Abstract
In animal models of heart failure (HF), myocardial metabolism shifts from the normal preference for high-energy fatty acid (FA) metabolism towards the more efficient fuel, glucose. However, FA (vs. glucose) metabolism generates more ATP/mole; thus FA metabolism may be especially advantageous in HF. Sex modulates myocardial blood flow (MBF) and substrate metabolism in normal humans. Whether sex affects MBF and metabolism in patients with HF is unknown. We studied 19 well-matched men and women with nonischemic HF with similar ejection fractions (all ≤ 35%). MBF and myocardial substrate metabolism were quantified using positron emission tomography. Women had higher MBF (mL/g/min), FA uptake (mL/g/min), utilization (nmol/g/min) (P<0.005, <0.005, <0.05, respectively) and trended towards higher FA oxidation than men (P=0.09). These findings were independent of age, obesity, and insulin resistance. There were no sex-related differences in fasting myocardial glucose uptake or metabolism. In an exploratory analysis of the longitudinal follow-up of these subjects (mean 7 y), we found that 4 men had a major cardiovascular event, while one woman died of non-cardiac causes. Higher MBF related to improved event-free survival (HR=0.31, P=0.02). In sum, in nonischemic HF, women have higher MBF and FA uptake and metabolism than men, and these changes are not due to differences in other variables that can affect myocardial metabolism (e.g., age, obesity, or insulin resistance). Moreover, higher MBF portends a better prognosis. These sex-related differences should be taken into account in the development and targeting of novel agents aimed at modulating in MBF and metabolism in HF.
Keywords: sex, myocardial blood flow, myocardial fatty acid metabolism, myocardial glucose metabolism, heart failure
Sex affects many clinical aspects of heart failure (HF). Sex affects etiology, risk factor susceptibilities, (16) responses to therapy, and outcomes (17). Women are more likely to suffer from nonischemic cardiomyopathy and have a higher likelihood of being obese and inactive (17). In large clinical trials, men and women do not reap the same benefits from the standard HF medical therapies (13). Most importantly, women with HF – especially nonischemic HF – have a 33% better survival than men after onset of HF (17). Whether sex affects myocardial metabolism and blood flow in nonischemic heart failure is unknown.
Results from studies in male animal models with HF are fairly consistent, showing that HF shifts myocardial metabolism away from FA and towards glucose metabolism (11). Results from studies on the effect of HF on human myocardial metabolism, however, are mixed. While some show a switch to glucose, others do not (11, 20). Since glucose is a more oxygen-efficient fuel than fatty acids, it has been suggested that this shift towards more glucose metabolism is beneficial. While this may be advantageous in the setting of ischemia (with limited oxygen) (27), theoretically it might be disadvantageous in nonischemic conditions. Beta-oxidation of long-chain FAs results in far more adenosine triphosphate (ATP) produced (e.g., ~129 ATP/palmitate) than glucose oxidation (~36–38 ATP net). Thus, it is not surprising then that the normal adult heart, with its demand for ~5 kg of ATP/d, uses predominantly FAs for fuel (11). In humans without HF, women have higher myocardial blood flow (MBF) and myocardial FA metabolism than age-matched men (24). However, because of the major metabolic shift seen in animal models of HF and in some human studies, it cannot be assumed that the sex-related differences in MBF and myocardial substrate metabolism seen in normal subjects automatically extend to patients with HF.
Given the inextricable links between myocardial metabolism, blood flow, and function (and between blood flow, function and outcomes), we aimed to determine whether there are sex-related differences in myocardial metabolism and MBF in nonischemic HF. We hypothesized that women with nonischemic HF would have greater myocardial FA uptake and metabolism and MBF than men with HF. In exploratory analyses, we proposed to evaluate the effect of sex on myocardial glucose uptake and metabolism and determine if there are any relationships between MBF or metabolism measures and outcomes over a ~7 y follow-up.
METHODS
Study subjects
In this prospective study we enrolled and screened 25 subjects with documented nonischemic cardiomyopathy. All subjects were recruited by posted advertisements and letters to cardiologists at Washington U. School of Medicine. If patients fit the entry criteria for the study and they agreed to participate, they were enrolled. Four women were excluded because they were found to have ejection fractions over 45% and 2 women were excluded because diabetes was diagnosed. Nineteen individuals with nonischemic HF, 10 women and 9 men, finished the study. All underwent a medical history, physical exam, and blood tests for fasting chemistries, insulin levels, and lipid panels. Subjects were ambulatory, in NYHA Class II-III HF, aged 20–60 years, and able to lie flat for the 4 h positron emission tomography (PET) study. All subjects were on stable HF regimens. Subjects were excluded if they had major organ system dysfunction (other than HF), coronary artery disease, diabetes mellitus, or if they were lactating, or were unable to give informed consent. Because of the radiation exposure with PET, each premenopausal woman was required to assure the study team that she had appropriate birth control (abstinence or contraception) and to undergo a urine pregnancy test before the PET. One premenopausal woman was taking depo-provera 150 mg every 13 weeks, and one was taking ethinyl estradiol and levonorgestrel for birth control. All subjects had an ejection fraction ≤ 35% by echocardiography and were diagnosed with a nonischemic cardiomyopathy by cardiac catheterization. Thus, all patients had heart failure with reduced ejection fraction. No subject had a hypertrophic or restrictive cardiomyopathy or pericardial constriction. The Human Research Protection Office at the Washington University School of Medicine approved this study, and all subjects gave informed consent prior to participation.
Experimental procedure
Subjects were admitted to the General Clinical Research Center at Washington University School of Medicine the night prior to the study and underwent an overnight 12 h fast. The following morning, an 18- or 20-gauge catheter was inserted into an antecubital vein for radiopharmaceutical injection. All were studied at 8 AM to avoid circadian variation in metabolism (29). Subjects underwent PET imaging with a conventional commercially available tomograph (Siemens Systems, Iselin, New Jersey). Hemodynamics were obtained throughout the study. At predetermined points during the PET fasting plasma glucose (measured using a glucose analyzer, Yellow Springs Instruments, Yellow Springs, OH), insulin (measured using a radioimmunoassay Linco Research, St. Charles, MO), and free FAs were quantified (using an enzymatic colorimetric kit, NEFA C kit; WAKO Chemicals, Richmond, VA). Radiolabeled metabolites were also measured at predetermined intervals and were used for compartmental modeling of the tracer kinetics. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from the fasting glucose and insulin levels (18).
Cardiac PET imaging and analyses
MBF was measured using 15O-water, FA fractional uptake, utilization and oxidation were measured using 1-11C-palmitate, and myocardial glucose utilization, glycolysis, glucose oxidation, and glycogen storage rates were measured using 1-11C-glucose, as previously described (9, 24). MBF was quantified in all 19 subjects, FA metabolism in all but 1 woman (there was a problem verifying the quality of the C-11 palmitate for that single study and the subject did not want to return for a repeat study), and glucose metabolism in 15 (8 men, 7 women). The modeling of myocardial substrate metabolism is well-validated, previously published, and is described by the following equations (25):
Myocardial FA utilization represents total FA metabolism by the heart because back-diffusion of FAs is accounted for in the kinetic modeling. Myocardial FA utilization can be further broken down into its components:
Similarly, our group has validated the compartmental modeling that allows us to measure glucose uptake, glucose utilization, and the components of glucose utilization - glycolysis and glycogen storage rates (9). Glycolysis can also be further broken down using kinetic modeling into the portion accounted for by full glucose oxidation as detailed by Herrero et al.. {Herrero et al., 2007, #2144}. (9).
Echocardiography
Subjects underwent a complete 2D and Doppler echocardiographic exam after the PET measurement of MBF. LV end-diastolic and end-systolic volumes were calculated by the method of discs. End-systolic volume was indexed to body surface area (BSA)1.19, as suggested by de Simone et al.(5) to mitigate the known sex-related differences in LV volumes. The modified Simpson’s method was used to calculate LV ejection fraction. Cardiac index = ((LV end-diastolic volume – end-systolic volume)* heart rate)/BSA. LV mass was indexed to the height (m)2.7. Stroke work = stroke volume * mean arterial pressure.
Follow-up
An “event” was considered to be the composite of all-cause death, cardiac transplant, or placement on a LV assist device or extracorporeal membrane oxygenation (ECMO). Subjects’ actuarial and medical status was confirmed using their medical record, the heart failure registry at Washington University School of Medicine, and the Social Security Death Index. All subjects were followed until first event occurrence or last available confirmed event-free observation date.
Statistical Analyses
Excel (14.4.2) and SAS were used for the statistical analyses. Comparisons of continuous variables between men and women were made with Student’s t test with or without a Satterthwaite correction for unequal variances, as appropriate. Fisher’s exact tests were used for comparison of categorical variables. Linear regressions between age, BMI, HOMA-IR (homeostasis model assessment of insulin resistance) and the myocardial endpoints were performed. Multivariable regression analyses forcing the independent variables age and obesity into the model with the variable sex were performed to determine the independent predictors of MBF, myocardial FA uptake, oxidation, FA utilization, and glucose utilization. The association between clinical outcome and each myocardial endpoint was examined by a univariable Cox proportional hazards models. The hazard ratio was reported from each of these models and describes the risk of event occurrence, with values greater than 1 indicating higher risk and values < 1 indicating lower risk. A value of P<0.05 was considered statistically significant.
RESULTS
Subject characteristics (Tables 1 and 2)
Table 1.
Subject Characteristics
| Men | Women | p Value | |
|---|---|---|---|
| N | 9 | 10 | |
| Age (yrs) | 42±3 | 41±3 | 0.86 |
| Race (% Caucasian) | 89% | 60% | 0.06 |
| Body mass index (kg/m2) | 31±2 | 35±8 | 0.25 |
| Body surface area (m2) | 2.12±0.08 | 1.89±0.08 | 0.07 |
| Total cholesterol (mg/dL) | 169±15 | 178±27 | 0.77 |
| Low-density lipoprotein (mg/dL) | 107±12 | 106±27 | 0.99 |
| High-density lipoprotein (mg/dL) | 37±4 | 53±10 | 0.17 |
| Triglycerides (mg/dL) | 125±41 | 98±12 | 0.48 |
| HOMA-IR | 2.55±0.51 | 4.40±1.77 | 0.35 |
| Plasma glucose during PET (µmol/mL) | 5.03±0.10 | 4.69±0.15 | 0.30 |
| Fasting plasma FA (nmol/mL) | 624±67 | 706±95 | 0.49 |
| Fasting insulin levels (µU/mL) | 10.0±2.6 | 22.2±8.7 | 0.30 |
| Beta-blocker use | 6 (67%) | 8(80%) | NS |
| ACE-I/ARB use | 9(100%) | 10(100%) | NS |
Data are expressed as mean ± standard error.
HOMA=homeostasis model assessment of insulin resistance; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; PET = Positron emission tomography
Table 2.
Hemodynamics, LV Structure, and Function
| Men | Women | p Value | |
|---|---|---|---|
| N | 9 | 10 | |
| Hemodynamics | |||
| Systolic blood pressure (mm Hg) | 106±4 | 108±6 | 0.79 |
| Diastolic blood pressure (mm Hg) | 65±2 | 62±4 | 0.42 |
| Mean arterial pressure (mm Hg) | 79±2 | 77±4 | 0.74 |
| Heart rate (bpm) | 70±6 | 68±3 | 0.72 |
| Rate-pressure product (mm Hg*bpm) | 6580±835 | 6084±534 | 0.66 |
| Cardiac structure and function | |||
| End-systolic volume indexed to BSA1.19 | 24±4 | 23±2 | 0.83 |
| LV mass index (g/m2.7) | 85±8 | 67±5 | 0.07 |
| Cardiac output (L/min) | 4.4±0.7 | 3.1±0.2 | 0.12 |
| Cardiac index (L/min/m2) | 2.1±0.3 | 1.7±0.1 | 0.30 |
| Stroke work (ml*mmHg) | 4728±786 | 3488±260 | 0.16 |
| LV ejection fraction | 25±3 | 29±2 | 0.29 |
SBP=systolic blood pressure, DBP=diastolic blood pressure, MAP=mean arterial SBP = Data are expressed as mean ± standard error. LV=left ventricular
There were no significant differences between the men and women in age, BMI, or ejection fraction. There were also no differences in weight, race, BSA, cholesterol levels, or insulin resistance as measured by HOMA-IR. Importantly, there were no significant differences in beta-blocker or angiotensin antagonism therapy use since the former may decrease FA metabolism and the latter may increase it (11). No subject was taking any hypoglycemic therapy. There were no significant differences in fasting plasma substrate or insulin levels during the PET studies. Blood pressure, heart rate and rate-pressure product during the PET studies were not different between the men and the women. The men trended toward greater LV mass index, which was expected because men are known to have higher LV mass than women even after indexing to body surface area (BSA). LV chamber size indexed to BSA1.19, which decreases sex-related differences in chamber size, was not different between the men and women. All measures of cardiac systolic function were similar between the sexes.
Myocardial Blood Flow
MBF was significantly higher in women (Figure 1) despite no difference in hemodynamics between the sexes (Table 2). MBF did not correlate with age, obesity, or HOMA-IR. Sex was the only independent predictor of MBF in multivariable modeling (R2=0.26, P =0.01).
Figure 1. Sex differences in myocardial blood flow (MBF) in heart failure.
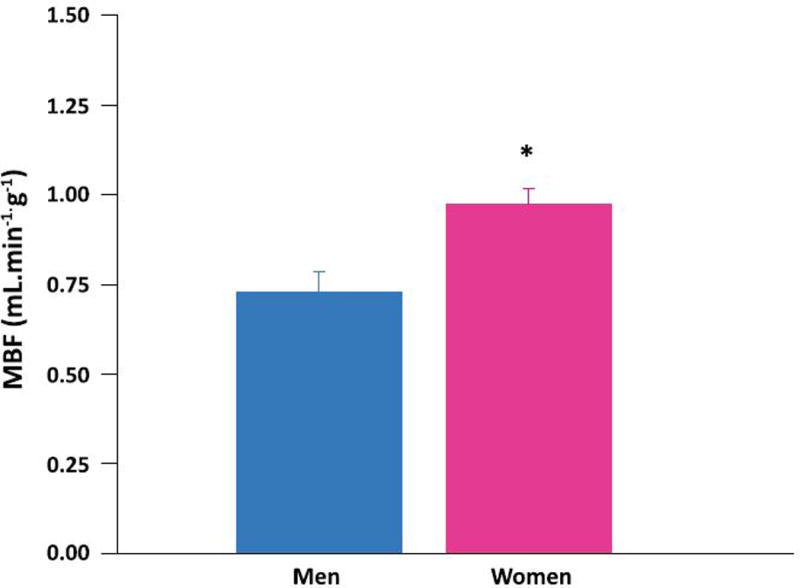
Myocardial blood flow (MBF) average in 9 men (blue bars) and 10 women (pink bars) with non-ischemic heart failure. *MBF higher in women than men (p < 0.005). Data are expressed as mean ± standard error.
Myocardial fatty acid and glucose substrate metabolism
Women had higher myocardial FA uptake (Figure 2 top panel) and higher myocardial FA utilization than men (Figure 2 middle panel). FA oxidation trended higher in the women than in the men (Figure 2 bottom panel). There were no correlations between age, obesity, or insulin resistance and any measure of fractional FA uptake or metabolism. Sex was the only independent predictor of myocardial FA uptake and metabolism. Sex accounted for 35% of the variation in FA uptake (P = 0.005) and 25% of FA utilization (P = 0.03). There were no differences in myocardial glucose uptake, utilization, glycolysis, glycogen deposition or glucose oxidation rates in the fasted condition between the sexes (Table 3). There were no independent predictors of glucose uptake or metabolism.
Figure 2. Sex differences in myocardial fatty acid (FA) uptake and metabolism in heart failure.
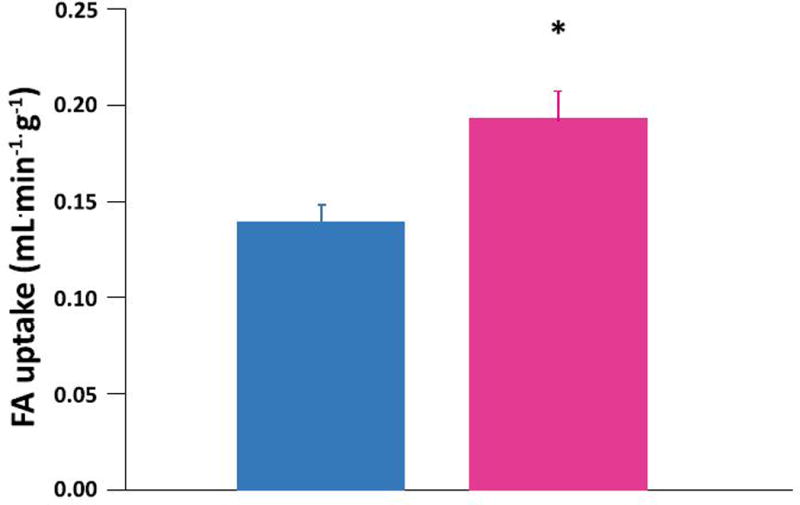
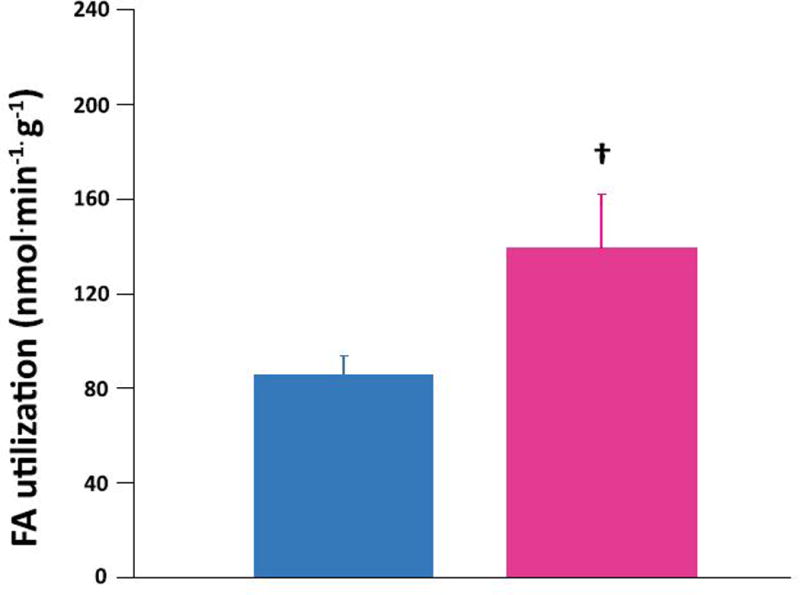
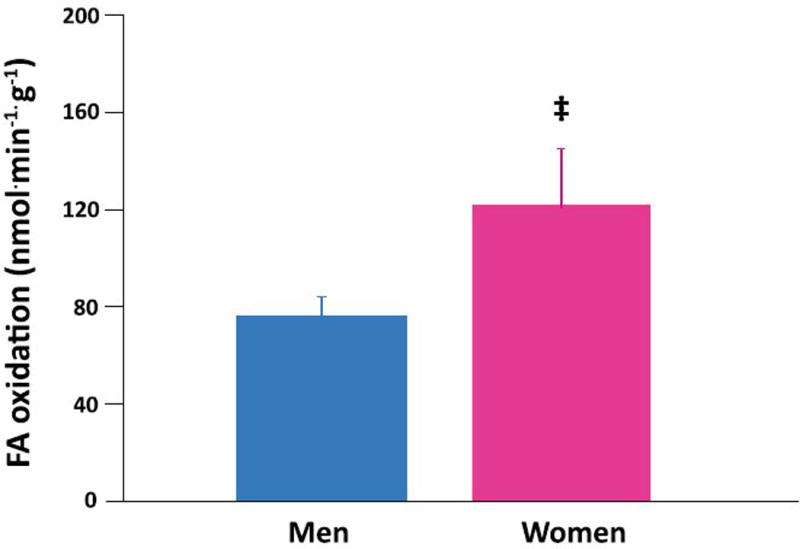
Myocardial FA uptake (top panel), utilization (middle panel), and oxidation (bottom panel) averages in 9 men (blue bars) and 9 women (pink bars) with non-ischemic heart failure. Women higher than men: *p<0.005, †p<0.05, ‡ p=0.09. Data are expressed as mean ± standard error.
Table 3.
Measures of myocardial glucose uptake and metabolism
| Men | Women | p Value | |
|---|---|---|---|
| N | 8 | 7 | |
| Glucose uptake (mL/g/min) | 0.034±0.005 | 0.045±0.010 | 0.33 |
| Glucose utilization (nmol/g/min) | 167 ± 26 | 214±50 | 0.65 |
| Glycolysis (nmol/g/min) | 40 ± 9 | 76±24 | 0.16 |
| Glucose oxidation (nmol/g/min) | 36±8 | 60±19 | 0.24 |
| Glycogen deposition (nmol/g/min) | 127±18 | 138±29 | 0.75 |
Data are expressed as mean ± standard error.
Post-hoc analyses
Because menopausal status may affect myocardial metabolism (10), we performed post-hoc analyses excluding the 2 postmenopausal women. The premenopausal women with HF left in the analyses (highest age 43 y) still had higher MBF (p<0.01) and myocardial FA uptake (p<0.01) than the men. Myocardial FA utilization trended higher in the premenopausal women with HF than the men (p=0.08). Myocardial FA oxidation differences became less significant (p=0.16) with the decreased numbers of subjects analyzed.
Follow-up, events, and correlations with event-free survival
The mean follow-up was 2452 d or ~7 y with a range of 724 to 5337 d. Only men (N=4) had cardiovascular events in the follow-up period. One man underwent ECMO, then LV assist device placement, and eventual transplant. (The first event date – the date of the ECMO – was the event date). Two other men underwent LV assist device placement and eventual transplant. One man underwent LV assist device placement and later died. One woman died of a non-cardiovascular cause (metastatic breast cancer). There were no significant differences in age, ejection fraction, or LV end-diastolic diameter between those who had a cardiovascular event and those who did not. Higher MBF (per 0.1mL/kg/min increase) related positively to event-free survival (HR=0.309, P=0.02, 95% confidence intervals 0.11, 0.85). Although only men suffered a cardiovascular event, in the analysis of all-cause event rates between the men and women was not statistically significant (hazard ratio [HR]: men (vs. women) 3.86, P=0.23. Myocardial FA and glucose uptake, utilization, and oxidation were not related to differences in event-free survival.
DISCUSSION
To our knowledge, this study is the first – in animals or humans – to show that sex influences both MBF and substrate metabolism in non-ischemic HF. Women with non-ischemic HF had greater MBF, FA uptake and overall utilization and trended towards higher FA oxidation than men with HF. Glucose uptake was not different between the sexes in absolute terms. Moreover, sex was the only independent predictor of MBF, and myocardial FA uptake and utilization. These findings cannot be attributed to differences in age, obesity status, HOMA-IR, hemodynamics, ejection fraction, or the plasma substrate environment. In our exploratory analyses, higher MBF was a predictor of event-free survival.
MBF was higher in women with HF than in men with HF. However, MBF was relatively low in both our HF women and HF men in comparison with well-established values in the literature from our group and others (20, 24). A low MBF even in the absence of coronary artery disease-related HF is consistent with previous literature (21). Multiple mechanisms may contribute to this, including an imbalance of nitric oxide/reactive oxygen species as well as neurohumoral activation (1). Although one might speculate that the women’s higher MBF may be solely the result of estrogen’s effects on nitric oxide synthase and conduit artery function (14) this is unlikely because normal postmenopausal women taking estrogen (replacement therapy) do not have higher MBF than men (10). In addition, estrogen replacement does not increase MBF in postmenopausal women (23). In HF, female sex also affects calcium handling, extracellular matrix expression, and other parameters, which may indirectly affect myocardial perfusion (26). Interestingly, in a purely exploratory follow-up of our subjects, we found that the 4 persons (all men) with the lowest MBF levels had either undergone transplant, ventricular assist device, or had died over a mean of 7 years. This is partly consistent with the findings from a study by Neglia et al. (21). In that study, the authors found that patients with a lower resting MBF tended to have a higher risk of a composite endpoint including worsening ejection fraction and cardiac death, however, the finding was not statistically significant. They did find that lower myocardial blood flow after dipyridamole independently predicted these endpoints (21). Taken together, our findings and those of Neglia et al., suggest that differences in MBF are important, not just descriptive, even in patients without coronary artery disease. It further suggests that MBF may be a target for treatment even in nonischemic HF.
The women also had higher myocardial FA uptake than men. At first glance this may appear to be due to the women’s higher MBF because it is used in the calculation of FA uptake. However, there is generally a reciprocal relationship between MBF and FA extraction by the heart (8). Thus, despite a higher MBF, FA extraction by the heart was better maintained in the women, leading to a higher FA uptake. This higher FA uptake in the women could not be explained by differences in age, obesity status, or HOMA-IR. Although there are no reports to our knowledge of sex-related differences in FA transporters in the heart, women do appear to have higher FA transporter/CD36 gene and protein expression in skeletal muscle (3, 12). Taken together with our findings, it is intriguing to speculate that a similar difference may exist in the myocardium in HF. Interestingly, the levels of FA uptake rates that were seen in the men and women with HF in this study appear to be lower than what has been reported in studies of normal controls for each sex (25). Thus, the heart’s intrinsic ability to extract FAs appears to be working better in the women than in the men, though neither appears to be extracting FAs normally.
Women with HF also had higher FA utilization than men. The higher FA uptake in women contributed to the higher FA utilization. The slightly (though not statistically) higher plasma FA levels in women also contributed to higher FA utilization. Higher peripheral plasma FA availability is concordant with studies in humans show that sex affects percent fat mass, whole body lipolytic rates, FA rate of appearance (22) and very low-density lipoprotein kinetics - with women having higher levels of each (19). Healthy women also have a higher ‘set point’ for the rate of FA release in relation to their fuel requirements than men (19). Our results in HF patients suggest that some of these sex-related differences may still hold true in nonischemic cardiomyopathy. Some of the myocardial FA metabolism differences may be related to sex hormone differences between the men and women. For example, estrogen is well-known to increase plasma triglyceride levels. However, there is increasing evidence that many metabolic genes are expressed/function in a “sexually dimorphic manner”, which may also contribute to the sex-related differences noted in the current study (19). Although the HF women had higher FA utilization levels that the men, it is still important to put these findings in context. To that end, neither men nor women of current HF subjects had rates as high as healthy subjects in the literature (24).
Although myocardial FA oxidation mirrored FA utilization in both sexes, oxidation only trended higher in women compared with men. This is partly consistent with findings in subjects without HF, in which most FAs that enter myocardial cells are oxidized immediately and female sex independently predicted higher FA oxidation. (24). While our exploratory analysis in our small study did not show a relationship between measures of FA oxidation and outcomes, an interventional study in humans with nonischemic HF has shown that decreasing FA availability, uptake, and overall oxidation worsens LV function (28). Studies in murine models engineered to have increased myocardial FA oxidation (those with cardiac-specific deletion acetyl CoA carboxylase 2) have improved LV function (15), while those with decreased FA oxidation (carnitine palmitoyl transporter 1 haploinsufficient mice) have worse function in response to pressure overload (7). Taken together, these data suggest that, contrary to dogma, a shift away from myocardial FA metabolism in the non-ischemic failing heart may be maladaptive rather than adaptive. This is also contrary to studies that show that the FA oxidation inhibitor, trimetazidine, helps cardiac function in HF (6). The reason for this discrepancy is not completely clear. However, as yet there is no large, multicenter, randomized trial with a FA oxidation inhibitor therapy, and it may be that trimetazidine also has other beneficial mechanisms of action.
Interestingly, the men and women in the current study had rates of glucose uptake and metabolism that were not statistically different. This is despite the fact that the women had a higher rate of FA metabolism. Thus, it appears that FA and glucose metabolism are not perfectly counterbalanced, which is consistent with other studies done in the resting, fasted state (24, 25). Although glucose utilization rates were similar in the sexes, the HF patients’ glucose utilization rate were slightly lower than their normal counterparts.(24). These lower than normal glucose utilization rates in the present study may appear to contradict findings from a previous, smaller study by our group (4). In the previous study, patients with idiopathic dilated cardiomyopathy exhibited higher rates of myocardial glucose utilization than normal controls. However, 75% of normal controls were women (who may be expected to have lower rates of myocardial glucose utilization) while only 43% of the dilated cardiomyopathy patients were women. This gender disparity may explain the apparent discrepancy between the previous study (4) and the current one.
Limitations
Although this study had a relatively small number of patients, we proved our primary hypotheses regarding MBF and FA uptake and metabolism. The lack of a difference in our exploratory aim to determine if there was a difference in myocardial glucose metabolism, may have been due to small numbers (type II error). However, based on our current study’s results, and estimating the pooled (i.e., men and women) standard deviation as 116, it would take at least 194 subjects - 102 men and 92 women - to show a difference. Thus, any clinical impact of sex-related myocardial glucose metabolism differences would appear to be far less than that of the MBF and FA metabolism differences. The small sample size increases the confidence bounds on the magnitude of the effect of sex on our primary endpoints (MBF and FA uptake and metabolism), but it has no impact on the degree of significance of our findings. Our findings should not be extrapolated to patient populations that were not included in this study (e.g., patients with ischemia). Larger, longitudinal studies are needed to test our speculations on the possible association between a more normal metabolic pattern (i.e. more FA use) and better outcomes in nonischemic cardiomyopathy. The current study was not designed to include normal control patients and so cannot address the question as to whether or not there is an interaction between HF status and sex in the determination of MBF or FA metabolism.
Conclusions
Sex significantly affects cardiac perfusion and metabolism in HF. Moreover, female sex — independent of age, adiposity, or insulin resistance — predicts higher MBF, FA uptake and metabolism in patients with non-ischemic systolic HF. Consistent with the literature, we found that women had fewer cardiovascular events. A higher resting MBF is associated with improved event-free survival, suggesting that MBF may be a novel target for treatment of nonischemic HF with additional vasodilator therapy. In this new era of personalized medicine, it is important to understand that not all non-ischemic systolic HF is the same. In the development of new MBF and metabolic modulator therapy to improve function and survival in HF, trials and therapy should be tailored to each sex. Given that a higher MBF has been associated with improved event-free survival in general, larger longitudinal studies are needed to determine if the sex-related differences in MBF and/or FA metabolism are markers of, or contribute to women’s well-known survival advantage.
New and Noteworthy.
This is the first report in animals or humans with heart failure that there are sex-related differences in myocardial blood flow, fatty acid uptake and utilization. These differences are independent of age, obesity, or insulin resistance. Our exploratory analysis is the first to show that high resting MBF portends a good prognosis.
Acknowledgments
Funding sources:
There is no significant relationship with industry relating to this publication
The authors wish to thank the study subjects for their participation, Douglas L. Mann, MD and Kitty O’Callaghan for editorial assistance, and Ava Ysaguirre for assistance with manuscript preparation. We also wish to thank Karen Gillis Taylor for graciously allowing us to use her artwork on our recruitment flyers and posters.
GRANTS
Barnes-Jewish Hospital Foundation Grant (St. Louis, Missouri); American Society of Nuclear Cardiology (Bethesda, Maryland), Mentors in Medicine Grant (St. Louis, Missouri); and from the National Institutes of Health (Bethesda, Maryland): P30DK056341 (NORC), M01-RR000036-475265 (ICTS), and P60-DK020579 (DRTC), R01HL58878.
Footnotes
All authors participated in the performance of the study, the interpretation of the data, and edited/approved the final version of the manuscript. AK, CHL, KBS, EN, and LRP participated in the statistical analyses. AK, CHL, ARC, and LRP wrote the initial drafts. AK, ARC, and LRP prepared the figures. AK, CHL, KBS, EN, VGD, RJG, and LRP participated in the conception and the study design.
Clinical Trials #: NCT00776035
DISCLOSURES
None related to this work. All authors have approved the final article.
References
- 1.Bauersachs J, Schafer A. Endothelial dysfunction in heart failure: mechanisms and therapeutic approaches. Curr Vasc Pharmacol. 2004;2:115–124. doi: 10.2174/1570161043476447. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 3.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr and Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 5.de Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR, Greco R, Witt S, Contaldo F. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95:1837–1843. doi: 10.1161/01.cir.95.7.1837. [DOI] [PubMed] [Google Scholar]
- 6.Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 7.He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126:1705–1716. doi: 10.1161/CIRCULATIONAHA.111.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrero P, Kim J, Sharp TL, Engelbach JA, Lewis JS, Gropler RJ, Welch MJ. Assessment of myocardial blood flow using 15O-water and 1-11C-acetate in rats with small-animal PET. J Nucl Med. 2006;47:477–485. [PubMed] [Google Scholar]
- 9.Herrero P, Kisrieva-Ware Z, Dence CS, Patterson B, Coggan AR, Han DH, Ishii Y, Eisenbeis P, Gropler RJ. PET measurements of myocardial glucose metabolism with 1-11C-glucose and kinetic modeling. J Nucl Med. 2007;48:955–964. doi: 10.2967/jnumed.106.037598. [DOI] [PubMed] [Google Scholar]
- 10.Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, Gropler RJ. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005;12:574–581. doi: 10.1016/j.nuclcard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Kadkhodayan A, Coggan AR, Peterson LR. A “PET” area of interest: myocardial metabolism in human systolic heart failure. Heart Fail Rev. 2013;18:567–574. doi: 10.1007/s10741-012-9360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol (1985) 2004;97:1209–1218. doi: 10.1152/japplphysiol.01278.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kimmelstiel C, Goldberg RJ. Congestive heart failure in women: focus on heart failure due to coronary artery disease and diabetes. Cardiology. 1990;77(Suppl 2):71–79. doi: 10.1159/000174655. [DOI] [PubMed] [Google Scholar]
- 14.Koh KK, Cardillo C, Bui MN, Hathaway L, Csako G, Waclawiw MA, Panza JA, Cannon RO. Vascular effects of estrogen and cholesterol-lowering therapies in hypercholesterolemic postmenopausal women. Circulation. 1999;99:354–360. doi: 10.1161/01.cir.99.3.354. [DOI] [PubMed] [Google Scholar]
- 15.Kolwicz SCJ, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 17.Lund LH, Mancini D. Heart failure in women. Med Clin North Am. 2004;88:1321–45. xii. doi: 10.1016/j.mcna.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135:681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 20.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–8. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 21.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori P, Sorace O, Carpeggiani C, Poddighe R, L’Abbate A, Parodi O. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–193. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson LR, Eyster D, Davila-Roman VG, Stephens AL, Schechtman KB, Herrero P, Gropler RJ. Short-term oral estrogen replacement therapy does not augment endothelium-independent myocardial perfusion in postmenopausal women. Am Heart J. 2001;142:641–647. doi: 10.1067/mhj.2001.118111. [DOI] [PubMed] [Google Scholar]
- 24.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–581. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regitz-Zagrosek V, Oertelt-Prigione S, Seeland U, Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ J. 2010;74:1265–1273. doi: 10.1253/circj.cj-10-0196. [DOI] [PubMed] [Google Scholar]
- 27.Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann N Y Acad Sci. 2005;1047:208–218. doi: 10.1196/annals.1341.019. [DOI] [PubMed] [Google Scholar]
- 28.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 29.Young ME. Circadian rhythms in cardiac gene expression. Curr Hypertens Rep. 2003;5:445–453. doi: 10.1007/s11906-003-0051-8. [DOI] [PubMed] [Google Scholar]


