Abstract
Objectives
Low back pain (LBP) causes more than 2.5 million visits to US EDs annually. LBP patients are often treated with non-steroidal anti-inflammatory drugs and benzodiazepines. The former is an evidence-based intervention while the efficacy of the latter has not been established. We compared pain and functional outcomes one week and three months after ED discharge among patients randomized to a one week course of naproxen + diazepam versus naproxen + placebo.
Methods
Randomized, double-blind, comparative efficacy clinical trial conducted in an urban healthcare system. Patients presenting with acute, non-traumatic, non-radicular LBP of no more than two weeks duration were eligible for enrollment immediately prior to discharge from an ED if they had a score greater than five on the Roland-Morris Disability Questionnaire (RMDQ), a validated 24-item inventory of functional impairment due to LBP. Higher scores on the RMDQ indicate greater functional disability. The primary outcome in the trial was improvement on the RMDQ between ED discharge and one week later. Secondary outcomes included pain intensity one week and three months after ED discharge, as measured on a four point descriptive scale (severe, moderate, mild, none). All patients were given 20 tablets of naproxen 500mg, to be taken twice a day, as needed for LBP. Additionally, patients were randomized to receive either 28 tablets of diazepam 5 mg or identical placebo, to be taken as one or two tablets every 12 hours as needed for LBP. All patients received a standardized 10 minute LBP educational session prior to discharge. Using a between-group mean difference of five RMDQ points, a previously validated threshold for clinical significance, we calculated the need for at least 100 patients with primary outcome data.
Results
Enrollment began in June 2015 and continued for 9 months. 545 patients were screened for eligibility. 114 patients met selection criteria and were randomized. Baseline demographic characteristics were not substantially different between the two groups. 112 patients (98%) provided one week outcome data. The mean RMDQ of patients randomized to naproxen + diazepam improved by 11 (95%CI: 9, 13) as did the mean RMDQ of patients randomized to naproxen + placebo [11 (95%CI: 8, 13)]. At one week follow-up, 18/57 (32%, 95%CI: 21, 45%) diazepam patients reported moderate or severe LBP versus 12/55 (22%, 95%CI: 13, 35%) placebo patients. At three month follow-up, 6/50 (12%, 95%CI: 5, 24%) diazepam patients reported moderate or severe LBP versus 5/53 (9%, 95%CI: 4, 21%) placebo patients. Adverse events were reported by 12/57 (21%, 95%CI: 12, 33%) diazepam patients and 8/55 (15%, 95%CI: 7, 26%) placebo patients.
Conclusion
Among ED patients with acute, non-traumatic, non-radicular LBP, naproxen + diazepam did not improve functional outcomes or pain when compared to naproxen + placebo one week and three months after ED discharge.
Introduction
Background
Low back pain is responsible for 2.4% of visits to US emergency departments (ED) resulting in 2.7 million visits annually.(1) Pain outcomes for these patients are generally poor.(2) One week after an ED visit in an unselected low back pain (LBP) population, 70% of patients report persistent back-pain related functional impairment and 69% report continued analgesic use.(2) Three months later, 48% report functional impairment and 46% report persistent analgesic use. Among the subset of ED patients who present with acute, new-onset LBP, outcomes are generally better--most will recover, though more than 20% of this group will also report moderate or severe LBP three months later and 30% will report LBP-related functional impairment.(3)
Importance
Non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line therapy for patients with acute LBP. (4)However, it is not clear if the addition of other classes of therapeutic agents to NSAIDS can further improve LBP outcomes. Benzodiazepines are often mentioned as useful for these patients and are used in 300,000 US ED visits for LBP annually, although scant evidence exists to determine the appropriateness of this approach. (5, 6) Efficacy of benzodiazepines, if any, may be related to direct or centrally-mediated action on skeletal muscle or may work, entirely or in part, by mitigating patient anxiety about the condition.(7)
Goals of this Investigation
Because of the poor pain and functional outcomes that persist beyond an ED visit for musculoskeletal LBP, we conducted a double-blind, randomized clinical trial to evaluate whether combining a benzodiazepine with an NSAID is more efficacious than NSAID monotherapy for the treatment of acute, non-traumatic, non-radicular LBP. Specifically, we wished to evaluate the following hypothesis: A daily regimen of naproxen + diazepam would provide greater relief of functional impairment due to LBP than naproxen + placebo, as measured by improvement in the Roland Morris Disability Questionnaire one week after an ED visit.
Methods
Study design and setting
This was a randomized, double-blind, ED-based comparative efficacy study conducted in two EDs of an urban healthcare system. We enrolled patients during an ED visit for acute musculoskeletal LBP and followed them by telephone seven days and three months later. Every patient received standard-of-care therapy, consisting of naproxen and a low back pain education session, in addition to either diazepam or placebo. The Albert Einstein College of Medicine Institutional Review Board reviewed and approved this study. We obtained written consent from all participants. The study was registered at http://clinicaltrials.gov (NCT02646124). Enrollment commenced in June 2015 and continued for nine months.
We conducted this study in two EDs of Montefiore Medical Center, an urban teaching medical center, with 178,000 adult visits annually. Salaried, trained, fluently bilingual (English and Spanish) research associates staffed the ED 24 hours per day, seven days per week during the accrual period.
Selection of participants
Our goal was to include a broad representation of patients with musculoskeletal back pain who were likely to respond to the investigational medications. We included adults aged 21-69 years who presented to the ED primarily for management of acute LBP, defined as pain originating between the lower border of the scapulae and the upper gluteal folds. The primary clinical diagnosis, at the conclusion of the ED visit, was required to be a diagnosis consistent with non-traumatic, non-radicular, musculoskeletal LBP. We only included patients who were to be discharged home, and those who had functionally impairing back pain, defined as a score of > 5 on the Roland-Morris Disability Questionnaire (RMDQ). The RMDQ is a validated 24 item questionnaire commonly used to measure LBP and related functional impairment on which 0 represents no impairment and 24 represents maximum impairment (Appendix 1).
We excluded patients from participation for radicular pain, defined as pain radiating below the gluteal folds in a dermatomal distribution, pain duration >2 weeks (336 hours), or a baseline LBP frequency of once per month or more frequently. We required the absence of non-musculoskeletal etiology of low back, such as urinary tract infection or influenza like illness. Patients with direct trauma to the back within the previous month were excluded, as were those who were unavailable for follow-up, those who were pregnant or breast-feeding, those patients with a chronic pain syndrome, defined as use of any analgesic medication on a daily or near-daily basis, and those who were allergic to or intolerant of the investigational medications. We did not exclude patients for use of an NSAID prior to ED presentation. Finally, patients could only be enrolled once.
Interventions
The pharmacist performed randomization in blocks of four based on a random number sequence generated at http://randomization.com. Patients were randomized in a 1:1 manner to one of two interventions:
The benzodiazepine arm: Naproxen 500mg tablets taken twice per day + diazepam 5mg, taken as one or two tablets every 12 hours
The control arm: Naproxen 500mg tablets taken twice per day + placebo, taken as one or two tablets every 12 hours
In an effort to maximize effectiveness while minimizing side effects, we instructed patients to take one or two pills of the investigational medication every 12 hours. If one tablet of the investigational medication afforded sufficient relief, then there was no need for the patient to take the second tablet. However, if the patient had not experienced sufficient relief within 30 minutes of taking one investigational medication tablet, they were instructed to take the second tablet. We gave all study patients 20 naproxen tablets, a ten day supply, and 28 tablets of the investigational medication, enough to last seven days if the patient took the maximum dose of two tablets every twelve hours.
Methods and measurements
The pharmacists masked diazepam and placebo by placing tablets into identical capsules, which were packed with scant amounts of lactose and sealed. They performed the masking within the pharmacies, secure locations inaccessible to ED personnel. We then presented patients with two containers of medication. The container with the naproxen, labeled in a typical manner, was not masked. The second container, holding diazepam or placebo, was labeled as investigational medication.
Prior to discharge, research personnel delivered verbally to each participant a 10-minute educational intervention, based on NIAMS's five page “What is back pain?” information sheet from the National Library of Medicine's Fun Facts: An Easy-to-Read Series of Publications for the Public (available at http://www.niams.nih.gov/Health_Info/Back_Pain/back_pain_ff.asp). We informed each participant that carefully chosen exercises and stretches may help pain and prevent future occurrences and that hot or cold packs, physical therapy, massage therapy, and acupuncture may help some patients.
Research associates, who were blinded to study assignment, performed the follow-up phone calls.
Outcomes
The primary outcome for this study was improvement on the Roland Morris Disability Questionnaire (RMDQ) between ED discharge and the 7day telephone follow-up. A five-point improvement on this scale is generally considered a clinically significant improvement.(8) Secondary outcomes one week and three months after ED discharge were as follows: One week after ED discharge, we determined 1) participants' worst pain during the previous 24 hours, using a four item ordinal scale (severe, moderate, mild, or none—dichotomized as severe/moderate versus mild/none for analysis); 2) the frequency of LBP during the previous 24 hours using a five item scale (not at all, rarely, sometimes, usually, always—trichotomized as not at all/rarely versus sometimes versus usually/always for analysis); 3) the frequency of any analgesic or LBP medication use during the previous 24 hours (dichotomized as use versus no use); 4) satisfaction with treatment, as measured by response to the question, “The next time you have back pain, do you want to take the same medications you've been taking this past week?”; 5) the number of days following ED discharge the participant was able to return to usual activities; and 6) the frequency of visits to any clinician during the follow-up period. We determined how frequently participants used naproxen and the investigational medication by asking them to categorize their use of each as more than once per day, once per day, sometimes, only once, or never. Three months after ED discharge, we determined 1) participants' absolute RMDQ; 2) their worst LBP during the previous 72 hours, using the same ordinal scale as above; 3) the frequency of LBP during the previous 72 hours using the same scale as above; and 4) the frequency of use of any LBP medication during the previous 72 hours, again dichotomized as use versus no use. Adverse events were ascertained by asking patients to report any symptoms from the medications. We specifically asked participants to describe whether or not the medications made them tired or dizzy, or irritated their stomachs. For these latter three symptoms, participants were asked to use the descriptions “ a lot” “ a little”, or “none.” These measures have all been used previously.(3)
Analysis
The primary analysis was intention-to-treat. All eligible participants with available outcome data were analyzed based on group assignment. The primary outcome was a comparison of the change in RMDQ between baseline and one week. These results are reported as means with 95%CI, and difference between the means of the two comparison groups, with 95%CI. Dichotomous secondary outcomes are reported as proportions and difference between proportions with 95%CI.
We based our assumptions on a recently completed RCT of LBP treatment.(3) The mean improvement in RMDQ among those who received naproxen alone was 10.2 (standard deviation of 8.9). A widely accepted minimum clinically important improvement of 5 points on the RMDQ (8) therefore would have required those randomized to diazepam to demonstrate a mean improvement of 15.2 on the RM scale. We believed that if one group had a decrease in the RMDQ that was 5 points (or more) greater than the other group, this would be a clinically significant difference between the groups. Using a standard 2-tailed alpha of 0.05 and a beta of 0.20, we determined the need for 50 subjects in each arm. To account for protocol violations and lost-to-follow-up, we planned to enroll 115% of our calculated sample size or 16 additional patients.
Results
Characteristics of study subjects
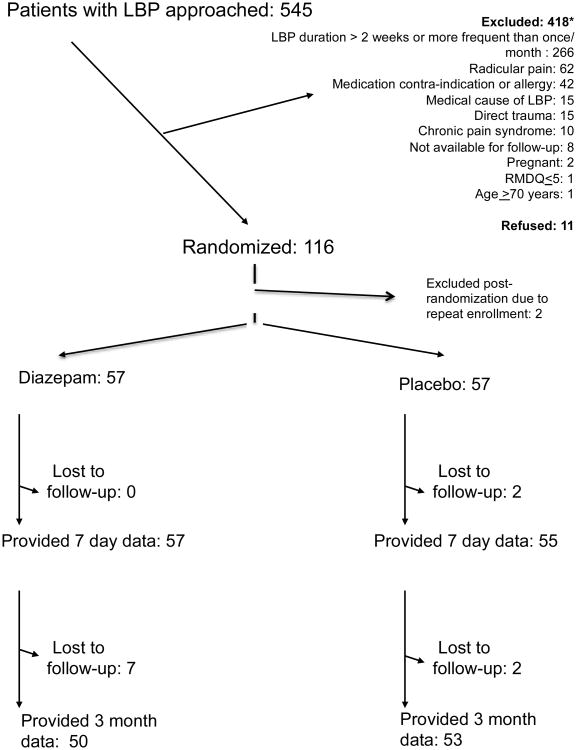
During the study period, we approached 545 patients with low back pain for participation and randomized 114 eligible patients (Figure 1). Baseline characteristics were comparable between the groups. (Table 1) The median initial RMDQ of 18 demonstrated substantial baseline functional impairment upon presentation. Most patients had pain for no more than two or three days before presenting to the ED.
Figure 1. CONSORT flow diagram.
* 4 patients were excluded for more than one reason
Table 1. Baseline characteristics.
| Variable | Naproxen + diazepam (n= 57) | Naproxen + placebo (n= 57) |
|---|---|---|
| Age in years, mean (SD) | 34 (12) | 38 (12) |
| Sex | ||
| Men | 30 (53%) | 33 (58%) |
| Women | 27 (47%) | 24 (42%) |
| Work status | ||
| Unemployed | 11 (19%) | 3 (5%) |
| Student | 1 (2%) | 6 (11%) |
| <30 hours/ week | 6 (11%) | 4 (7%) |
| ≥30 hours/ week | 39 (68%) | 44 (77%) |
| Median RMDQ at time of ED visit (IQR) | 18 (16, 21) | 18 (15, 20) |
| Median duration of LBP prior to presentation to ED, hours (IQR) | 72 (24, 108) | 48 (12, 96) |
| Previous episodes of LBP | ||
| Never before | 28 (50%) | 22 (39%) |
| Few times before | 25 (45%) | 29 (52%) |
| At least once/ year | 3 (5%) | 5 (9%) |
| Missing | 1 | 1 |
| Depression screen positive1 | 3/57 (5%) | 2/57 (4%) |
Values are n (%) unless otherwise stated.
RMDQ: Roland Morris Disability Questionnaire. This is a 24 item instrument measuring low back pain related functional impairment. On this instrument, 0 represents no low back pain related functional impairment and 24 represents maximum functional impairment.
- a) Before your back pain began, how often were you bothered by little pleasure or interest in doing things?
- b) Before your back pain began, how often were you bothered by feeling down, depressed, or hopeless? Patients who responded to either question “More than half the days” or “Nearly every day” were considered to screen positive for depression.
We discuss the presence or absence of spasm at baseline and discordance in work status in Appendix 2.
Main results
One week after the ED visit, patients randomized to diazepam improved by a mean of 11 (95%CI: 9, 13) RMDQ points while placebo patients improved by 11 (95%CI: 8, 13) (95%CI for mean difference of 0.3: -2.8, 3.5). The between-group difference achieved neither clinical nor statistical significance. Secondary outcomes were also comparable between the groups (Table 2).
Table 2. One-week outcomes among study participants who completed one-week follow-up.
| Outcome variable | Naproxen + diazepam (n= 57) | Naproxen + placebo (n=55) | Difference between diazepam and placebo % (95%CI) |
|---|---|---|---|
| Worst LBP during previous 24 hours | |||
| Mild/ none | 39 (68%) | 43 (78%) | - 10% (-26, 7%) |
| Moderate/ Severe | 18 (32%) | 12 (22%) | |
| Frequency of LBP during previous 24 hours | |||
| Never/ rarely | 28 (49%) | 30 (56%) | -6% (-25, 12%)3 |
| Sometimes | 16 (28%) | 11 (20%) | |
| Frequently/ always | 13 (23%) | 13 (24%) | |
| missing | 1 | ||
| Use of medication for LBP during the 24 hours prior to one week follow-up | |||
| No meds | 31 (54%) | 30 (55%) | 0 % (-19, 18%) |
| Took meds | 26 (46%) | 25 (46%) | |
| Desires same medications during subsequent episode of LBP 1 | |||
| Yes | 44 (77%) | 37 (70%) | 7% (-9, 24%)4 |
| No | 9 (16%) | 12 (23%) | |
| Not sure | 4 (7%) | 4 (8%) | |
| missing | 2 | ||
| Median number of days until able to return to usual activities (IQR) 2 | 4 (2, >7) | 5 (2, >7) | -0.4 (-0.6, 1.4)5 |
Data in the fourth column have been rounded to the nearest integer.
Participants were asked: “The next time you have back pain, do you want to take the same medications you've been taking this past week?”
Patients who had not yet recovered at the time of the one week phone call were categorized as >7 days.
(Never/rarely) versus (sometimes/frequently/always)
(Yes) versus (no/not sure)
Difference in mean number of days.
We detail use of off-protocol medication in Appendix 3.
A large majority of patients used naproxen at least once per day (Table 3). Use of the investigational medication (diazepam or placebo) among the study cohort was less common. Most of our patients did not visit another healthcare provider within one week of ED discharge (Table 3).
Table 3. Use of investigational medication and healthcare resources within one week of ED discharge.
| Outcome | Naproxen + diazepam, n (%) N=57 | Naproxen + placebo, n (%) N=55 |
|---|---|---|
| Frequency of naproxen use | ||
| More than once/ day | 45 (79%) | 34 (63%) |
| Once/ day | 6 (11%) | 13 (24%) |
| Sometimes | 1 (2%) | 2 (4%) |
| Only once | 5 (9%) | 3 (6%) |
| Never | 0 (0%) | 2 (4%) |
| missing | 0 | 1 |
| Frequency of placebo/ diazepam use | ||
| More than once/ day | 21 (38%) | 21 (38%) |
| Once/day | 18 (32%) | 16 (29%) |
| Sometimes | 9 (16%) | 5 (9%) |
| Only once | 6 (11%) | 5 (9%) |
| Never | 2 (4%) | 8 (15%) |
| missing | 1 | 0 |
| Healthcare resources utilized | ||
| No visit to any clinician | 49 (88%) | 40 (77%) |
| Subsequent ED visit | 3 (5%) | 2 (4%) |
| Primary care | 1 (2%) | 6 (12%) |
| MD specialist1 | 1 (2%) | 1 (2%) |
| Complementary therapy2 | 2 (4%) | 3 (6%) |
| missing | 1 | 3 |
Orthopedics
Physical therapy, chiropractor
Adverse events were relatively infrequent and comparable between the groups (Table 4). Other than the symptoms reported in Table 4, no more than one participant reported any other adverse event. There were no serious or unexpected adverse events.
Table 4. Adverse medication effects.
| Adverse event | Naproxen + diazepam n/ N (%) | Naproxen + placebo n/ N (%) | Difference between diazepam and placebo % (95%CI) |
|---|---|---|---|
| Any adverse event | 12/ 57 (21%) | 8/ 52 (15%) | 6% (-9, 20%) |
| Tired (a lot)1 | 4/ 56 (7%) | 1/ 52 (2%) | 5% (-2, 13%) |
| Dizzy (a lot) 1 | 1/ 56 (2%) | 0/ 51 (0%) | 2% (-2, 5%) |
| Stomach irritation (a lot) 1 | 1/ 57 (2%) | 1/ 52 (2%) | 0% (-5, 5%) |
At the seven day follow-up, we asked study participants specifically whether or not they experienced dizziness, feeling tired, and stomach irritation. They were asked to choose among the following options: “no,” “a little,” “a lot.”
By three months after the ED visit, most patients had recovered completely (Table 5). Similar to the findings at one week follow-up, differences in three month pain or functional outcomes between groups were neither clinically nor statistically significant.
Table 5. Three month outcomes.
| Outcome variable | Naproxen + diazepam (n=50) | Naproxen + placebo (n=53) | Difference between diazepam and placebo % (95%CI) |
|---|---|---|---|
| Median RMDQ (IQR) | 0 (0, 1) | 0 (0, 6) | -2.0 (-4.2, 0.3)1 |
| Worst LBP during previous 72 hours | |||
| Mild/none | 44 (88%) | 48 (91%) | -3% (-15, 9%) |
| Moderate/ severe | 6 (12%) | 5 (9%) | |
| Frequency of LBP during previous 72 hours | |||
| Never/ rarely | 42 (84%) | 42 (79%) | 5% (-10, 20%)2 |
| Sometimes | 7 (14%) | 5 (9%) | |
| Frequently/ always | 1 (2%) | 6 (11%) | |
| Use of medication for LBP within 72 hours | |||
| No meds | 42 (84%) | 47 (89%) | -5% (-18, 9%) |
| Took meds | 8 (16%) | 6 (11%) |
RMDQ: Roland Morris Disability Questionnaire. This is a 24 item instrument measuring low back pain related functional impairment. On this instrument, zero represents no LBP related functional impairment and 24 represents maximum functional impairment.
Difference between mean three month RMDQ scores
(Never/ rarely) versus (sometime/ frequently/ always)
Limitations
The first limitation is that in the interest of maintaining homogeneity for this study, we screened but did not include many patients because they did not meet our strict entry criteria. Thus, the study participants represent only a subset of patients who present to the ED with acute non-traumatic, non-radicular LBP. These results therefore cannot be generalized to patients with other types of back pain, nor do the findings extend to those suffering from chronic LBP.
A second limitation is that we conducted this study in one urban healthcare system serving a socio-economically depressed population. Because back pain outcomes may be associated with socio-economic variables, our results can be generalized most appropriately to EDs that serve similar disadvantaged patient populations.
A third limitation is that we tested the combination of diazepam with naproxen, not diazepam alone. Thus, we do not know how diazepam would have fared by itself.
A forth limitation is that we did not insist that patients take these medications on a standing schedule but instead allowed them to take the medications on an as needed basis. Therefore, it is possible that the true efficacy of diazepam was missed because of insufficient dosing. However, our study more closely mirrors the clinical reality of emergency practice.
Finally, we did not use presence or absence of muscle spasm on clinical exam as an entry criterion because the clinical significance of this finding is uncertain.(9) Furthermore, it cannot be assessed pragmatically in a reliable and accurate manner. It is plausible that patients with true muscular spasm may have fared better with the active medication.
Discussion
Diazepam is currently used in about 300,000 visits for LBP to US EDs annually.(1) Given the frequent usage of diazepam, there is a surprising paucity of evidence regarding its efficacy. We identified four studies in which diazepam was compared with placebo for LBP and one in which it was compared to aspirin. Brotz prescribed physiotherapy and diclofenac to 60 patients hospitalized with lumbar disc prolapse, and then randomized the patients into placebo and diazepam study arms. When compared with diazepam, the placebo group was found to have a shorter hospital stay and a higher probability of >50% reduction in pain by day seven. However, there were no differences in functional outcome.(10) Hingorani randomized 50 hospitalized patients with various etiologies of acute LBP to diazepam or placebo and found no difference in subjective or objective outcomes.(5) Brown randomized 49 patients with chronic back or neck pain to diazepam, cyclobenzaprine groups or placebo and used a global outcome measure that encompassed change in level of pain, spasm, mobility, tenderness to palpation, and restriction in activities. The cyclobenzaprine patients had better outcomes than the diazepam patients, who had better outcomes than patients randomized to placebo.(11) Basmajian randomized 105 patients with neck or back pain to diazepam, cyclobenzaprine, and placebo and found no statistically significant differences among the groups.(12)
Our data contribute to a growing body of literature suggesting that in general, most medications do not improve acute LBP. We demonstrated previously that adding cyclobenzaprine or oxycodone/acetaminophen to naproxen is unlikely to benefit patients with new-onset non-radicular LBP.(3) Similarly, patients with non-radicular LBP appear to receive no benefit from either corticosteroids(13) or acetaminophen(14). Complementary therapies, including acupuncture,(15) yoga,(16) and massage(17) may be offered but have been inadequately studied to assess efficacy in an acute LBP population. Spinal manipulation is unlikely to benefit ED patients with acute LBP who are well managed medically.(18) Physical therapy may be useful for some.(19) Emergency physicians should counsel their patients that passage of time will bring improvement and eventual relief to most patients.
Overall, one week and three month outcomes in this study were generally better than in other ED-based work.(2, 3, 13, 20) This is partly explained by our selection criteria, which excluded patients with chronic or frequent episodic LBP, who have been shown to have worse outcomes.(21) However, in all ED-based studies, 25-40% of patients with acute, new-onset LBP report moderate or severe LBP one week after ED discharge and 10-25% of these patients report moderate or severe pain three months later. Ideally, patients at higher risk of poor outcome should be targeted for close follow-up with the goal of preventing the transition from acute to chronic pain. Unfortunately, it is difficult to predict which individual patients with acute LBP are at risk of poor outcomes.
Enrollment in this study did not commence until an individual was ready for discharge from the ED. Therefore, we do not know if diazepam may have a role in the acute management of acute LBP, i.e., whether diazepam can increase the likelihood of discharge among those patients who arrive in the ED with marked functional impairment due to low back pain of sufficient severity that hospitalization may be necessary. Also, we excluded from participation those patients with chronic or frequent episodic LBP. A systematic review suggests that these patients are at increased risk of poor outcomes if they are prescribed benzodiazepines.(4)
In conclusion, diazepam does not appear to confer any benefit beyond that of placebo when added to naproxen for the treatment of non-radicular, non-traumatic acute LBP.
Supplementary Material
Acknowledgments
This publication was supported in part by the Harold and Muriel Block Institute for Clinical and Translational Research at Einstein and Montefiore grant support (UL1TR001073)
Footnotes
We will present this study at the American College of Emergency Physicians research forum in Las Vegas NV in October, 2016
We have no conflicts of interest to report.
BWF, CS, EJG conceived the study and designed the trial. BWF, EI, CS, EZ supervised the conduct of the trial and data collection. BWF, EI, JZ managed the data, including quality control. BWF analyzed the data. BWF and NK drafted the manuscript, and all authors contributed substantially to its revision. BWF takes responsibility for the paper as a whole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman BW, Chilstrom M, Bijur PE, Gallagher EJ. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine. 2010;35(24):E1406–11. doi: 10.1097/BRS.0b013e3181d952a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman BW, O'Mahony S, Mulvey L, Davitt M, Choi H, Xia S, et al. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Annals of emergency medicine. 2012;59(2):128–33 e3. doi: 10.1016/j.annemergmed.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Friedman BW, Dym AA, Davitt M, Holden L, Solorzano C, Esses D, et al. Naproxen With Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA. 2015;314(15):1572–80. doi: 10.1001/jama.2015.13043. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, Casey D, Cross JT, Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–91. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hingorani K. Diazepam in backache. A double-blind controlled trial. Ann Phys Med. 1966;8(8):303–6. [PubMed] [Google Scholar]
- 6.van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM Cochrane Back Review G. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976) 2003;28(17):1978–92. doi: 10.1097/01.BRS.0000090503.38830.AD. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Peterson K. Drug Class Review: Skeletal Muscle Relaxants: Final Report. Portland (OR) 2005 [PubMed] [Google Scholar]
- 8.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25(24):3115–24. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75(1):1–17. doi: 10.1016/S0304-3959(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 10.Brotz D, Maschke E, Burkard S, Engel C, Manz C, Ernemann U, et al. Is there a role for benzodiazepines in the management of lumbar disc prolapse with acute sciatica? Pain. 2010;149(3):470–5. doi: 10.1016/j.pain.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Brown BR, Jr, Womble J. Cyclobenzaprine in intractable pain syndromes with muscle spasm. JAMA. 1978;240(11):1151–2. [PubMed] [Google Scholar]
- 12.Basmajian JV. Cyclobenzaprine hydrochloride effect on skeletal muscle spasm in the lumbar region and neck: two double-blind controlled clinical and laboratory studies. Arch Phys Med Rehabil. 1978;59(2):58–63. [PubMed] [Google Scholar]
- 13.Friedman BW, Holden L, Esses D, Bijur PE, Choi HK, Solorzano C, et al. Parenteral corticosteroids for Emergency Department patients with non-radicular low back pain. The Journal of emergency medicine. 2006;31(4):365–70. doi: 10.1016/j.jemermed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Williams CM, Maher CG, Latimer J, McLachlan AJ, Hancock MJ, Day RO, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586–96. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 15.Furlan AD, van Tulder M, Cherkin D, Tsukayama H, Lao L, Koes B, et al. Acupuncture and dry-needling for low back pain: an updated systematic review within the framework of the cochrane collaboration. Spine. 2005;30(8):944–63. doi: 10.1097/01.brs.0000158941.21571.01. [DOI] [PubMed] [Google Scholar]
- 16.Cramer H, Lauche R, Haller H, Dobos G. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29(5):450–60. doi: 10.1097/AJP.0b013e31825e1492. [DOI] [PubMed] [Google Scholar]
- 17.Furlan AD, Imamura M, Dryden T, Irvin E. Massage for low back pain: an updated systematic review within the framework of the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34(16):1669–84. doi: 10.1097/BRS.0b013e3181ad7bd6. [DOI] [PubMed] [Google Scholar]
- 18.Juni P, Battaglia M, Nuesch E, Hammerle G, Eser P, van Beers R, et al. A randomised controlled trial of spinal manipulative therapy in acute low back pain. Ann Rheum Dis. 2009;68(9):1420–7. doi: 10.1136/ard.2008.093757. [DOI] [PubMed] [Google Scholar]
- 19.Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560–71. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman BW, Esses D, Solorzano C, Choi HK, Cole M, Davitt M, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine. 2008;33(18):E624–9. doi: 10.1097/BRS.0b013e3181822711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman BW, Mulvey L, Davitt M, Choi H, Esses D, Bijur PE, et al. Predicting 7-day and 3-month functional outcomes after an ED visit for acute nontraumatic low back pain. Am J Emerg Med. 2012;30(9):1852–9. doi: 10.1016/j.ajem.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.