Abstract
Background
Several new prostate cancer treatments emerged since 2000, including two radiotherapies with similar efficacy at the time of their introduction: intensity-modulated radiotherapy (IMRT) and stereotactic body radiation therapy (SBRT). We sought to compare their early adoption patterns and identify factors associated their use.
Methods
Using Surveillance, Epidemiology, and End Results (SEER)-Medicare, we identified prostate cancer patients treated with radiation during the five years after IMRT introduction (2001–2005) and five years after SBRT introduction (2007–2011). Our outcome was the use of new radiation therapy (i.e., IMRT or SBRT) compared with the existing standard radiation therapies at that time. We fit a series of multivariable hierarchical logistic regression models accounting for patients nested within health service areas to examine factors associated with new radiation therapy use.
Results
In 2001–2005, 5680 men (21%) received IMRT compared with standard radiation (n=21,555). Men receiving IMRT were older, had higher grade tumors, and lived in more populated areas (p<0.05). In 2007–2011, 595 men (2%) received SBRT compared with standard radiation (n=28,255). Men receiving SBRT were more likely to be white, had lower grade tumors, lived in more populated areas, and were more likely to live in the Northeast (p<0.05). Adjusting for cohort demographic and clinical factors, the early adoption rate for IMRT was substantially higher than for SBRT (44% versus 4%, p<0.01).
Conclusions
There is a stark contrast in the adoption rates of IMRT and SBRT at the time of their introduction. Further investigation of the nonclinical factors associated with this difference is warranted.
Keywords: prostate cancer, intensity-modulated radiotherapy, stereotactic body radiation treatment, technology, adoption
Condensed abstract
There is a stark contrast in the adoption rates of intensity-modulated radiotherapy (IMRT) and stereotactic body radiation treatment (SBRT) for prostate cancer at the time of their introduction. Further investigation of the nonclinical factors associated with this difference is warranted.
INTRODUCTION
Many prostate cancer treatments have emerged over the past several years, including two radiation therapy options: intensity-modulated radiotherapy (IMRT) and stereotactic body radiation therapy (SBRT). When IMRT entered the market in 2001, it offered conceptual advances over its predecessor, 3-dimensional conformal therapy, in terms of increased precision but had yet to accrue long-term data regarding its comparative cancer control, complications, or mortality.1–3 Similarly, when SBRT entered the market in 2007, it offered conceptual advances over IMRT in terms of a shorter duration of treatment and had also not yet accrued long-term data regarding its comparative effectiveness.4, 5 Despite these similarities, early data suggest that these treatments were adopted at different rates.6, 7
Yet our understanding of the context surrounding the differential adoption of these two treatments remains limited. For instance, it is unknown how much the initial trajectories of adoption differed for these two treatments. Further, it is unclear if the factors that influenced the adoption of IMRT also influenced the adoption of SBRT. For example, did the patients who received early IMRT have similar sociodemographics and tumor characteristics as those who received early SBRT? Were the regions that adopted IMRT shortly after its introduction the same as the regions that adopted SBRT? Were the physicians who were considered “early adopters” the same for each treatment? Understanding the clinical and nonclinical factors that influence the early adoption of these treatments is an important first step in ultimately identifying targets to drive physicians towards a particular technology.
To address these knowledge gaps, we conducted a retrospective cohort study to examine the trends in the early adoption of IMRT and SBRT and to investigate factors associated with their early use.
METHODS
Data Source and Study Population
We used Surveillance, Epidemiology, and End Results (SEER)-Medicare data to identify men aged 66 years or older diagnosed with prostate cancer between 2001 and 2011. SEER is a nationally representative population-based registry that comprises approximately 26% of the United States’ population and can be linked to Medicare claims.8
Using the Medicare Provider Analysis and Review (MEDPAR), outpatient, and carrier files, we further identified men primarily treated with radiation (i.e., SBRT, IMRT, external beam radiation, and brachytherapy) within the first 12 months of diagnosis, according to prior methods.9 We included only fee-for-service beneficiaries eligible for both Medicare Parts A and B from 12 months prior until 12 months after diagnosis. We excluded men who were 65 years old to ensure accurate comorbidity estimation using Medicare claims for the 12-month period prior to diagnosis.10 We included men who had prostate cancer as their first and only cancer and excluded those with metastatic disease.
Defining periods of early adoption
Since we were specifically interested in the early adoption patterns of IMRT and SBRT, we limited analyses to the five years after each treatment’s introduction. The early IMRT adoption period was 2001–2005 and the early SBRT adoption period was 2007–2011. We started the early IMRT adoption period in 2001 and the early SBRT adoption period in 2007 due to the introduction of their respective Healthcare Common Procedure Coding System (HCPCS) codes during those years.11, 12 We investigated starting the adoption periods in 2000 and 2006, but very few IMRT patients were identified in 2000 and, similarly, very few SBRT patients were identified in 2006.
Outcome
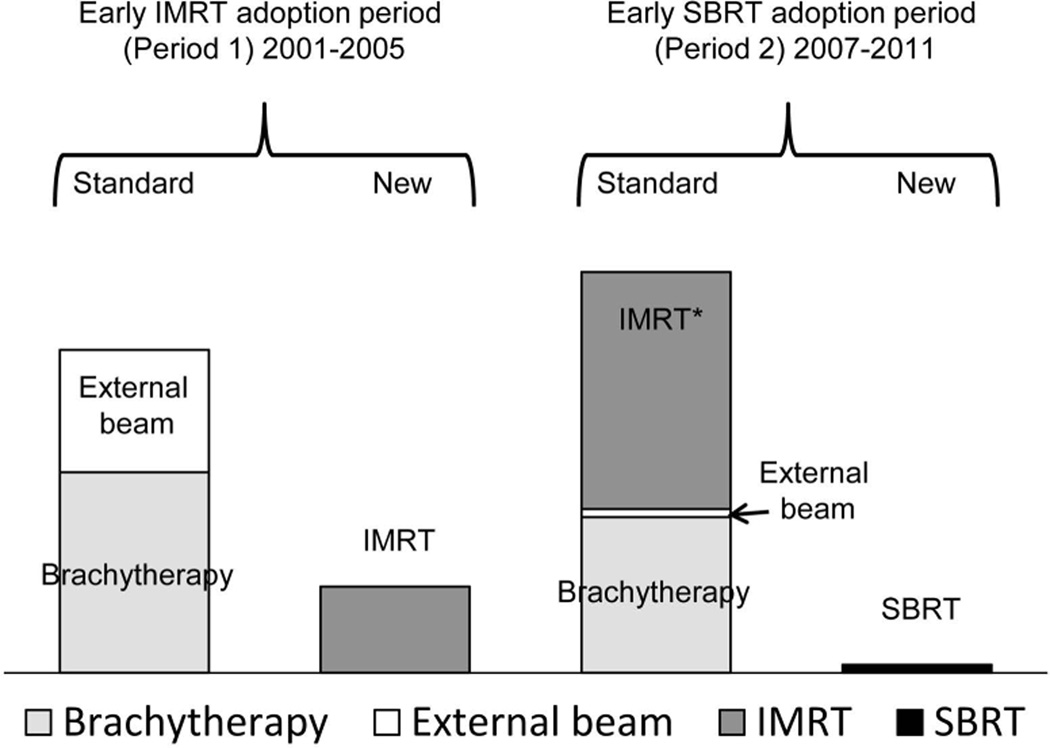
The outcome variable was the annual adoption probability of the new radiation therapy compared with the existing standard radiation. We defined existing standard radiation based on the predominant treatments at the start of the time period, which included external beam radiation and brachytherapy from 2001–2005 and external beam radiation, brachytherapy, and IMRT from 2007–2011 (Figure 1). We included IMRT as a standard radiation treatment during the early SBRT adoption period since it was the most common radiation treatment used for prostate cancer at that time.6 IMRT precisely delivers high doses of radiation over approximately 40 treatment sessions and is generally thought to be appropriate for both patients with low- and high-risk disease.2 SBRT also delivers high doses of radiation, but condensed into 5 treatment sessions (i.e., hypofractionation) and is thought to be most suitable for patients with lower risk disease.4
Figure 1. Explanation of the radiation treatments during the early IMRT adoption period (period 1) and the early SBRT adoption period (period 2).
IMRT is the new treatment in period 1; SBRT is the new treatment in period 2. The total proportion of treatments during each treatment period is 100%.
*During the early SBRT adoption period, IMRT was considered a standard radiation treatment since it was the most common radiation treatment used for prostate cancer at that time.
Abbreviations: IMRT; intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment
Statistical Analyses
Patient/Regional factors
We first compared demographic and clinical characteristics of patients treated with new radiation treatments and standard radiation treatments using chi-square tests for each period. We then fit multivariable hierarchical logistic regression models to examine factors associated with new radiation therapy use in both the early IMRT adoption period (period 1) and the early SBRT adoption period (period 2).13 We used the National Center for Health Statistics’ Health Service Areas as a random effect to account for the clustering of patients within markets.14 We considered using the physician as the clustering variable. However due to concerns for nonrandom missing data (potentially due to the transition from Unique Physician Identification Numbers to National Provider Identifiers in 2007), we chose the Health Service Area instead. In addition, physicians are clustered within markets or practices and there is reason to think that behavior is driven by practice group as much as individual physician preferences.15 Patient-level covariates considered to be factors associated with treatment type included age, race/ethnicity, marital status, comorbidity, tumor grade, year after treatment introduction, population, education, median income, and geographic region. Information about population, education, and median income were obtained from the 2010 United States’ Census. Variables in the final model were selected based on univariable analysis results (p<0.10) and/or based on clinical judgment. Race and ethnicity were self-reported by the patient and were examined because they can influence cancer treatment.16 Comorbidity was measured using the Klabunde modification of the Charlson index.12 Tumor grade was used instead of D’Amico’s disease risk classification because variables such as Gleason score were not available in SEER-Medicare prior to 2004.17 We also did not incorporate PSA values due to issues with misclassification in SEER.18 After fitting the model, we calculated the adjusted annual probability of IMRT and SBRT use during their respective early adoption years. We then fit another multivariable hierarchical logistic regression model adjusting for the same covariates as in our main model to examine how IMRT or SBRT use in a Health Service Area the year prior to treatment influenced the odds of a patient in that Health Service Area undergoing IMRT or SBRT in the subsequent year.
Physician factors
Next, we identified the treating physician and evaluated for an association between a physician’s treatment pattern during the early IMRT and the early SBRT adoption periods. To do this, we first identified the treating physician (i.e., the radiation oncologist) as we have done previously.19 Briefly, we identified the Unique Physician Identification Number and/or the National Provider Identifier in the Medicare claims. We then determined the treating physician by identifying the radiation oncologist who performed the clinical planning and simulation for the treatment.20 To examine how the pattern of new radiation therapy use among physicians differed across periods 1 and 2, we excluded those who did not treat patients during both time periods. We used a Pearson's chi-squared test with Yates' continuity correction to test whether or not the proportion of SBRT adopters in period 2 was higher among the physicians who used IMRT compared with standard radiation in period 1. Lastly, we examined the distribution of treating physicians among Health Service Areas.
Additional analyses
We performed two sensitivity analyses to assess the robustness of our results. First, since brachytherapy involves seed implantation and may be viewed differently from the other radiation treatments that all involve external beams, we repeated the analyses after removing brachytherapy from the standard treatment group. Second, since IMRT became the predominant radiation therapy within four years of its introduction,6 we performed a sensitivity analysis looking at a three-year period of early adoption to examine whether later adopters of IMRT could have biased the results. In both instances, our findings were qualitatively unchanged, so we only present the results from our primary analyses.
We performed all data management and analyses in SAS v9.4 (SAS Institute, Cary, NC) and R v13.2 (R Foundation for Statistical Computing, Vienna, Austria), respectively, using the compareGroups for descriptive tables, ggplot2 for graphics, and lme4 for fitting hierarchical logistic models.21, 22 All tests were two-sided, and the probability of a type I error was set at 0.05. The University of Pittsburgh institutional review board exempted this study from full board review.
RESULTS
Patient/Regional factors
The demographics and clinical characteristics of the study population are summarized in Table 1. There were 27,235 patients identified during the early IMRT adoption period (2001–2005), of whom 21,555 (79%) received standard radiation and 5680 (21%) received IMRT. During the early SBRT adoption period (2007–2011), there were 28,850 patients of whom 28,255 (98%) received standard radiation and 595 (2%) received SBRT.
Table 1.
Demographics and clinical characteristics of the study population
| Characteristics | Early IMRT adoption period (Period 1) |
Early SBRT adoption period (Period 2) |
||||
|---|---|---|---|---|---|---|
| Standard (n=21,555) |
IMRT (n=5680) |
P Value* | Standard (n=28,255) |
SBRT (n=595) |
P Value* | |
| Patient factors | ||||||
| Age, years (%) | <0.001 | 0.045 | ||||
| 66–69 | 4931 (23) | 990 (17) | 6290 (22) | 148 (25) | ||
| 70–74 | 8109 (38) | 1969 (35) | 10,472 (37) | 239 (40) | ||
| 75–79 | 6274 (29) | 1884 (33) | 7904 (28) | 141 (24) | ||
| 80+ | 2241 (10) | 837 (15) | 3589 (13) | 67 (11) | ||
| Race/ethnicity (%) | <0.001 | <0.001 | ||||
| White | 18,228 (85) | 4742 (84) | 23,061 (82) | 528 (89) | ||
| Black | 2177 (10) | 519 (9) | 3140 (11) | 43 (7) | ||
| Other | 1150 (5) | 419 (7) | 2054 (7) | 24 (4) | ||
| Marital Status (%) | 0.002 | 0.004 | ||||
| Married | 16,099 (75) | 4120 (73) | 19,424 (69) | 430 (72) | ||
| Not married | 3917 (18) | 1092 (19) | 5096 (18) | 114 (19) | ||
| Unknown | 1539 (7) | 468 (8) | 3735 (13) | 51 (9) | ||
| Comorbidity (%) | 0.002 | 0.75 | ||||
| 0 | 14,734 (68) | 3793 (67) | 17,168 (61) | 370 (62) | ||
| 1 | 4740 (22) | 1252 (22) | 6836 (24) | 141 (24) | ||
| 2 or more | 2081 (10) | 635 (11) | 4251 (15) | 84 (14) | ||
| Tumor grade (%) | <0.001 | <0.001 | ||||
| Well/moderately differentiated | 15,555 (72) | 3109 (55) | 11,060 (39) | 334 (56) | ||
| Poorly/undifferentiated | 6000 (28) | 2571 (45) | 17,195 (61) | 261 (44) | ||
| Regional factors | ||||||
| Population of county of residence (%) | <0.001 | <0.001 | ||||
| 1,000,000 or more | 11,295 (52) | 3283 (58) | 15,238 (54) | 376 (63) | ||
| 250,000 to 999,999 | 3749 (17) | 1093 (19) | 5182 (18) | 91 (15) | ||
| 0 to 249,999 | 6511 (30) | 1304 (23) | 7835 (28) | 128 (22) | ||
|
At least a high school education in ZIP code of residence (%) |
0.88 | 0.003 | ||||
| Low (0–75) | 2626 (12) | 687 (12) | 3554 (13) | 50 (8) | ||
| High (>75) | 18,929 (88) | 4993 (88) | 24,701 (87) | 545 (92) | ||
|
Median household income in ZIP code of residence, $ (%) |
<0.001 | <0.001 | ||||
| 40,000 or less | 4126 (19) | 968 (17) | 5086 (18) | 78 (13) | ||
| > 40,000–60,000 | 7966 (37) | 1832 (32) | 10,131 (36) | 144 (24) | ||
| > 60,000 | 9463 (44) | 2880 (51) | 13,038 (46) | 373 (63) | ||
| Geographic region(%)** | <0.001 | <0.001 | ||||
| Northeast | 5039 (23) | 1722 (30) | 6991 (25) | >282 (>47) | ||
| South | 5783 (27) | 1149 (20) | 7689 (27) | 140 (24) | ||
| Central | 4530 (21) | 776 (14) | 4594 (16) | <15 (<3) | ||
| West | 6203 (29) | 2033 (36) | 8981 (32) | 158 (27) | ||
| Year after introduction (%) | <0.001 | <0.001 | ||||
| 1 | 5806 (27) | 196 (3) | 6699 (24) | 61 (10) | ||
| 2 | 5578 (26) | 683 (12) | 5907 (21) | 93 (16) | ||
| 3 | 4178 (19) | 1090 (19) | 5533 (20) | 105 (18) | ||
| 4 | 3394 (16) | 1657 (29) | 5129 (18) | 157 (26) | ||
| 5 | 2599 (12) | 2054 (36) | 4987 (18) | 179 (30) | ||
Abbreviations: IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment
Percentages might not sum to 100 because of rounding
P values generated from chi-square tests
Exact numbers not shown in all cells in order to be compliant with SEER-Medicare guidelines
The results of our multivariable hierarchical logistic regression models are summarized in Table 2. In period 1, several patient factors were associated with a higher likelihood of receiving IMRT compared with the standard, including older age and poorly/undifferentiated tumors. Several regional factors were associated with a lower likelihood of receiving IMRT compared with the standard, including residing in a county with a smaller population, living in an area with a lower median income, and living outside the northeast. During period 1, the likelihood of receiving IMRT compared to the existing standard increased over time (odds ratio [OR] 29.52; 95% confidence interval (CI), 25.10–34.72 [year after introduction: 5 vs. 1]).
Table 2.
Estimated effect (adjusted OR* and 95% CI) of each predictor on the use of new technology (IMRT or SBRT) versus the standard: Results of a multivariable hierarchical logistic regression analysis
| Predictor | IMRT vs. Standard (Period 1) |
P value | SBRT vs. Standard (Period 2) |
P value |
|---|---|---|---|---|
| Patient factors | ||||
| Age, years | <0.001 | 0.22 | ||
| 66–69 | 1 | 1 | ||
| 70–74 | 1.30 (1.18–1.43) | 1.00 (0.81–1.25) | ||
| 75–79 | 1.55 (1.41–1.72) | 0.81 (0.63–1.03) | ||
| 80+ | 1.73 (1.55–1.96) | 0.94 (0.69–1.28) | ||
| Race/ethnicity | 0.51 | 0.02 | ||
| White | 1 | 1 | ||
| Black | 0.93 (0.82–1.06) | 0.71 (0.50–0.99) | ||
| Other | 0.97 (0.83–1.13) | 0.63 (0.40–0.97) | ||
| Marital Status | 0.83 | 0.09 | ||
| Married | 1 | 1 | ||
| Not married | 1.02 (0.94–1.12) | 1.04 (0.84–1.30) | ||
| Unknown | 1.03 (0.90–1.17) | 0.72 (0.53–0.98) | ||
| Comorbidity | 0.76 | 0.25 | ||
| 0 | 1 | 1 | ||
| 1 | 1.01 (0.93–1.10) | 1.01 (0.82–1.24) | ||
| 2 or more | 1.04 (0.93–1.17) | 0.82 (0.63–1.05) | ||
| Tumor grade | <0.001 | <0.001 | ||
| Well/moderately differentiated | 1 | 1 | ||
| Poorly/undifferentiated | 1.60 (1.49–1.72) | 0.48 (0.40–0.57) | ||
| Regional factors | ||||
| Population of county of residence | <0.001 | 0.002 | ||
| 1,000,000 or more | 1 | 1 | ||
| 250,000 to 999,999 | 0.81 (0.63–1.03) | 0.35 (0.20–0.62) | ||
| 0 to 249,999 | 0.64 (0.51–0.80) | 0.52 (0.31–0.87) | ||
|
At least a high school education in ZIP code of residence |
0.07 | 0.21 | ||
| Low (0–75) | 1 | 1 | ||
| High (>75) | 1.13 (0.99–1.28) | 1.26 (0.87–1.82) | ||
|
Median household income in ZIP code of residence, $ |
0.03 | 0.005 | ||
| 40,000 or less | 1 | 1 | ||
| > 40,000–60,000 | 0.89 (0.79–1.00) | 0.94 (0.68–1.31) | ||
| > 60,000 | 0.99 (0.86–1.13) | 1.40 (0.99–1.98) | ||
| Geographic region | 0.055 | <0.001 | ||
| Northeast | 1 | 1 | ||
| South | 0.45 (0.21–0.96) | 0.35 (0.13–0.93) | ||
| Central | 0.39 (0.17–0.87) | 0.01 (<0.01–0.07) | ||
| West | 0.69 (0.30–1.56) | 0.28 (0.10–0.79) | ||
| Year after introduction | <0.001 | <0.001 | ||
| 1 | 1 | 1 | ||
| 2 | 4.02 (3.39–4.75) | 1.81 (1.30–2.53) | ||
| 3 | 8.98 (7.62–10.57) | 2.33 (1.68–3.22) | ||
| 4 | 17.24 (14.67–20.26) | 3.73 (2.74–5.07) | ||
| 5 | 29.52 (25.10–34.72) | 4.59 (3.39–6.21) |
The effect of each predictor was adjusted for all other predictors in the model.
Abbreviations: CI, confidence interval; EBRT, external beam radiation therapy; IMRT, intensity-modulated radiotherapy; OR, odds ratio; SBRT, stereotactic body radiation treatment
In period 2, several patient factors were associated with a lower likelihood of receiving SBRT compared with the standard, including non-white race and poorly/undifferentiated tumors. Several regional factors were associated with a lower likelihood of receiving SBRT compared with the standard, including residing in a county with a smaller population and living outside the northeast. During period 2, the likelihood of receiving SBRT compared to the existing standard increased over time, although not as rapidly as IMRT in period 1 (OR 4.59; 95% CI, 3.39–6.21 [year after introduction: 5 vs. 1]).
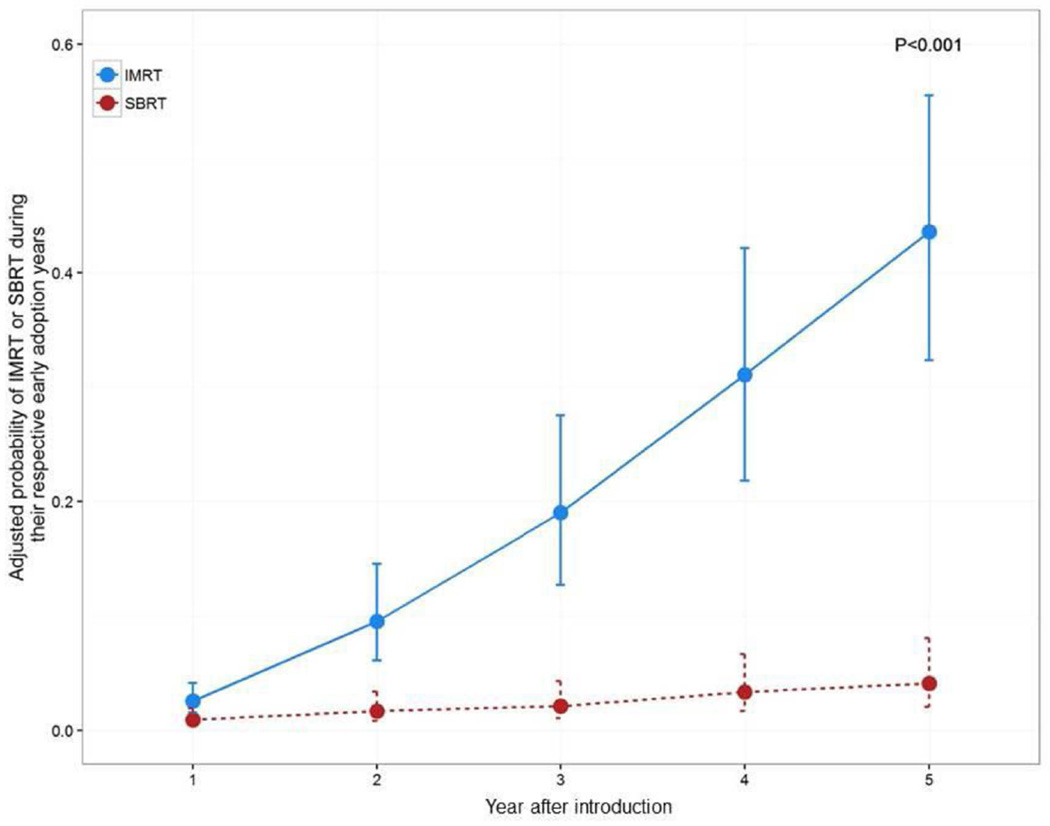
The adjusted annual probability of receiving IMRT was significantly higher than that of SBRT during their respective early adoption years (p<0.001) (Figure 2). In five years, the probability of IMRT use increased from 3% to 44%. Conversely, SBRT use only increased from 1% to 4%. In the early IMRT adoption period, the likelihood of receiving IMRT was significantly higher if IMRT was used in that Health Service Area the year prior (OR 2.25; 95% CI, 1.92–2.63). The same pattern was observed for SBRT use in the early SBRT adoption period (OR 2.14; 95% CI, 1.54–2.97).
Figure 2. Adjusted* probability of IMRT and SBRT during their respective early adoption years.
P-value generated from a model with an interaction term (year after introduction by treatment period)
Abbreviations: IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment
*Adjusted for age, race, marital status, comorbidity, tumor grade, population of county of residence, education in ZIP code of residence, median income in ZIP code of residence, and region.
Physician factors
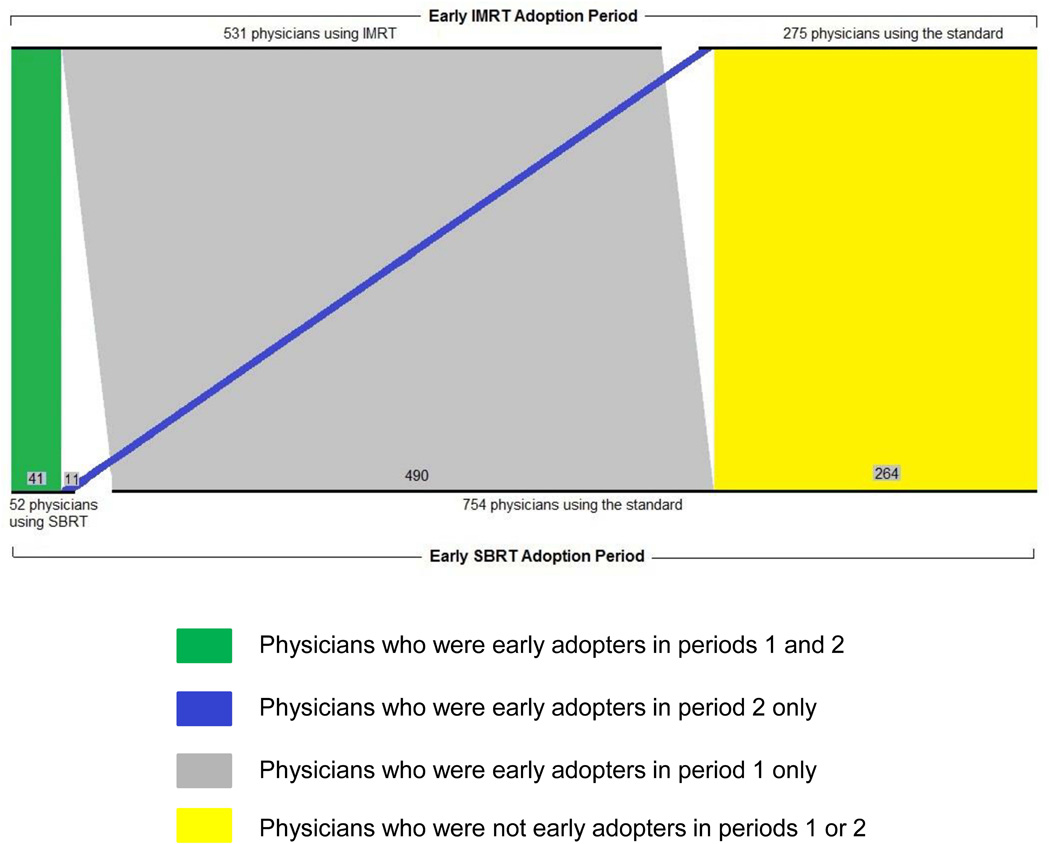
The adoption patterns among treating physicians are shown in Figure 3. There were a total of 806 physicians who treated prostate cancer patients with radiation therapy across both periods 1 and 2. In period 1, 531 physicians were early adopters of IMRT. Among these physicians, 8% (n=41) were also early adopters of SBRT in period 2. In period 1, 275 physicians were not early adopters of IMRT (i.e., they used standard radiation). Among these physicians, 4% (n=11) were early adopters of SBRT in period 2. This difference (8% versus 4%) in the proportion of early SBRT adopters in period 2 approached statistical significance (p=0.06). The 52 physicians who used SBRT were dispersed across 27 Health Service Areas. The distribution of these physicians as well as those using the other radiation treatments is shown in the supporting material.
Figure 3. Adoption patterns among physicians who used radiation therapy in the early IMRT adoption period (period 1) and the early SBRT adoption period (period 2).
Parallel set plot showing the 806 physicians who treated prostate cancer patients with radiation therapy in both the early IMRT adoption period (period 1) and the early SBRT adoption period (period 2).
In period 1, 531 physicians were early adopters of IMRT. Among these physicians, 8% (n=41) were also early adopters of SBRT in period 2. In period 1, 275 physicians were not early adopters of IMRT (i.e., they used standard radiation). Among these physicians, 4% (n=11) were early adopters of SBRT in period 2. This difference (8% versus 4%) in the proportion of early SBRT adopters in period 2 approached statistical significance (p=0.06).
Abbreviations: IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment
DISCUSSION
IMRT’s diffusion was brisk, accounting for 44% of radiation treatment within five years of introduction, while SBRT’s diffusion was slow, accounting for 4% of radiation within five years. Patients were more likely to receive both IMRT and SBRT compared to the standard radiation therapies at those times if they lived in more populated areas and lived in the northeast as opposed to other regions. IMRT was more likely to be given to older patients and those with poorly differentiated tumors whereas SBRT was more likely to be given to white patients and those with well differentiated tumors.
One plausible explanation for the observed differences in the patients who received these treatments is the contrasting radiation delivery methods of IMRT and SBRT. A potential advantage of IMRT is the ability to deliver increased doses of radiation with better sparing of normal tissues like the bladder and rectum, thus improving cancer control with decreased toxicity.1, 2 With the potential to decrease side effects, it is reasonable that providers would prefer to treat older patients with IMRT, thinking it would be a gentler form of radiation. In addition, since higher doses of radiation decrease biochemical recurrence,23 IMRT may be more appealing for patients with higher grade tumors.
Unlike the patients who received IMRT, those getting SBRT tended to have lower risk disease. Although SBRT delivers higher doses of radiation per session, which proponents feel improves cancer control,24 there may be some trepidation to using SBRT in higher risk disease due to the limited clinical evidence in this population.4 Moreover, there are a limited number of opportunities to target the cancer, resulting in a smaller margin for error. To the contrary, IMRT is administered with the same number of sessions as its predecessor 3-dimensional conformal therapy, so initially providers were more likely concerned with toxicity than cancer control.25
Another reason for the decreased use of SBRT among higher risk patients may relate to incentives set up by health policies at the time. During their introduction, both IMRT and SBRT were governed by local coverage determinations, which are decisions by Medicare administrative contractors about coverage for a service based on whether that service is considered reasonable and necessary.26 Prior work showed that local coverage determinations influence the adoption of SBRT to some extent.27 Based on the evidence available at the time, they often restricted the use of SBRT to low- and intermediate-risk prostate cancers, which would result in decreased use among patients with poorly differentiated tumors.
Physicians did not show a propensity for being “early adopters” across the two treatment periods. In Rogers’ Diffusion of Innovation theory, he describes five categories of adopters (i.e., innovators, early adopters, early majority, late majority, and laggards).28 We hypothesized that physicians who were early adopters of IMRT during period 1 would more likely become early adopters of SBRT during period 2. Although this was not the case, the propensity to be an early adopter in both periods approached significance (p=0.06) and this association may surface in future studies with larger sample sizes.
Despite contrasting reasons for the early adoption of IMRT and SBRT, both these modalities were used more in the northeast compared with other regions. Regional variation in treatment is well documented29, 30 and the northeast has shown an increased propensity for treatments in other conditions.31 Further, the northeast comprises several markets with high physician and hospital capacity,32 which generates market competition. For example, the northeast has a higher concentration of institutions with SBRT capabilities than other parts of the country.33, 34 This competition can foster the adoption of new technologies to gain an increased market share of patients and attract physicians, among other reasons.35, 36
Along with geographic region, there are other nonclinical factors that potentially influenced the differential adoption rates of these two treatments. Especially early in the SBRT adoption period, health policies and insurance providers often made it difficult to get SBRT approved for prostate cancer.27 Perhaps more importantly, there were substantial differences in physician reimbursements. IMRT had favorable reimbursement rates shortly after it was introduced. As a result, urologists began purchasing IMRT equipment and offering IMRT to their patients.37 Manufacturers aggressively marketed IMRT to urologists, claiming that treating 1.5 new patients monthly with IMRT could generate more than $425,000 in additional revenue per physician annually.38 To the contrary, total reimbursements for SBRT are lower than for IMRT.39 In the current fee-for-service payment model, each treatment session is reimbursed, which discourages the use of a treatment that comprises five sessions instead of 40. This discrepancy in reimbursement may also dissuade practices from making the initial investments in equipment and training needed to provide SBRT.
The faster uptake of IMRT likely occurred for both clinical and nonclinical reasons. Clinically, the delivery of IMRT was a more natural extension of its predecessor, 3-dimensional conformal therapy—both of which deliver roughly 40 sessions of radiation over eight weeks. At the time, it was also well documented that higher doses of radiation led to increased biochemical control,23 so IMRT was an easy sell for both patients and providers. Conversely, SBRT represented more of a paradigm shift in radiation delivery. It delivered higher doses of radiation in significantly fewer sessions (five instead of 40), and thus represented a new challenge not only technically but philosophically. For example, there are concerns about toxicity with a treatment that delivers higher doses of radiation in such few sessions.7, 39, 40 When making decisions, patients and providers may feel that more treatments with lower doses per treatment is a safer alternative when deciding between IMRT and SBRT in period 2, whereas no such trade off was perceived between 3-dimensional conformal therapy and IMRT in period 1, given that they both comprised the same number of treatments.
Our findings should be interpreted in the context of several limitations. First, the results from SEER-Medicare data may not be generalizable to non-SEER regions. For instance, SBRT is prevalent in Florida where there is a Multi-Institutional Registry for Prostate Cancer Radiosurgery (NCT01226004).41 Second, there is a small number of SBRT patients in our study. While we cannot capture all SBRT patients using SEER-Medicare data, our dataset contains 26% of the U.S. population and represents one of the largest cancer registries, which provides valuable data on tumor characteristics that are not provided with other national datasets.8 Third, we compared two novel technologies that emerged during different time periods, which can be confounded by several factors present in one time period but not the other. For example, more patients underwent active surveillance during the early SBRT adoption period, which could result in a population of radiation patients with more aggressive disease during this period. To help minimize this limitation, we avoided a “head-to-head” comparison of the two treatments and, instead, compared each treatment to the standard treatments that were present at that time. Fourth, there are several policy factors (e.g., local coverage determinations or certificate-of-need laws) that may influence the adoption of these treatments for which we could not account. Nonetheless, we adjusted for several patient, tumor, and market characteristics as well as accounted for the nesting of patients within health markets to help minimize confounding from these unmeasured characteristics, among others.
Despite these limitations, this study merits consideration for three reasons. First, this study provides new evidence demonstrating that many of the factors associated with the early adoption of IMRT and SBRT are different and include both clinical and nonclinical elements. Second, the varying rates of adoption among two treatments that both had limited long-term data at the time of their introduction emphasize the importance of critically examining the incentives and disincentives related to adoption and of revisiting effectiveness as more long-term data become available. Third, if longer term evidence supports SBRT as a comparable treatment, then transitioning towards SBRT may reduce the treatment burden for patients and lower the costs for our health care system.
Supplementary Material
In the Early IMRT adoption period, the 531 physicians using IMRT were dispersed over 115 Health Service Areas whereas the 275 physicians using the standard radiation treatments were dispersed over 93 Health Service Areas. In the Early SBRT adoption period, the 52 physicians using IMRT were dispersed over 27 Health Service Areas whereas the 754 physicians using the standard radiation treatments were dispersed over 136 Health Service Areas.
Abbreviations: IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment
Acknowledgments
Financial disclosures:
Bruce Jacobs is a consultant for ViaOncology
Amber Barnato is a former board member of the Society of Medical Decision Making
Funding:
Bruce Jacobs is supported in part by the National Institutes of Health Institutional KL2 award (KL2TR001856), the GEMSSTAR award (R03AG048091), the Jahnigen career development award, and the Tippins Foundation Scholar Award.
Jonathan Yabes is supported in part by the by the University of Pittsburgh Clinical and Translational Science Institute –Research Education and Career Development Core (UL1 TR000005)
Florian Schroeck is supported in part by the Department of Veterans Affairs VISN 1 Career Development Award
Jeremy Kahn is supported in part by a career development award from the National Institutes of Health (K24HL133444).
Amber Barnato is supported in part by the University of Pittsburgh Clinical and Translational Science Institute – CORE C: Research Education, Training, and Career Development (UL1-RR024153)
Footnotes
Disclaimer: The contents do not represent the views of the United States’ Department of Veterans Affairs or the United States’ Government.
Author contributions:
Bruce Jacobs: conception and design, data acquisition, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript, and statistical analyses
Jonathan Yabes: conception and design, data acquisition, data analysis and interpretation, critical revision of the manuscript, and statistical analyses
Samia Lopa: conception and design, data acquisition, data analysis and interpretation, critical revision of the manuscript, and statistical analyses
Dwight Heron: conception and design, data analysis and interpretation, critical revision of the manuscript, supervision
Chung-Chou Chang: conception and design, data acquisition, data analysis and interpretation, critical revision of the manuscript, and statistical analyses
Florian Schroeck: conception and design, data analysis and interpretation, critical revision of the manuscript
Justin Bekelman: conception and design, data analysis and interpretation, critical revision of the manuscript, supervision
Jeremy Kahn: conception and design, data analysis and interpretation, critical revision of the manuscript, supervision
Joel Nelson: conception and design, data analysis and interpretation, critical revision of the manuscript, supervision
Amber Barnato: conception and design, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript, supervision
REFERENCES
- 1.Vora SA, Wong WW, Schild SE, Ezzell GA, Halyard MY. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Jani AB, Su A, Milano MT. Intensity-modulated versus conventional pelvic radiotherapy for prostate cancer: analysis of acute toxicity. Urology. 2006;67:147–151. doi: 10.1016/j.urology.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Buyyounouski MK, Price RA, Jr, Harris EE, et al. Stereotactic body radiotherapy for primary management of early-stage, low- to intermediate-risk prostate cancer: report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Committee. Int J Radiat Oncol Biol Phys. 2010;76:1297–1304. doi: 10.1016/j.ijrobp.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 5.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs BL, Zhang Y, Skolarus TA, Hollenbeck BK. Growth of high-cost intensity-modulated radiotherapy for prostate cancer raises concerns about overuse. Health Aff (Millwood) 2012;31:750–759. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker BR, Basak R, Mohiuddin JJ, Chen RC. Use of stereotactic body radiotherapy for prostate cancer in the United States from 2004 through 2012. Cancer. 2016;122:2234–2241. doi: 10.1002/cncr.30034. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; 2010. [Google Scholar]
- 9.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 11.Current Procedural Terminology (CPT) 2001. Chicago, IL: AMA press; 2001. [Google Scholar]
- 12.Current Procedural Terminology (CPT) 2007. Chicago, IL: AMA press; 2007. [Google Scholar]
- 13.Allison PD. Logistic Regression Using SAS: Theory and Application. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- 14.National Center for Health Statistics. [accessed January 3, 2012]; Available from URL: http://www.cdc.gov/nchs/
- 15.Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians' practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889–905. doi: 10.1097/00005650-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Smedley BD, Stitch AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 17.SEER-Medicare linked database. [accessed December 3, 2016]; Available from URL: https://healthcaredelivery.cancer.gov/seermedicare/
- 18.PSA values and SEER data, 1973–2012. [accessed December 3, 2016]; Available from URL: https://seer.cancer.gov/data/psa-values.html.
- 19.Schroeck FR, Kaufman SR, Jacobs BL, et al. Technology diffusion and diagnostic testing for prostate cancer. J Urol. 2013;190:1715–1720. doi: 10.1016/j.juro.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack CE, Weissman G, Bekelman J, Liao K, Armstrong K. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subirana I, Sanz H, Vila J. Building bivariate tables: The compareGroups package for R. Journal of Statistical Software. 2014;57:1–16. [Google Scholar]
- 22.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 23.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 24.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 25.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:643–657. doi: 10.1016/j.clon.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Medicare Program Integrity Manual. [accessed April 2, 2014];Chapter 13-Local Coverage Determinations. Available from URL: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS019033.html.
- 27.Jacobs BL, Sunderland R, Yabes J, Nelson JB, Barnato AE, Bekelman J. Local coverage determination policy and the use of stereotactic body radiation therapy for prostate cancer. Urology Practice. 2015;2:304–311. doi: 10.1016/j.urpr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers E. Diffusion of Innovations. 5th. New York, NY: Free Press; 2003. [Google Scholar]
- 29.Wennberg J, Gittelsohn Small area variations in health care delivery. Science. 1973;182:1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 30.Cary KC, Punnen S, Odisho AY, Litwin MS, Saigal CS, Cooperberg MR. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the Urologic Diseases in America project. Prostate Cancer Prostatic Dis. 2015;18:149–154. doi: 10.1038/pcan.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003–2009. JAMA. 2012;307:1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 32.Goodman DC, Fisher E, Bronner KK. Hospital and physician capacity update: A brief report from the Dartmouth Atlas of Health Care. [accessed June 16, 2016]; Available from URL: http://www.dartmouthatlas.org/downloads/reports/Capacity_Report_2009.pdf. [PubMed] [Google Scholar]
- 33.Tipton K, Launders JH, Inamdar R, Miyamoto C, Schoelles K. Stereotactic body radiation therapy: scope of the literature. Ann Intern Med. 2011;154:737–745. doi: 10.7326/0003-4819-154-11-201106070-00343. [DOI] [PubMed] [Google Scholar]
- 34.American Hospital Association. AHA guide to the health care field. 2009 edition. Chicago: Health Forum; 2008. [Google Scholar]
- 35.Adler JT, Chang DC. Implications of Market Competition, Technology Adoption, and Cost for Surgical Patients. JAMA Surg. 2016;151:621. doi: 10.1001/jamasurg.2015.5554. [DOI] [PubMed] [Google Scholar]
- 36.Sethi RK, Henry AJ, Hevelone ND, Lipsitz SR, Belkin M, Nguyen LL. Impact of hospital market competition on endovascular aneurysm repair adoption and outcomes. J Vasc Surg. 2013;58:596–606. doi: 10.1016/j.jvs.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JM. Urologists' use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–1637. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 38.FAQ'S. [accessed January 27, 2012]; Available from URL: http://online.wsj.com/public/resources/documents/urorad-whois12082010.pdf. [Google Scholar]
- 39.Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol. 2014;32:1195–1201. doi: 10.1200/JCO.2013.53.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halpern JA, Sedrakyan A, Hsu WC, et al. Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer. 2016;122:2496–2504. doi: 10.1002/cncr.30101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ClinicalTrials.gov. [accessed November 30, 2015]; Available from URL: https://clinicaltrials.gov/ct2/show/NCT01226004?term=NCT01226004&rank=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the Early IMRT adoption period, the 531 physicians using IMRT were dispersed over 115 Health Service Areas whereas the 275 physicians using the standard radiation treatments were dispersed over 93 Health Service Areas. In the Early SBRT adoption period, the 52 physicians using IMRT were dispersed over 27 Health Service Areas whereas the 754 physicians using the standard radiation treatments were dispersed over 136 Health Service Areas.
Abbreviations: IMRT, intensity-modulated radiotherapy; SBRT, stereotactic body radiation treatment