Abstract
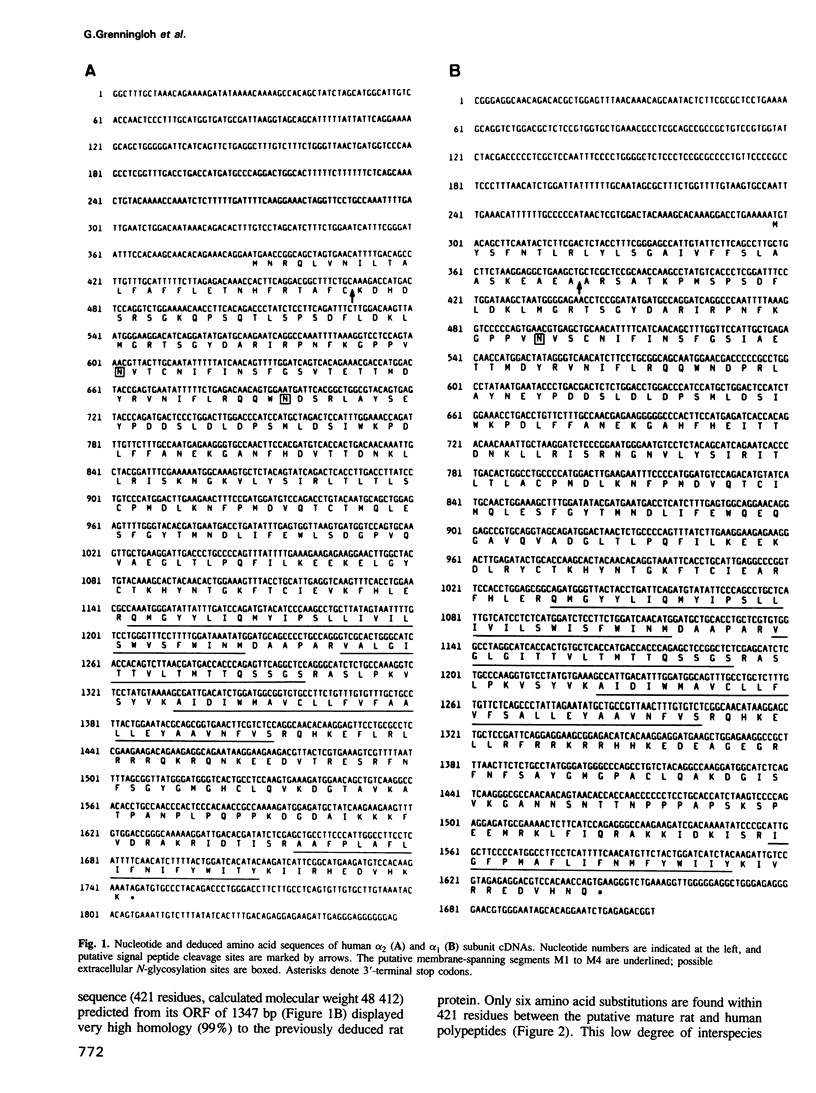
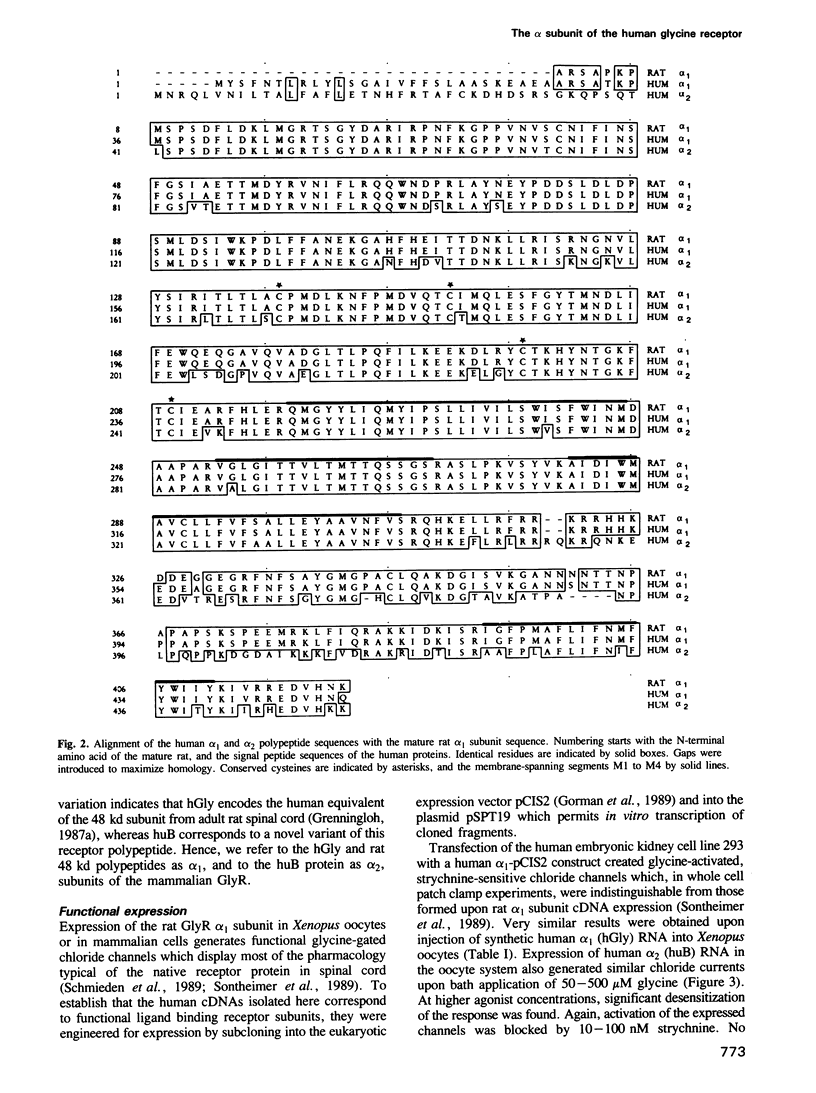
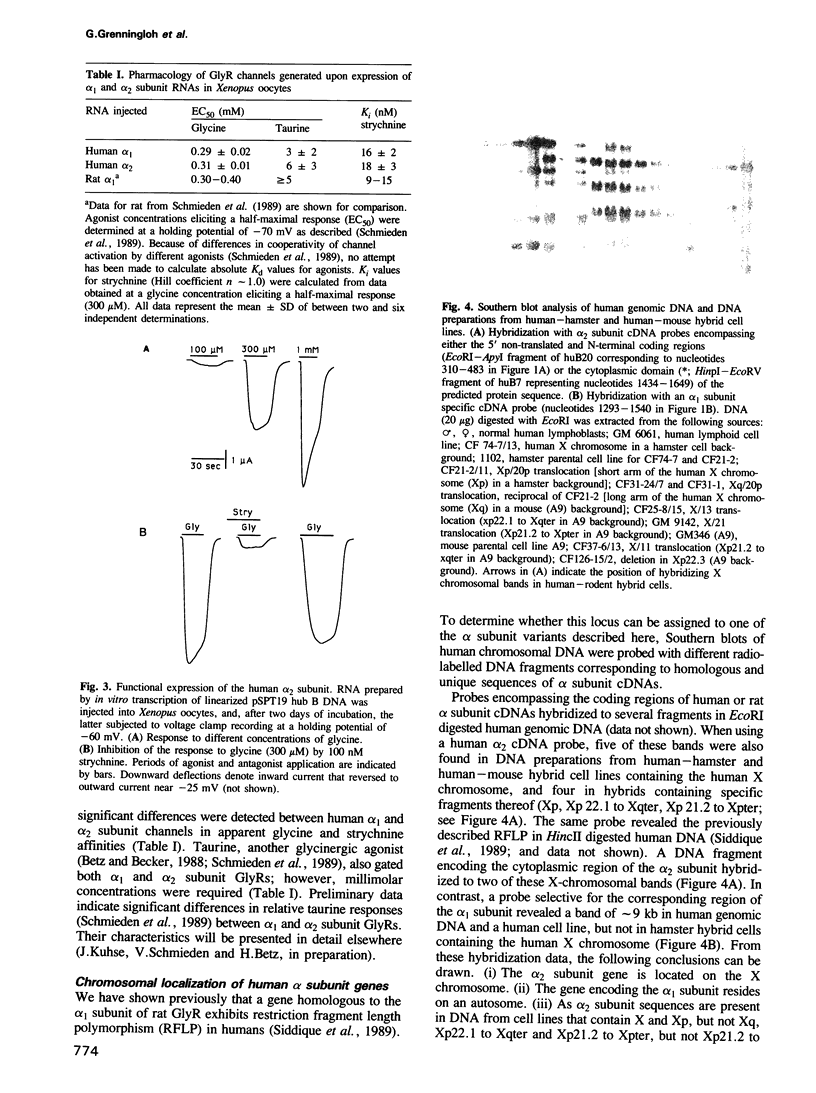
Two cDNAs encoding variants (alpha 1 and alpha 2) of the strychnine binding subunit of the inhibitory glycine receptor (GlyR) were isolated from a human fetal brain cDNA library. The predicted amino acid sequences exhibit approximately 99% and approximately 76% identity to the previously characterized rat 48 kd polypeptide. Heterologous expression of the human alpha 1 and alpha 2 subunits in Xenopus oocytes resulted in the formation of glycine-gated strychnine-sensitive chloride channels, indicating that both polypeptides can form functional GlyRs. Using a panel of rodent-human hybrid cell lines, the gene encoding alpha 2 was mapped to the short arm (Xp21.2-p22.1) of the human X chromosome. In contrast, the alpha 1 subunit gene is autosomally located. These data indicate molecular heterogeneity of the human GlyR at the level of alpha subunit genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi H., Miledi R. Heterogeneity of glycine receptors and their messenger RNAs in rat brain and spinal cord. Science. 1988 Oct 14;242(4876):270–273. doi: 10.1126/science.2845580. [DOI] [PubMed] [Google Scholar]
- Andermann F., Keene D. L., Andermann E., Quesney L. F. Startle disease or hyperekplexia: further delineation of the syndrome. Brain. 1980 Dec;103(4):985–997. doi: 10.1093/brain/103.4.985. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. GABA and glycine may share the same conductance channel on cultured mammalian neurones. Nature. 1979 Jan 18;277(5693):234–236. doi: 10.1038/277234a0. [DOI] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Boulter J., Wada E., Wada K., Swanson L. W., Patrick J., Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988 Mar;1(1):45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Lane P. W. Assignment of LH XVI to chromosome 3 in the mouse. J Hered. 1980 Sep-Oct;71(5):315–318. doi: 10.1093/oxfordjournals.jhered.a109378. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Gies D., McCray G., Huang M. The human cytomegalovirus major immediate early promoter can be trans-activated by adenovirus early proteins. Virology. 1989 Aug;171(2):377–385. doi: 10.1016/0042-6822(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Gundelfinger E., Schmitt B., Betz H., Darlison M. G., Barnard E. A., Schofield P. R., Seeburg P. H. Glycine vs GABA receptors. Nature. 1987 Nov 5;330(6143):25–26. doi: 10.1038/330025b0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Gundlach A. L., Dodd P. R., Grabara C. S., Watson W. E., Johnston G. A., Harper P. A., Dennis J. A., Healy P. J. Deficit of spinal cord glycine/strychnine receptors in inherited myoclonus of Poll Hereford calves. Science. 1988 Sep 30;241(4874):1807–1810. doi: 10.1126/science.2845573. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Hereditary "pure" spastic paraplegia: a clinical and genetic study of 22 families. J Neurol Neurosurg Psychiatry. 1981 Oct;44(10):871–883. doi: 10.1136/jnnp.44.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W., Betz H., Becker C. M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989 Sep;3(3):339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Molecular genetic studies in the neuropsychiatric disorders. Trends Neurosci. 1989 Apr;12(4):130–137. doi: 10.1016/0166-2236(89)90051-9. [DOI] [PubMed] [Google Scholar]
- Nadeau J. H. Maps of linkage and synteny homologies between mouse and man. Trends Genet. 1989 Mar;5(3):82–86. doi: 10.1016/0168-9525(89)90031-0. [DOI] [PubMed] [Google Scholar]
- Nef P., Oneyser C., Alliod C., Couturier S., Ballivet M. Genes expressed in the brain define three distinct neuronal nicotinic acetylcholine receptors. EMBO J. 1988 Mar;7(3):595–601. doi: 10.1002/j.1460-2075.1988.tb02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Gorman C. M., Kettenmann H., Seeburg P. H., Schofield P. R. Transient expression shows ligand gating and allosteric potentiation of GABAA receptor subunits. Science. 1988 Dec 2;242(4883):1306–1308. doi: 10.1126/science.2848320. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Pritchett D. B., Sontheimer H., Kettenmann H., Seeburg P. H. Sequence and expression of human GABAA receptor alpha 1 and beta 1 subunits. FEBS Lett. 1989 Feb 27;244(2):361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- Siddique T., McKinney R., Hung W. Y., Bartlett R. J., Bruns G., Mohandas T. K., Ropers H. H., Wilfert C., Roses A. D. The poliovirus sensitivity (PVS) gene is on chromosome 19q12----q13.2. Genomics. 1988 Aug;3(2):156–160. doi: 10.1016/0888-7543(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Siddique T., Phillips K., Betz H., Grenningloh G., Warner K., Hung W. Y., Laing N., Roses A. D. RFLPs of the gene for the human glycine receptor on the X-chromosome. Nucleic Acids Res. 1989 Feb 25;17(4):1785–1785. doi: 10.1093/nar/17.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Barnard E. A. A gamma-aminobutyric acid/benzodiazepine receptor complex from bovine cerebral cortex. Improved purification with preservation of regulatory sites and their interactions. J Biol Chem. 1984 Jun 10;259(11):7219–7223. [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Read A. P., Davies K. E., Whyte M. P., Weksberg R., Glorieux F., Davies M., Mountford R. C., Harris R., King A. Bridging markers defining the map position of X linked hypophosphataemic rickets. J Med Genet. 1987 Dec;24(12):756–760. doi: 10.1136/jmg.24.12.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. F., Heller A. H. Glycine receptor alteration in the mutant mouse spastic. Nature. 1982 Aug 12;298(5875):655–657. doi: 10.1038/298655a0. [DOI] [PubMed] [Google Scholar]
- Ymer S., Schofield P. R., Draguhn A., Werner P., Köhler M., Seeburg P. H. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989 Jun;8(6):1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




