Abstract
Background
Cortisol is the primary output of the hypothalamic-pituitary-adrenal (HPA) axis and is central to the biological stress response, with wide-ranging effects on psychiatric health. Despite well-studied biological pathways of glucocorticoid function, little attention has been paid to the role of genetic variation. Conventional salivary, urinary, and serum measures are influenced by diurnal variation and transient reactivity. Recently developed technology can be used to measure cortisol accumulation over several months in hair, thus indexing chronic HPA function.
Methods
In a socioeconomically diverse sample of 1,070 twins/multiples (ages 7.80–19.47 years), we estimated effects of sex, age, and socioeconomic status (SES) on hair concentrations of cortisol and its inactive metabolite, cortisone, along with their interactions with genetic and environmental factors. This is the first genetic study of hair neuroendocrine concentrations and the largest twin study of neuroendocrine concentrations in any tissue type.
Results
Glucocorticoid concentrations increased with age for females, but not males. Genetic factors accounted for approximately half of the variation in cortisol and cortisone. Shared environmental effects dissipated over adolescence. Higher SES was related to shallower increases in cortisol with age. SES was unrelated to cortisone, and did not significantly moderate genetic effects on either cortisol or cortisone.
Conclusions
Genetic factors account for sizable proportions of gluccocorticoid variation across the entire age range examined, whereas shared environmental influences are modest, and only apparent at earlier ages. Chronic glucocorticoid output appears to be more consistently related to biological sex, age, and genotype than to experiential factors that cluster within nuclear families.
Keywords: hair hormones, quantitative genetics, HPA axis, cortisol, cortisone, gene-age interaction
Introduction
The biological stress system produces a multifaceted regulatory response to physiological and psychological threats to homeostasis (Stratkis & Chrousos, 1995). Within seconds of stressor onset, the hypothalamic-pituitary-adrenal (HPA) axis releases corticotropin-releasing hormone from the hypothalamus, stimulating the pituitary to release adrenocorticotropic hormone, which in turn stimulates release of glucocorticoids (specifically, cortisol, in humans) from the adrenal cortex. Glucorticoids have wide-ranging effects on physiology, including suppressing immune function and gonadal function, stimulating cardiovascular function, and elevating blood glucose (Chrousos, 1995; Sapolsky et al. 2013). Moreover, glucocorticoids pass into the central nervous system, where there are several neural regions containing high densities of glucocorticoid receptors (Lupien et al. 2009).
As the key end-product of the HPA-axis stress response with highly active effects on brain function, cortisol has become a leading candidate physiological mechanism for the effects of both chronic and acute stress on psychiatric health and psychopathology. HPA dysregulation, as indexed by cortisol concentrations in serum, saliva, or urine, has been linked with chronic stressors and history of major trauma (Miller et al. 2007), and has been concurrently and prospectively associated with a range of psychiatric symptomologies and disorders, including depression, anxiety, and posttraumatic stress disorder (Heim et al. 2008; Lupien et al. 2009; Vrshek-Schallhorn et al. 2012). Animal models that experimentally manipulate the social environment have found that HPA dysregulation is associated with brain atrophy and with suppression of signals for neurogenesis and synapse formation (Meaney, 2003). Moreover, in animal models, administering exogenous glucocorticoids produces similar deleterious effects on neural structure (Lupien et al. 2009).
Overcoming Methodological Challenges in Measuring HPA Function using Hair Sample
That cortisol output follows a pattern of diurnal variation complicates research on the role of chronic HPA function in stress and psychopathology. Diurnal variation typically begins with cortisol levels rising in the early morning, peaking immediately after waking, and declining through the day and evening. Cortisol levels measured in blood and saliva closely track diurnal patterns of output, and urinary levels reflect output over periods of 12–48 hours (Russell et al. 2015). Because of this diurnal variation, single samples of cortisol in bodily fluids confound temporally stable individual differences in basal cortisol levels with intra-individual fluctuations. Researchers, therefore, typically attempt to index chronic levels of HPA function by taking repeated salivary samples across the day and over multiple days (Adam & Kumari, 2009), a costly approach that imposes participant burden, and carries the risk of participant non-compliance and dropout. Although repeated measurements of cortisol across the day are necessary for estimating elements of diurnal rhythm such as the cortisol awakening response or diurnal cortisol decline (Stalder et al. 2016), they only provide an indirect index of long-term average or basal cortisol output.
Recently, researchers have developed methods for the analysis of cortisol accumulations in hair samples collected noninvasively at the time of the laboratory visit (Gao et al. 2015). Cortisol is hypothesized to be incorporated into the hair primarily via passive diffusion from blood capillaries surrounding the hair follicle (Stalder & Kirschbaum, 2012). Hair cortisol captures the accumulation of free cortisol over several months (Russell et al. 2015). Internal consistency estimates as estimated using duplicate sampling are above .90 (Stalder et al. 2012), month long test-retest consistencies are above .80 (Short et al. 2016), and yearlong test-retest consistencies are over .70 (Stalder & Kirschbaum, 2012). Hair cortisol concentrations have been estimated to correspond at over .60 with estimates of total cortisol output from thrice daily sampling of saliva taken over a one month period (Short et al. 2016), but convergent validity is much lower for urinary sampling (Sauvé et al. 2007; Short et al. 2016) and for salivary estimates taken over periods of 3–4 days (Xie et al. 2011), which are currently considered best practices. Hair cortisol concentrations are robust to a number of possible confounds, including natural hair color, oral contraceptive use, smoking, use of everyday hair products, and frequency of hair washes (Dettenborn et al. 2012). Hair cortisol is associated, as expected, with known disrupters of normal HPA functions, including shift work, Cushing syndrome, and posttraumatic stress disorder (Staufenbiel et al. 2013). Some studies have also reported a negative association between hair cortisol and socioeconomic status in children (Vaghri et al. 2013; Rippe et al. 2016; Vliegenthart et al. 2016) and in adults (Serwinski et al. 2016). However, null associations between socioeconomic status and hair cortisol have also been reported (Bosma et al. 2015; Staufenbiel et al. 2015).
Unclear Role of Genetic Variation in HPA Axis Function
Although variation in HPA axis output is most commonly discussed as a biomarker for exposure to environmental stress, genetic variation is also a potential contributor to heterogeneity in HPA axis output (Miller et al. 2007; Stratakis et al. 1997; Lupien et al. 2009). Indeed, the biological pathways from gene sequence to cortisol production, reception, and regulation are well-studied, and polymorphisms in these cortisol-relevant genes may account for heterogeneity in HPA function and cortisol output (Ising & Holsboer, 2006; Redei, 2009; Cole, 2010). However, biomarkers of HPA function are not currently available in samples of genotyped individuals that are sufficiently large for a well-powered genetic association study of quantitative traits (Velders et al. 2011; Bolton et al. 2014).
For many psychiatrically-relevant phenotypes, only a small subset of the specific genetic polymorphisms that constitute genetic risk have been identified, but vast literatures from twin and family designs provide precise, replicable estimates of heritability and genetic covariance with other phenotypes. For HPA axis function, however, even this basic information is lacking, as there has been relatively little research from genetic epidemiology on glucocorticoid output. A 2003 review identified only 12 genetically-informed studies of cortisol (Bartels et al. 2003). These studies were limited by failures to account for the cortisol diurnal rhythm, small sample sizes (no study exceeded 150 twin pairs), and a lack of methodological consistency. There have been a handful of more recent twin studies of salivary cortisol, most notably a study of 700 individuals from 309 twin families (Kupper et al. 2005), and another study of 446 twin pairs (Van Hulle et al. 2012). Overall, heritability estimates of salivary cortisol have been moderate, with the largest heritability estimates found for samples taken at waking (~30–40%), and somewhat lower for estimates taken later in the day (~0–20%). Whether these estimates, which may be downwardly biased by transient fluctuations in cortisol, generalize to overall cortisol output over a period of months is an open question.
Genetic influences on chronic HPA function may vary across subgroups. In animal models, marked sex differences exist in HPA reactivity, in the direction of greater HPA activity in females compared to males. Moreover, sex hormones may modulate HPA function (McCormick & Mathews, 2007; Viau & Meaney, 2013). Low socioeconomic status has also been linked to HPA dysregulation (Dowd et al. 2009), and genetically-influenced heterogeneity in reaction norms to stressful socioeconomic contexts (i.e., a “diathesis-stress” pattern) would be expected to produce a link between low socioeconomic status and increased heritability of cortisol (Monroe & Simons, 1991). Age has been reported to be associated with HPA function, in the direction of greater glucocorticoid output in adolescents compared to children and adults (McCormick & Mathews, 2007). Age may also modulate genetic and environmental influences on HPA activity, because of heterogeneity in exposure and responsivity to long-term stressors over time (Miller et al. 2007). Finally, genetic influence on HPA function may be activated by biological changes associated with puberty, and by stressful challenges associated with navigating social transitions across development. Overall, the extent to which individual differences in chronic HPA axis function reflect genetic differences between people, and the extent to which this genetic influences vary across subgroups, is critical information for researchers attempting to understand the relationships between genotype, chronic environmental stress, and psychiatric outcomes.
Goals of the Current Study
Using data from an ethnically and socioeconomically diverse population-based sample of over 1,000 3rd to 12th grade twins, the current article reports results from a genetic epidemiological study of influences on long-term HPA function, as indexed with endocrine assays of hair (Stalder & Kirschbaum, 2012). We examine both cortisol, the primary active glucocorticoid in humans, and cortisone. In humans, cortisol is metabolized into the inactive cortisone form, which can, in turn, be converted back to active cortisol (Quinkler & Stewart, 2013; Stewart et al. 2013; Rippe et al. 2016). We estimate main effects of biological sex, family socioeconomic status, and age on hair cortisol and cortisone, and interactions effects of these three factors with one another and with latent genetic and environmental components of hair cortisol and cortisone variation and covariation. This is, to our knowledge, the first genetic epidemiological study of hair markers of neuroendocrine hormones, the largest twin study of neuroendocrine concentrations in any tissue type, and among the largest studies of neuroendocrine concentrations in hair to date (see Feller et al. 2014; Rippe et al. 2016; Staufenbiel et al. 2015 for other large-scale studies of hair cortisol in singletons).
Methods and Materials
Twin pairs were recruited from the Texas Twin Project (Harden et al. 2013), an ongoing study of school-age twins and multiples, residing in the Austin and Houston, Texas metropolitan areas. Participants ranged in age from 7.80 to 19.47 years of age (M = 12.42, SD = 2.78). The research was approved by the Institutional Review Boards at the University of Texas at Austin and the University of Houston. Informed parental consent and informed consent/assent was obtained for all participants. Two female participants reported endocrine disorders and were excluded from analyses. The final sample consisted of 1,141 individuals forming 607 pairs from 556 unique families. In order for a pair to be included in the current analyses, at least one member must have provided a usable hair sample. Of the 1,141 individuals in the sample, 1,070 individuals provided usable hair samples for cortisol and cortisone assay. Of the 607 pairs included, there were 533 pairs in which both members provided usable hair samples. Two families had two sets of twins, one family had quadruplets who contributed six pairwise contributions, 21 families had triplets that contributed three pairwise comparisons, and two families had triplets where only one triplet provided hair resulting in two pairwise combinations. The final sample consisted of 188 monozygotic (MZ) pairs (110 female, 78 male) and 419 dizygotic (DZ) pairs (114 female, 82 male, 223 opposite-sex). Sixty-five percent (65%) of the sample was non-Hispanic white, 5% of participants were African American, 18% of participants were Hispanic, and 12% of participants were another race/ethnicity or multiple race/ethnicities. Of the participating families, 34% reported receiving some form of means-tested public assistance, including food stamps, since the twins’ birth.
As this is an ongoing study, sample sizes increase annually. We decided to conduct the current analyses at this point in time because, at a sample size of over 600 pairs, we were positioned to publish the largest genetic epidemiological study of hormone concentrations in any tissue type. An a priori power analysis indicated that, with 600 pairs, a bivariate ACE model could detect genetically-, shared environmentally-, and nonshared environmentally-mediated correlations of r = .225 at α< .05, with 85% or greater power.
Measures
Zygosity
Opposite-sex pairs were classified as DZ. For same-sex pairs, zygosity was assessed using a questionnaire concerning the twin’s physical similarities (e.g., facial appearance) and the frequency that they are mistaken for one another (Rietveld et al. 2000). Twins over 14 years old completed the zygosity questionnaire, and at least one parent and two research assistants completed the questionnaire for all twin pairs. Responses from all raters were entered into a latent class analysis (LCA) to obtain the above classifications. LCA of physical similarity ratings has been reported to accurately determine zygosity greater than 99% of the time, as validated by genotyping (Heath et al. 2003).
Hair Steroid Analyses
Hair samples were collected to determine cortisol and cortisone concentrations. On the day of the appointment, participants were instructed not to use any hair products that are not rinsed out of the hair. Samples were only collected if the participants’ hair was at least 3 cm in length. A section of hair strands approximately 3 mm in diameter was cut as close to the scalp as possible from a posterior vertex position (i.e., the center of the back of the head). Samples were analyzed at the laboratory of one of the authors (CK) using liquid chromatography-tandem mass spectrometry, as previously described (Gao et al. 2015). The 3 cm hair segment closest to the scalp was used for analyses. This hair segment is taken to represent cortisol and cortisone secretion over the most recent 3-month period (Stalder & Kirschbaum, 2012).
Socioeconomic Status (SES)
Years of parental education were averaged together and standardized; log-income was standardized; and the transformed education and income variables were averaged and standardized to create an SES composite. SES composites were available for 1,016 of the 1,070 participants who provided usable hair samples.
Data Preparation
Age was centered at 8 years to reflect its lowest observed integer value, sex was effects coded (female = −.5, male = .5), and SES was standardized. To correct positive skew, log and square root transformations were applied to cortisol and cortisone, respectively. Outliers were separately winsorized for males and females by replacing extreme values with the highest observed scores within 3 SDs of the mean. This involved replacing 19 female and 8 male outliers for cortisol, and 10 female and 11 male outliers for cortisone. As assays are performed annually, outcomes were residualized for the year the hair samples were assayed (treated as a nominal variable) to control for batch effects. Finally, the transformed winsorized cortisol and cortisone values were standardized relative to the respective standard deviations of their residuals from regressions of cortisol and cortisone on age and age-squared.
Model Estimation
Models were estimated with full information maximum likelihood using Mplus (Muthén & Muthén, 1998). For descriptive statistics, phenotypic models were fit using the complex survey option to correct standard errors for nesting of individuals within families. For the biometric models, the complex survey option was used to correct standard errors for the dependency between sibling pairs within triplet and quadruplet sets. Nested models were compared using Satorra-Bentler scaled chi-square difference tests (Satorra, 2000). Models were also compared using the Akaike Information Criterion (AIC; Akaike, 1974) and the Bayesian Information Criterion (BIC).
Results
Descriptive Statistics
Variable means, phenotypic correlations, and cross-twin correlations are reported in Table 1. Results from a series of step-wise regressions in which age, SES, sex, and their interactions were used to predict cortisol and cortisone are presented in Table 2. Main effects were initially examined in isolation, followed by two-way interactions and the three-way interaction (age × sex × SES). As quadratic effects of age were not significant for either cortisone or cortisol in either the sex-pooled data or sex-specific analyses, age2 was not included in the stepwise regression models reported here. For cortisol and cortisone, there were significant effects of age and an age × sex interaction. Females had lower levels of cortisol and cortisone relative to males at age 8, but female hormone levels were more strongly related to age such that concentrations were slightly higher in females than in males by age 18 (Figure 1). A regression model that included only SES indicated it was not associated with cortisol or cortisone. A regression model that included an age × SES interaction indicated that SES moderated age-gradients in cortisol, but not cortisone. Individuals in low SES environments had lower levels of cortisol at age 8, but the effect of age on increasing cortisol was heightened at low levels of SES such that cortisol was highest in the low SES group by age 18 (Figure 1). A three-way age × sex × SES interaction was not significant.
Table 1.
Descriptive statistics, phenotypic correlations, and cross-twin correlations
| Males | Females | ||||
|---|---|---|---|---|---|
|
| |||||
| Means (+/− 1 SD range) | |||||
| cortisol (pg/mg) | 3.39 (1.08, 10.69) | 2.95 (0.92, 9.44) | |||
| cortisone (pg/mg) | 6.50 (2.28, 12.90) | 5.21 (1.42, 11.40) | |||
| Age (years) | 12.08 (9.44, 14.72) | 12.65 (9.80, 15.48) | |||
|
| |||||
| Phenotypic Correlations (95% CI’s) | |||||
|
| |||||
| cortisol-cortisone | .54 (.44, .65) | .57 (.48, .65) | |||
| SES-cortisol | .02 (−.08, .12) | .04 (−.04, .12) | |||
| SES-cortisone | −.06 (−.15, .03) | .00 (−.07, .08) | |||
| age-cortisol | .01 (−.06, .08) | .23 (.15, .30) | |||
| age-cortisone | .06 (−.01, .14) | .30 (.23, .36) | |||
|
| |||||
| Cross-Twin Correlations (95% CI’s) | |||||
|
| |||||
| MZ | DZ | MZ | DZ | DZ Opposite-Sex | |
|
| |||||
| cortisol-cortisol | .74 (.55, .93) | .53 (.34, .72) | .58 (.44, .73) | .34 (.17, .51) | .26 (.12, .41) |
| cortisone-cortisone | .56 (.40, .72) | .47 (.33, .62) | .49 (.35, .64) | .36 (.20, .53) | .22 (.04, .40) |
| cortisol-cortisone | .44 (.28, .60) | .21 (.06, .37) | .22 (.10, .34) | .32 (.19, .45) | .06 (−.05, .17) |
Note. Sample means and +/−1 SD range were computed after cortisol and cortisone were square root and log transformed, respectively, and winsorized. We then exponentiated (cortisol) or squared (cortisone) the calculated values in order to return them to their original metric. Cross-twin and phenotypic correlations were estimated from models that controlled for sex-specific linear effects of age. For cross-twin and phenotypic correlations cortisone and cortisol were square root and log transformed, respectively, and winsorized.
Table 2.
Results from series of stepwise regressions predicting cortisol and cortisone
| Cortisol
|
Cortisone
|
|||||
|---|---|---|---|---|---|---|
| b | SE | p-value | b | SE | p-value | |
| 1. Age | 0.05 | 0.01 | < .001 | 0.07 | 0.01 | < .001 |
|
| ||||||
| 2. Sex | 0.12 | 0.07 | .094 | 0.25 | 0.07 | < .001 |
|
| ||||||
| 3. SES | 0.02 | 0.04 | .626 | −0.04 | 0.04 | .300 |
|
| ||||||
| 4. Age | 0.04 | 0.01 | .002 | 0.07 | 0.01 | < .001 |
| 4. Sex | 0.47 | 0.14 | .001 | 0.64 | 0.13 | < .001 |
| 4. Age × Sex | −0.08 | 0.03 | .002 | −0.08 | 0.03 | .001 |
|
| ||||||
| 5. Age | 0.05 | 0.01 | < .001 | 0.07 | 0.01 | < .001 |
| 5. SES | 0.17 | 0.09 | .043 | −0.01 | 0.07 | .933 |
| 5. Age × SES | −0.03 | 0.02 | .040 | −0.01 | 0.01 | .644 |
|
| ||||||
| 6. Sex | 0.15 | 0.07 | .028 | 0.26 | 0.07 | < .001 |
| 6. SES | 0.02 | 0.04 | .570 | −0.04 | 0.04 | .347 |
| 6. Sex × SES | −0.01 | 0.08 | .970 | −0.04 | 0.07 | .578 |
|
| ||||||
| 7. Age | 0.05 | 0.01 | .001 | 0.07 | 0.01 | < .001 |
| 7. SES | 0.19 | 0.08 | .015 | 0.03 | 0.07 | .668 |
| 7. Sex | 0.51 | 0.14 | < .001 | 0.60 | 0.13 | < .001 |
| 7. Age × SES | −0.04 | 0.02 | .017 | −0.01 | 0.01 | .343 |
| 7. Age × Sex | −0.07 | 0.03 | .005 | −0.07 | 0.03 | .006 |
| 7. Sex × SES | −0.05 | 0.08 | .542 | −0.07 | 0.07 | .331 |
|
| ||||||
| 8. Age | 0.05 | 0.01 | < .001 | 0.07 | 0.01 | < .001 |
| 8. SES | 0.19 | 0.08 | .019 | 0.03 | 0.07 | .671 |
| 8. Sex | 0.52 | 0.14 | < .001 | 0.60 | 0.13 | < .001 |
| 8. Age × SES | −0.04 | 0.02 | .029 | −0.01 | 0.01 | .357 |
| 8. Age × Sex | −0.08 | 0.03 | .003 | −0.07 | 0.03 | .007 |
| 8. Sex × SES | −0.16 | 0.16 | .296 | −0.07 | 0.14 | .617 |
| 8. Age × SES × Sex | 0.03 | 0.03 | .354 | < 0.01 | 0.03 | .996 |
Note. Numbering in the left-hand column indicates predictors that were included in the same regression model. Age was centered about the lowest observed integer value of 8 years. Cortisol and cortisone were log and square root transformed, respectively, and winsorized. SES, cortisol and cortisone were standardized to have a mean of 0 and SD of 1. Sex was effects coded (female = −.5, male = .5). p-value = two-tailed probability of type-I error.
Figure 1.
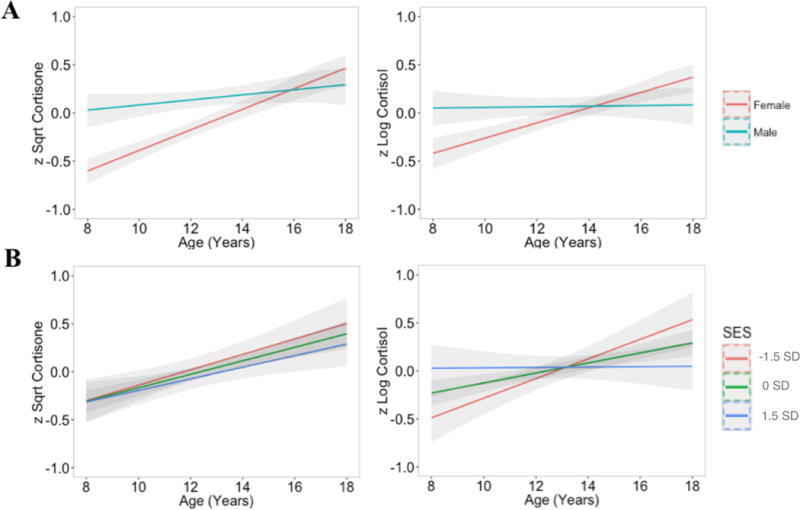
Moderated age trends in mean levels of cortisone and cortisol by sex (row A) and standardized SES at low (−1.5 SD), average (0 SD), and high (+1.5 SD) levels. (row B). As described in the analyses section, cortisone and cortisol were square root and log transformed, respectively, winsorized and standardized. Gray bands represent 95% confidence intervals.
Race/ethnicity differences in hormone levels were tested by entering three dummy-coded variables into a linear regression with Caucasian participants as the reference group. There was a significant effect of African-American race on higher cortisol (d =.78, SE = .24, p = .002), but not for Hispanic ethnicity (d = −.02, SE = .10, p = .89) or for the Other/multiple race/ethnicity variable (d = −.05, SE = .12, p = .65). There were significant effects of Hispanic ethnicity (d =.22, SE = .10, p = .04) and Other/multiple race/ethnicity (d =.25, SE = .11, p = .03) on higher cortisone, but not of African-American race (d =.24, SE = .19, p = .22).
Moderated Biometric Models
We estimated a series of three-group (MZ, same-sex DZ, opposite-sex DZ) bivariate correlated biometric factors models (Figure S1) to estimate additive genetic (A), shared environmental (C), and non-shared environmental (E) variance components (Figure S1). Within a phenotype, the A factor is fixed to be correlated at 1.0 and 0.5 in MZ and same sex DZ twins, respectively. The C factor is, by definition, fixed to correlate at 1.0 within a phenotype in all same-sex twin pairs. As E factor captures all variance not shared between MZ twins, including error variance, they are not correlated within phenotypes. The acn, ccn, ecn and ac, cc, ec coefficients are regression effects of A, C, and E influences on cortisone and cortisol respectively. The ra, rc, and re parameters represent correlations between the A, C, E variance components of cortisol with those of cortisone. Genetic, shared environmental, and nonshared environmental covariances are calculated as acn × ra × ac, ccn × rc × cc, and ecn × re × ec, respectively.
We report results of both parametric moderation models and nonparametric moderation models. Parametric moderation models (Purcell, 2002) allow for sets of the acn, ccn, ecn, ac, cc, ec, and ra, rc, and re parameters to vary as functions of the moderator tested in the form p = p0 + p1*m, where p is the parameter and m is the moderator. Nonparametric (LOSEM) models provide locally weighted estimates of model parameters along a continuous moderator, such as age (Briley et al. 2015). This is achieved LOSEM using a weighting kernel and bandwidth that gives observations in closer proximity to the focal value of the moderator greater weight. Multiple structural equation models are estimated that differ only with respect to the assigned focal value of the moderator (ranging from 8 to 18 years for age and −2SD to +2SD for SES, both at intervals of .10). All biometric models included main effects of race (Caucasian coded as reference group. Below we report separate moderation models for sex, age and SES. However, results were highly similar when all three moderators were simultaneously considered.
Moderation by Sex
The initial bivariate model allowed for quantitative, qualitative, and sex-specific mean age differences. Model fit was examined by sequentially removing: (i) qualitative sex differences in which the within- and across-phenotype cross-twin genetic correlations are freely estimated for opposite-sex pairs, (ii) quantitative sex differences, in which the genetic and environmental effects on each phenotype are allowed to differ by sex and (iii) sex-specific age trends in mean levels of each phenotype. Fit indices and parameter estimates are summarized in Table S1. There was no evidence of qualitative sex differences in cortisol or cortisone, but there was evidence for quantitative sex differences in cortisone. Table 3 reports unstandardized parameter estimates from the model that included quantitative sex differences in cortisone. Across males and females, 65% of the total variability in cortisol was explained by additive genetic effects. For cortisone, additive genetic and non-shared environmental effects both explained large portions of variability for males (h2 = 44% and e2 = 35%) and females (h2 = 47% and e2 = 47%). Shared environmental effects accounted for 21% of the variance in cortisone in males, but only 6% in females.
Table 3.
Unstandardized Parameter Estimates from Sex, Age, and SES Moderation Models.
| Parameter
|
Preferred Sex Moderation Model
|
Preferred Age Moderation Model
|
Preferred SES Model (No Moderation) |
Full SES Moderation
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variance in Cortisone | Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value |
| Main A effect (ACN0) | .68 | (.49, .86) | < .001 | .42 | (.09, .74) | .04 | .63 | (.41, .84) | < .001 | .70 | (.55, .86) | < .001 |
| A × Sex Interaction (ACN1) | −.05 | (−.25, .14) | .64 | – | – | – | – | – | – | – | – | – |
| A × Age Interaction (ACN1) | – | – | – | .03 | (−.14, .08) | .25 | – | – | – | – | – | – |
| A × SES Interaction (ACN1) | – | – | – | – | – | – | – | – | – | −.13 | (−.24, −.01) | .08 |
| Main C effect (CCN0) | .35 | (.04, .65) | .06 | .67 | (.42, .92) | < .001 | .41 | (.15, .67) | .009 | .26 | (−.05, .58) | .17 |
| C × Sex Interaction (CCN1) | .19 | (.01, .37) | .08 | – | – | – | – | – | – | – | – | – |
| C × Age Interaction (CCN1) | – | – | – | −.05 | (−.10, .01) | .14 | – | – | – | – | – | – |
| C × SES Interaction (CCN1) | – | – | – | – | – | – | – | – | – | .12 | (.03, .20) | .02 |
| Main E effect (ECN0) | .64 | (.57, .71) | < .001 | .57 | (.46, .69) | < .001 | .63 | (.55, .71) | < .001 | .61 | (.54, .67) | < .001 |
| E × Sex Interaction (ECN1) | −.13 | (−.25, −.01) | .07 | – | – | – | – | – | – | – | – | – |
| E × Age Interaction (ECN1) | – | – | – | .02 | (−.01, .05) | .13 | – | – | – | – | – | – |
| E × SES Interaction (ECN1) | – | – | – | – | – | – | – | – | – | .04 | (−.03, .10) | .37 |
| Main Sex effect | .63 | (.43, .83) | < .001 | .62 | (.42, .81) | < .001 | .55 | (.36, .75) | < .001 | .56 | (.36, .77) | < .001 |
| Main Age effect | .08 | (.06, .10) | < .001 | .08 | (.06, .10) | < .001 | .08 | (.06, .10) | < .001 | .08 | (.05, 10) | < .001 |
| Age × Sex Interaction | −.06 | (−.10, −.01) | .04 | −.06 | (−.10, −.01) | .03 | −.04 | (−.08, < .01) | .14 | −.04 | (−.08, < .01) | .11 |
| Main SES effect | – | – | – | – | – | – | −.01 | (−.07, .06) | .84 | −.01 | (−.07, .06) | .92 |
| Race (Hispanic) | .31 | (.13, .49) | .01 | .30 | (.11, .48) | .01 | .21 | (.03, .40) | .06 | .21 | (.02, .40) | .06 |
| Race (African American) | .33 | (.02, .65) | .08 | .33 | (.02, .65) | .08 | .26 | (−.05, .58) | .17 | .28 | (−.04, .61) | .15 |
| Race (Other) | .11 | (−.08, .30) | .34 | .10 | (−.09, .29) | .40 | .09 | (−.10, .28) | .42 | .10 | (−.09, .28) | .40 |
|
| ||||||||||||
| Variance in Cortisol | ||||||||||||
|
| ||||||||||||
| Main A effect (AC0) | .79 | (.67, .91) | < .001 | .79 | (.50, 1.07) | < .001 | .72 | (.55, .89) | < .001 | .75 | (.65, .84) | < .001 |
| A × Age Interaction (AC1) | – | – | – | −.02 | (−.07, .03) | .49 | – | – | – | – | – | – |
| A × SES Interaction (AC1) | – | – | – | – | – | – | – | – | – | −.03 | (−.17, .12) | .77 |
| Main C effect (CC0) | −.06 | (−1.15, 1.03) | .93 | .69 | (.38, .99) | < .001 | .32 | (.03, .60) | .07 | .09 | (−.19, .37) | .59 |
| C × Age Interaction (CC1) | – | – | – | −.13 | (−.17, −.05) | < .001 | – | – | – | – | – | – |
| C × SES Interaction (CC1) | – | – | – | – | – | – | – | – | – | .24 | (.10, .38) | .004 |
| Main E effect (EC0) | .58 | (.52, .65) | < .001 | .69 | (.56, .82) | < .001 | .58 | (.51, .65) | < .001 | .57 | (.51, .64) | < .001 |
| E × Age Interaction (EC1) | – | – | – | −.02 | (−.04, .01) | 0.16 | – | – | – | – | – | – |
| E × SES Interaction (EC1) | – | – | – | – | – | – | – | – | – | −.02 | (−.10, .06) | .66 |
| Main Sex effect | .57 | (.35, .79) | < .001 | .54 | (.33, .75) | < .001 | .55 | (.36, .75) | < .001 | .54 | (.33, .76) | < .001 |
| Main Age effect | .05 | (.03, .07) | < .001 | .05 | (.03, .07) | < .001 | .05 | (.02, .07) | .001 | .05 | (.02, .07) | < .001 |
| Age × Sex Interaction | −.07 | (−.11, −.02) | .009 | −.06 | (−.10, −.02) | .01 | −.05 | (−.09, −.02) | .02 | −.05 | (−.09, −.01) | .03 |
| Main SES effect | – | – | – | – | – | – | .04 | (−.03, .10) | .38 | .02 | (−.04, .09) | .58 |
| Race (Hispanic) | .02 | (−.13, .18) | .83 | .04 | (−.12, .19) | .70 | .02 | (−.14, .17) | .86 | .01 | (−.14, .17) | .90 |
| Race (African American) | .81 | (.44, 1.19) | < .001 | .83 | (.47, 1.12) | < .001 | .66 | (.26, 1.06) | .01 | .68 | (.28, 1.08) | .01 |
| Race (Other) | −.08 | (−.29, .13) | .51 | −.08 | (−.27, .11) | .50 | −.10 | (−.32, .11) | .43 | −.09 | (−.31, .13) | .50 |
|
| ||||||||||||
| Cortisone with Cortisol | ||||||||||||
|
| ||||||||||||
| Main A Correlation (ra0) | .78 | (.55, .99) | < .001 | .74 | (.08, 1.40) | .07 | .73 | (.45, 1.01) | < .001 | .65 | (.44, .87) | < .001 |
| rA × Age Interaction (ra1) | – | – | – | < .01 | (−.10, .11) | .96 | – | – | – | – | – | – |
| rA × SES Interaction (ra1) | – | – | – | – | – | – | – | – | – | −.03 | (−.38, .32) | .89 |
| Main C Correlation (rc0) | 2.76 | (−50.26, 55.77) | .93 | .58 | (.09, 1.06) | .05 | .23 | (−.65, 1.11) | .67 | 1.33 | (.01, 2.64) | .10 |
| rC × Age Interaction (rc1) | – | – | – | −.10 | (−.27, .08) | .35 | – | – | – | – | – | – |
| rC × SES Interaction rc1) | – | – | – | – | – | – | – | – | – | −.99 | (−2.17, .19) | .17 |
| Main E Correlation (re0) | .42 | (.30, .54) | < .001 | .19 | (−.03, .40) | .15 | .40 | (.26, .53) | < .001 | .41 | (.29, .54) | < .001 |
| rE × Age Interaction (re1) | – | – | – | .06 | (.03, .09) | .00 | – | – | – | – | – | – |
| rE × SES Interaction (re1) | – | – | – | – | – | – | – | – | – | <.01 | (−.17, .16) | .99 |
Note. 95% CI = 95% confidence interval. p-value = two-tailed probability of Type-I error. A = additive genetic; C = shared environment; E = non-shared environment. Results are presented for preferred (based on model comparisons) age and sex moderation models. Results are presented for the preferred age, sex, and SES moderation models based on model comparisons. The preferred sex model allowed for moderation of only cortisone ACE estimates, while the preferred age moderation model estimated age moderation for cortisol, cortisone, and the ACE correlations. The preferred SES model did not allow for moderation by SES of any parameters; however, results are also provided for a model that allowed SES to moderate cortisol, cortisone, and the ACE correlations. Sex was effects coded (female = −.5, male = .5) such that the main effects parameters of A, C, and E represent population-mean effects (assuming an equal sex distribution in the population) and interaction effects of sex by A, C, and E represent the sex difference in the corresponding parameter value. Age was centered at 8 years of age to reflect the lowest observed integer value in the sample. Thus the main effects parameters of A, C, and E represent model-implied biometric effects at age 8 years, and interaction effects of age by A, C, and E represent the difference in the corresponding parameter value for each additional year of age.
Moderation by Age
Model fits for the parametric age moderation models are reported in the top portion of Table S2. The full age moderation model was the best fitting model. Unstandardized parameter estimates for this model are reported in Table 3; see Figure S2 for age trends in model-implied variance accounted for by genetic and environmental factors. For cortisol, non-shared environmental influences were relatively constant across the age range, whereas additive genetic effects gradually decreased with age. Shared environmental influences on cortisol decreased through age 14, at which point they fixated at zero.
Although parametric results seemed to indicate a re-emergence of shared environment influences in the late teenage years, nonparametric results indicated no such re-emergence (Figure 2). For cortisone, the additive genetic factor explained increasing variability with age, and shared environmental factor explained decreasing variability with age that were estimated near 0 by age 18. The effect of non-shared environment on cortisone was moderate and relatively stable across the age range. Across the age range, the association between cortisol and cortisone was approximately 50% attributable to shared genetic etiology. The remaining cortisol-cortisone covariation shifted from being shared environmentally mediated in the younger, preadolescent years, to being non-shared environmentally mediated in the older, adolescent years.
Figure 2.
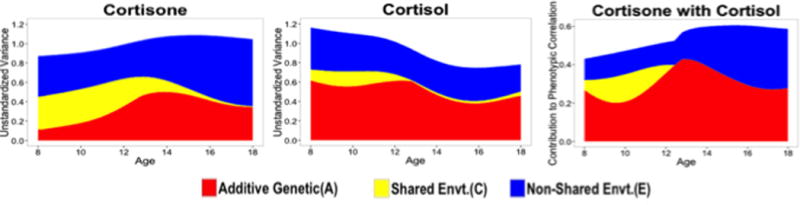
Model-implied age trends in additive genetic (A), shared environmental (C), and nonshared environmental (E) contributions to variance in cortisol, cortisone and their bivariate association using nonparametric LOSEM.
As phenotypic analyses indicated sex differences in age trends in cortisone and cortisol, we also fit parametric moderation models that included sex differences in these age moderation effects. This three-way interaction, however, was not significant.
Moderation by SES
Model fits for parametric SES moderation models are reported in the bottom portion of Table S2. Removing moderation by SES of ACE influences on cortisol, cortisone, or cross-trait ACE correlation estimates each did not significantly decrease model fit relative to the baseline model, nor did removing all moderation by SES. Inspection of individual parameter estimates in the full moderation model (Table 3) revealed significant positive moderation of shared environmental effects by SES for both cortisol and cortisone (Figure S3) and nonsignificant negative moderation of genetic effects by SES. Results were very similar when a phenotypic age × SES interaction was also included in the model. Similarly, nonparametric analyses indicated a trend of heightened genetic influence and reduced shared environmental influences on cortisol and cortisone at lower SES (Figure S3). This is consistent with a diathesis-stress hypothesis, which predicts that genetic influences are stronger under higher stress conditions. However, post-hoc multiple-group tests of these differences indicated that they were not statistically significant (Table S3). Even larger sample sizes than those implemented here may be necessary in order to test definitively for SES moderation.
Discussion
In a socioeconomically diverse sample of over 1,000 7 through 19-year-old twins, we estimated genetic and environmental contributions to child and adolescent hair cortisol and cortisone, allowing for moderation by age, sex, and family socioeconomic status. We found moderate genetic influences on both hormones, with some indication of stronger shared environmental influences on male cortisone than on female cortisone. Shared environmental influences on both cortisol and cortisone dissipated with age. We found that SES was positively related to cortisol under approximately 13 years of age, whereas SES was negatively related to cortisol at over ~13 years of age. SES was unrelated to cortisone. The mechanisms underlying this SES × age pattern on cortisol are unknown. However, one possibility is that patterns of cortisol output change with age as long-term stress accumulates over years. It is also possible that adolescence-related increases in cortisol output are more pronounced for lower SES individuals as the result of more dramatic changes in socioecological stress during that transition period. We also found marked sex differences in age trends. At age 8 years, females evince lower average levels of both hair cortisol and cortisone than males, but females increase in glucocorticoid concentrations over the course of adolescence more rapidly than males, such that by age 18 years females have slightly higher mean levels of both hormones. Previous large-scale studies of hair cortisol and cortisone have either focused on adults (Feller et al. 2014; Staufenbiel et al. 2015) or on six year olds (Rippe et al. 2016), and have therefore been unable to examine age trends in chronic HPA function. The sex differences in age trends described here may provide one plausible biological mechanism for the escalation of internalizing psychopathology in females over the course of adolescence (Hankin et al. 1998; Natsuaki et al. 2009).
One previous large-scale study reported a correlation of .55 between hair cortisol and cortisone in adults (Feller et al. 2014), which is very similar to the values that we report in the current child and adolescent sample (.51 in both males and females). Biometric decompositions indicated that approximately half of this correlation is attributable to shared genetic etiology. The source of the remaining, environmentally-driven covariation between cortisol-cortisone changed dynamically with age, shifting from family-level (shared) influences prior to ~age 10 years, to twin-specific (non-shared) environments thereafter.
It is useful to consider possible mechanisms for genetic influence on HPA activity. Most obviously, polymorphisms in the genes involved in glucocorticoid synthesis, release, and metabolism may be related to homeostatic levels of circulating cortisol. However, although cortisol and cortisone are molecular phenotypes, the pathways between genotype and hormonal concentrations may be less direct. Genetic differences between people also shape the likelihood that they will experience stressful events. For example, neighborhood quality, life events, and relationship disruption have all been shown to be heritable, i.e., systematically associated with genetically-influenced individual differences (Jocklin et al. 1996; Bemmels et al. 2012; Sariaslan et al. 2016). Additionally, genetic variation in other biological pathways, not directly involved in glucocorticoid metabolism, may shape psychological factors that, in turn, influence how stressful events are interpreted and coped with. Therefore, genetic influences on phenotypes measured “under the skin” may nevertheless be translated via environmental, “outside the skin” pathways (Kendler et al. 2012). Finally, HPA axis changes may be an outcome of disease processes, such as major depression (Frodl & O’Keane, 2013).
Limitations
The current study presents the first rigorous examination of age trends in, sex differences in, SES differences in, and genetic and environmental effects on cortisol and cortisone over middle childhood and adolescence. Nevertheless, there are several limitations that must be acknowledged. First, although hair cortisol has substantial benefits relative to more conventional technologies with respect to indexing long-term HPA function, we have only recently started to measure diurnal glucocorticoid output in saliva alongside long-term HPA function in hair in this sample. Indeed, it is possible that SES affects HPA function by way of specific components of diurnal variation (Desantis et al. 2015). Additionally, while the broad child-adolescent age range was valuable for studying age-related differences in HPA function, it is less well suited for obtaining precise estimates of associations within a narrow age group. Moreover, although this is the largest twin study of hormones to date, we found substantial complexity in associations involving age, sex, and SES. Even larger samples may be necessary to obtain precise conditional estimates of genetic and environmental effects within subgroups.
Conclusion
In conclusion, this study is the first to estimate the magnitude of genetic influences on long-term glucocorticoid output and to examine how genetic influences differ with age, sex, and socioeconomic status. Further research spanning levels of measurement and explanation will be needed to understand the mechanisms of these genetic influences.
Supplementary Material
Acknowledgments
Financial Support
This research was supported by the National Institutes of Health grants R01HD083613, R21HD081437, and and R21AA023322. LEE was supported by a National Science Foundation Graduate Research Fellowship. The Population Research Center at the University of Texas at Austin is supported by NIH grant R24HD042849
Footnotes
Conflict of Interest
The authors do not report any biomedical financial interests or potential conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bemmels HR, Burt SA, Legrand LN, Iacono WG, McGue M. The heritability of life events: An adolescent twin and adoption study. Twin Research and Human Genetics. 2008;11:257–265. doi: 10.1375/twin.11.3.257. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, Anderson A, Huffman J, Wilson JF, Campbell H, Rudan I. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genetics. 2014;10:e1004474. doi: 10.1371/journal.pgen.1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H, Golsteyn B, Groffen D, Schils T, Stalder T, Syurina E, Borghans L, Feron F. The socioeconomic patterning of perceived stress and hair cortisol in Dutch 10–12 year olds. Journal of Public Health and Epidemiology. 2015;4:195–197. [Google Scholar]
- Briley DA, Harden KP, Bates TC, Tucker-Drob EM. Nonparametric estimates of gene × environment interaction using local structural equation modeling. Behavior Genetics. 2015;45:581–596. doi: 10.1007/s10519-015-9732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress: Basic mechanisms and clinical implications. New York Academy of Sciences; 1995. [Google Scholar]
- Cole SW. Elevating the perspective on human stress genomics. Psychoneuroendocrinology. 2010;35:955–962. doi: 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis AS, Kuzawa CW, Adam EK. Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. American Journal of Human Biology. 2015;27:458–467. doi: 10.1002/ajhb.22668. [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. Introducing a novel method to assess cumulative steroid concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 2012;15:348–353. doi: 10.3109/10253890.2011.619239. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. International Journal of Epidemiology. 2009;39:1–13. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132–140. doi: 10.1016/j.psyneuen.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of Disease. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Gao W, Kirschbaum C, Grass J, Stalder T. LC–MS based analysis of endogenous steroid hormones in human hair. The Journal of Steroid Biochemistry and Molecular Biology. 2015;162:92–99. doi: 10.1016/j.jsbmb.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM, Tackett JL. The Texas Twin Project. Twin Research and Human Genetics. 2013;16:385–390. doi: 10.1017/thg.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Nyholt DR, Neuman R, Madden PA, Bucholz KK, Todd RD, Nelson EC, Montgomery GW, Martin NG. Zygosity diagnosis in the absence of genotypic data: an approach using latent class analysis. Twin Research. 2003;6:22–26. doi: 10.1375/136905203762687861. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Ising M, Holsboer F. Genetics of stress response and stress-related disorders. Dialogues in Clinical Neuroscience. 2006;8:433–444. doi: 10.31887/DCNS.2006.8.4/mising. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocklin V, McGue M, Lykken DT. Personality and divorce: a genetic analysis. Journal of Personality and Social Psychology. 1996;71:288–299. doi: 10.1037//0022-3514.71.2.288. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15:181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology Biochemistry and Behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2003;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus: The comprehensive modeling program for applied researchers. Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child and Adolescent Psychology. 2009;38:513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene–environment interaction in twin analysis. Twin research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. The Journal of Clinical Endocrinology and Metabolism. 2013;88:2384–2392. doi: 10.1210/jc.2003-030138. [DOI] [PubMed] [Google Scholar]
- Redei EE. Molecular genetics of the stress‐responsive adrenocortical axis. Annals of Medicine. 2009;40:139–148. doi: 10.1080/07853890701724863. [DOI] [PubMed] [Google Scholar]
- Rietveld MJH, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Research. 2000;3:134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Rippe RC, Noppe G, Windhorst DA, Tiemeier H, van Rossum EF, Jaddoe VW, Verhulst FC, Bakermans-Kranenburg MJ, van Ijendoorn MH, van den Akker EL. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology. 2016;66:56–64. doi: 10.1016/j.psyneuen.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Russell E, Kirschbaum C, Laudenslager ML, Stalder T, de Rijke Y, van Rossum EF, van Uum S, Koren G. Toward standardization of hair cortisol measurement: results of the first international interlaboratory round robin. Therapeutic Drug Monitoring. 2015;37:71–75. doi: 10.1097/FTD.0000000000000148. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sariaslan A, Fazel S, D’onofrio BM, Långström N, Larsson H, Bergen SE, Kuja-Halkola R, Lichtenstein P. Schizophrenia and subsequent neighborhood deprivation: revisiting the social drift hypothesis using population, twin and molecular genetic data. Translational Psychiatry. 2016;6:e796. doi: 10.1038/tp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A. Innovations in multivariate statistical analysis. Springer US; 2000. Scaled and adjusted restricted tests in multi-sample analysis of moment structures; pp. 233–247. [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human Hair as a biomarker of systemic exposure. Clinical & Investigative Medicine. 2007;30:183–191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Serwinski B, Salavecz G, Kirschbaum C, Steptoe A. Associations between hair cortisol concentration, income, income dynamics and status incongruity in healthy middle-aged women. Psychoneuroendocrinology. 2016;67:182–188. doi: 10.1016/j.psyneuen.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, Wadhwa PD, Buss C. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12–18. doi: 10.1016/j.psyneuen.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C. Analysis of cortisol in hair – State of the art and future directions. Brain. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockrya S, Smyth N, Evans P, Hellhammer DH, Miller R. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2012;37:602–610. doi: 10.1016/j.psyneuen.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Andela CD, Manenschijn L, Pereira AM, van Rossum EF, Biermasz NR. Increased hair cortisol concentrations and BMI in patients with pituitary-adrenal disease on hydrocortisone replacement. The Journal of Clinical Endocrinology & Metabolism. 2015;100:2456–2462. doi: 10.1210/jc.2014-4328. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BWJH, Spijker AT, Elzinga BM, van Rossum EFC. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone→ cortisol conversion in subjects with central adiposity. The Journal of Clinical Endocrinology & Metabolism. 1999;84:1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals of the New York Academy of Sciences. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Sarlis NJ, Berrettini WH, Badner JA, Chrousos GP, Gershon ES, Detera-Wadleigh SD. Lack of linkage between the corticotropin-releasing hormone (CRH) gene and bipolar affective disorder. Molecular Psychiatry. 1997;2:483–485. doi: 10.1038/sj.mp.4000268. [DOI] [PubMed] [Google Scholar]
- Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, Hertzman C. Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology. 2013;38:331–340. doi: 10.1016/j.psyneuen.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior. 2012;62:36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, Van Duijn CM. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, van Rossum EFC, Koper JW, Raat H, van den Akker ELT. Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. 2016;65:9–14. doi: 10.1016/j.psyneuen.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine. 2012;43:483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, Lu Z. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clinical Chemistry and Laboratory Medicine. 2011;49:2013–2019. doi: 10.1515/CCLM.2011.706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


