP.0b013e3181987730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libon DJ, Drabick DA, Giovannetti T, et al. Neuropsychological syndromes associated with Alzheimer’s/vascular dementia: a latent class analysis. J Alzheimers Dis. 2014;42:999–1014. doi: 10.3233/JAD-132147. [DOI] [PubMed] [Google Scholar]
- 15.Axelson D, Birmaher B, Strober, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 16.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 20.Hollingshead A. Four-factor Index of Social Status. New Haven: Yale University; 1975. [Google Scholar]
- 21.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability validity and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 22.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 23.Warshaw MG, Dyck I, Allsworth J, Stout RL, Keller MB. Maintaining reliability in a long-term psychiatric study: An ongoing inter-rater reliability monitoring program using the longitudinal follow-up evaluation. J Psychiatr Res. 2001;35:297–305. doi: 10.1016/s0022-3956(01)00030-9. [DOI] [PubMed] [Google Scholar]
- 24.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 26.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 27.Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 29.Kessels RPC, van Zandvoort MJE, Postma A, Kappelle LJ, de Haan EHF. The Corsi Block-Tapping task: Standardization and normative data. Appl Neuropsychol. 2000;7:252–258. doi: 10.1207/S15324826AN0704_8. [DOI] [PubMed] [Google Scholar]
- 30.Conners CK MHS Staff. Conners’ Continuous Performance test II: Computer program for Windows technical guide and software manual. North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- 31.Van Zwieten A, Meyer J, Hermens DF, et al. Social cognition deficits and psychopathic traits in young people seeking mental health treatment. PLoS One. 2013 doi: 10.1371/journal.pone.0067753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennertz L, An der Heiden W, Kronacher R, et al. Smaller than expected cognitive deficits in schizophrenia patients from the population-representative ABC catchment cohort. Eur Arch Psychiatry Clin Neurosci. 2016;266:423–431. doi: 10.1007/s00406-015-0625-x. [DOI] [PubMed] [Google Scholar]
- 33.Fervaha G, Zakzanis KK, Foussias G, et al. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014;71:1058–1065. doi: 10.1001/jamapsychiatry.2014.1105. [DOI] [PubMed] [Google Scholar]
- 34.Depp CA, Savla GN, de Dios LA, Mausbach BT, Palmer BW. Affective symptoms and intra-individual variability in the short-term course of cognitive functioning in bipolar disorder. Psychol Med. 2012;42:1409–1416. doi: 10.1017/S0033291711002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2:302–317. [Google Scholar]
- 36.Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 31.Pais Rs, Moreno-Barriuso N, Hernández-Porras I, López Ip, De Las Rivas J, Pichel JG. Transcriptome analysis in prenatal IGF1-deficient mice identifies molecular pathways and target genes involved in distal lung differentiation. PLoS One. 2013 doi: 10.1371/journal.pone.0083028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liepelt-Scarfone I, Graeber S, Feseker A, et al. Influence of different cut-off values on the diagnosis of mild cognitive impairment in Parkinson’s disease. Parkinsons Disease. 2011 doi: 10.4061/2011/540843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strejilevich SA, Samamé C, Martino DJ. The trajectory of neuropsychological dysfunctions in bipolar disorders: a critical examination of a hypothesis. J Affect Disord. 2015;175:396–402. doi: 10.1016/j.jad.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Biederman J, Petty CR, Wozniak J, et al. Impact of executive function deficits in youth with bipolar I disorder: a controlled study. Psychiatry Res. 2011;186:58–64. doi: 10.1016/j.psychres.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabares-Seisdedos R, Balanzá-Martínez V, Sánchez-Moreno J, et al. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J Affect Disord. 2008;109:286–299. doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]
- 42.Bonnín CM, Martínez-Arán A, Torrent C, et al. Clinical neurocognitive predictors of functional outcome in bipolar euthymic patients: a long-term follow-up study. J Affect Disord. 2010;121:156–160. doi: 10.1016/j.jad.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Kozicky JM, Torres IJ, Silveira LE, Bond DJ, Lam RW, Yatham LN. Cognitive change in the year after a first manic episode: association between clinical outcome and cognitive performance early in the course of bipolar I disorder. J Clinic Psychiatry. 2014;75:e587–593. doi: 10.4088/JCP.13m08928. [DOI] [PubMed] [Google Scholar]
- 44.Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134:91–103. doi: 10.1111/acps.12581. [DOI] [PubMed] [Google Scholar]
- 45.Cardoso T, Bauer IE, Meyer TD, Kapczinski F, Soares JC. Neuroprogression and cognitive functioning in bipolar disorder: A systematic review. Curr Psychiatry Rep. 2015;17:75. doi: 10.1007/s11920-015-0605-x. [DOI] [PubMed] [Google Scholar]
- 46.Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: review of the evidence. Neurosci Biobehav Rev. 2013;37:418–435. doi: 10.1016/j.neubiorev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Wegbreit E, Weissman AB, Cushman GK, et al. Facial emotion recognition in childhood-onset bipolar I disorder: an evaluation of developmental differences between youths and adults. Bipolar Disord. 2015;17:471–485. doi: 10.1111/bdi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Meter AR, Burke C, Kowatch RA, Findling RL, Youngstrom EA. Ten-year updated meta-analysis of the clinical characteristics of pediatric mania and hypomania. Bipolar Disord. 2016;18:19–32. doi: 10.1111/bdi.12358. [DOI] [PubMed] [Google Scholar]
- 49.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorder during adolescence and young adulthood in a community sample. Bipolar Disord. 2000;2:281–293. doi: 10.1034/j.1399-5618.2000.20309.x. [DOI] [PubMed] [Google Scholar]