Abstract
Syndecan-1 is considered a biomarker of injury to the endothelial glycocalyx following hemorrhagic shock, with shedding of sdc1 deleterious. Resuscitation with fresh frozen plasma (FFP) has been correlated with restitution of pulmonary sdc1 and reduction of lung injury, but the precise contribution of sdc1 to FFPs protection in the lung remains unclear. Human lung endothelial cells were used to assess the time and dose dependent effect of FFP on sdc1 expression and the effect of sdc1 silencing on in vitro endothelial cell permeability and actin stress fiber formation. Wild-type (WT) and syndecan-1−/− mice were subjected to hemorrhagic shock followed by resuscitation with lactated ringers (LR) or FFP and compared to shock alone and shams. Lungs were harvested after 3 hours for analysis of permeability, histology, and inflammation and for measurement of syndecan- 2 and 4 expression. In vitro, FFP enhanced pulmonary endothelial sdc1 expression in time- and dose-dependent manners and loss of sdc1 in pulmonary endothelial cells worsened permeability and stress fiber formation by FFP. Loss of sdc1 in vivo lead to equivalency between LR and FFP in restoring pulmonary injury, inflammation, and permeability after shock. Lastly, sdc1 −/− mice demonstrated a significant increase in pulmonary syndecan 4 expression after hemorrhagic shock and FFP based resuscitation. Taken together, our findings support a key role for sdc1 in modulating pulmonary protection by FFP after hemorrhagic shock. Our results also suggest that other members of the syndecan family may at least be contributing to FFP’s effects on the endothelium, an area that warrants further investigation.
INTRODUCTION
Trauma is the third leading cause of death across all age groups.1–2 Of these deaths, hemorrhage remains the number one cause of early trauma deaths, though reductions in early mortality have occurred from modern resuscitation strategies employing the early and empiric use of plasma.3–5 This decrease in mortality from plasma-based resuscitative strategies appears to extend beyond its ability to correct trauma-induced coagulopathy and provide hemorrhage control and is thought to involve repair of a dysfunctional endothelium that follows trauma and hemorrhage. This “endotheliopathy of trauma” is a systemic response by the injured endothelium resulting in abnormalities of coagulation, inflammation, and endothelial barrier integrity.6 Clinically, this is manifested as a pro-inflammatory state along with capillary leak and subsequent tissue edema, both of which may contributes to organ dysfunction and late deaths. Therefore, restoring the endothelium and barrier integrity is integral to further reducing hemorrhagic shock-related morbidity and mortality.
We have been interested in endothelial repair by plasma which we hypothesize is due in part to restoration of the glycocalyx and syndecan-1 (Sdc1) backbone on injured endothelial cells. The endothelial glycocalyx is a complex network of soluble components that projects from the endothelial surface and consists of proteoglycans and glycoproteins. The proteoglycans provide structural support for the glycocalyx and consist of a core protein, primarily syndecan, to which the glycosaminoglycans attach. For Sdc1, heparan sulfate chains are the main glycosaminoglycans.7 There are four members (syndecans1–4) that comprise the syndecan family. Sdc1 has been the focus of most studies as it has become a recognized systemic marker of damage to the glycocalyx. Syndecan 2 and 4, like Sdc1, are found on endothelial cells and in the lung, though little data exists on their contribution after shock. Injury to the glycocalyx results in shedding of the Sdc1 ectodomain after a variety of shock states.8–10 Shedding is considered deleterious as the Sdc1 ectodomain and its heparan sulfate side chains can function as danger-associated pattern molecules (DAMPs), contributing to sterile inflammation, immunosuppression, and auto-heparinization following trauma.11–13 Ectodomains have been detected in the blood of patients with sepsis, hemorrhagic shock and ischemia-reperfusion injury, and in fluids from infected or injured tissues.14 In injured patients, shedding is associated with enhanced shock, inflammation, coagulopathy, endothelial damage and is an independent predictor of morality.15–17 We and others have shown in the laboratory that plasma promotes endothelial restoration of Sdc1 and the glycocalyx following hemorrhagic shock.18–19 The specific contribution of Sdc1 to plasma’s pulmonary protection, however, remains unclear. We hypothesized that loss of Sdc1 would worsen pulmonary injury and abrogate protection by plasma.
MATERIALS AND METHODS
In vitro
Cell culture
Human lung microvascular endothelial cells (HLMECs, PromoCell) were grown to confluence in supplemented endothelial basic medium-2 (EBM-2, Lonza). Prior to experiments, cells (passages 5–10) were cultured under low serum conditions (0.5 % FBS) for 2 hours then incubated in EBM-2 containing 10% lactated Ringers (LR) or 10% fresh frozen plasma (FFP), as we have shown this concentration to be optimal.20
RNA silencing of Sdc1
Four sets of pre-designed human Sdc1 small-interference RNA (siRNA) pools and negative control siRNA (scrambled siRNA) were purchased from Dharmacon (Lafayette, CO). The sequences were as follows: 5’- ACGCAGCUCCUGACGGCUA -3’; 5’-GUAACGACAAUAAACGGUA -3’; 5’-CAUCAGGCCUCAACGACCA-3’; 5’-UGGGAAACUUGGCUCGAAU -3’. Transfections were performed with DharmaFECT 4 according to the manufacturer’s instructions. Immunoblot analysis was used to assess efficacy of downregulation of the RNA.
Endothelial permeability
Transwell permeability was assessed in confluent monolayers by adding fluorescein isothiocyanate (FITC)-dextran (40 kDa) to the upper chamber. After 30 min, samples were collected from the lower chamber and the fluorescent intensity determined using a fluorimeter. The fold change in FITC-dextran fluorescence intensity over controls was calculated as we have described.21
Actin stress fiber staining
Cells were grown on gelatin-coated 8-well chamber slides and fixed in 4% paraformaldehyde then permeabilized with 0.1% Triton X-100. After washing and blocking with 1% BSA, cells were incubated with 1 U/ml of Texas red-X phalloidin (Molecular Probes, ThermoFisher, Waltham, MA) which is specific for F-actin.
In vivo
Mouse model of hemorrhagic shock
All procedures performed were approved by the University of Texas Health Science Center at Houston or Maryland School of Medicine Animal Welfare Committees. The experiments were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum. Male wild type (WT) littermates and syndecan-1 null (Sdc1−/−) mice on the C57BL/6J background were used at 8–10 weeks of age and weighing approximately 25 grams. Our established mouse model of trauma-hemorrhagic shock was utilized.21 Under isoflurane anesthesia, a midline laparotomy incision was made, the intestines were inspected and then the incision was closed. The right femoral artery was cannulated for continuous hemodynamic monitoring and blood withdrawal or resuscitation. Mean arterial blood pressure was recorded via the femoral arterial line at baseline then every 5 minutes during the shock period. After a 10-minute period of equilibration, mice were bled to a mean arterial pressure (MAP) of 35 ± 5 mmHg which was maintained for 90 minutes. Shams underwent anesthesia and placement of catheters but were not subjected to laparotomy or hemorrhagic shock. Shock animals were resuscitated with either lactated Ringer’s at 3× shed blood volume or fresh frozen plasma at 1× shed blood and compared to animals that underwent shock alone as we have described.21,22 Following resuscitation, lines were removed and the animals awoken to more closely mimic an awake shock model. Three hours after the end of shock, animals were sacrificed by exsanguination under isoflurane anesthesia and lungs harvested for further analysis. This time point was chosen based on our previous investigation showing pulmonary protection and partial restitution of pulmonary Sdc1 by plasma at this time point.18,21 For FFP, single donor fresh frozen plasma was thawed, aliquoted, and then samples stored frozen at −20°C until ready for use.
Lung permeability
Evans blue dye extravasation was used as an indicator of vascular permeability. Animals received a bolus injection of 3% Evans blue (4 ml/kg) one hour prior to sacrifice. At sacrifice, animals were perfused via the right ventricle with 10 ml PBS to remove the intravascular dye from vessels. Lungs were harvested then incubated in formamide for 24 hour at 55°C to allow for dye extraction. After centrifugation, absorbance was measured in the supernatant at 620 nm using a plate reader.
Lung histopathologic injury
Lung tissue sections were stained with hematoxylin and eosin (H&E) and scored on a 3-point scale for alveolar thickness, capillary congestion, and cellularity as initially described by Hart et al and as we have reported.18,23 The overall lung injury score was calculated by averaging the three parameters.
Lung inflammation
Infiltration of neutrophils was assessed by myeloperoxidase (MPO) immunofluorescence staining. Paraffin-embedded tissue was cut into 5-µm-thick sections and then incubated with MPO primary antibody (1:100 MPO mouse monoclonal antibody; Abcam, Cambridge, MA) followed by incubation with secondary antibody (goat anti-mouse; AlexaFluor 568, Invitrogen). Two random images were taken from each lung section with a fluorescent microscope (Nikon Eclipse Ti) at 200 magnification and quantified using ImageJ software (NIH). Results are reported as relative fluorescence units (RFUs).
Pulmonary syndecan 2 and syndecan 4
As both syndecan 2 and 4 are found in endothelial cells and in the lung, lung tissues were homogenized for measurement of syndecan 2 and syndecan 4 by enzyme-linked immunosorbent assay according to manufacturer’s instructions (MyBioSource, San Diego, CA) or messenger RNA was isolated and the relative mRNA copy numbers quantified by real time PCR and normalized to actin gene expression (Thermo Fischer Scientific, Halethorpe MD).
Data Analysis
All data were analyzed by one way analysis of variance (ANOVA) with Bonferroni correction. P values < 0.05 were considered significant. Data are expressed as mean ± SEM. In vitro data was repeated in triplicates; n=at least 8/group for in vivo animal experiments.
RESULTS
In vitro
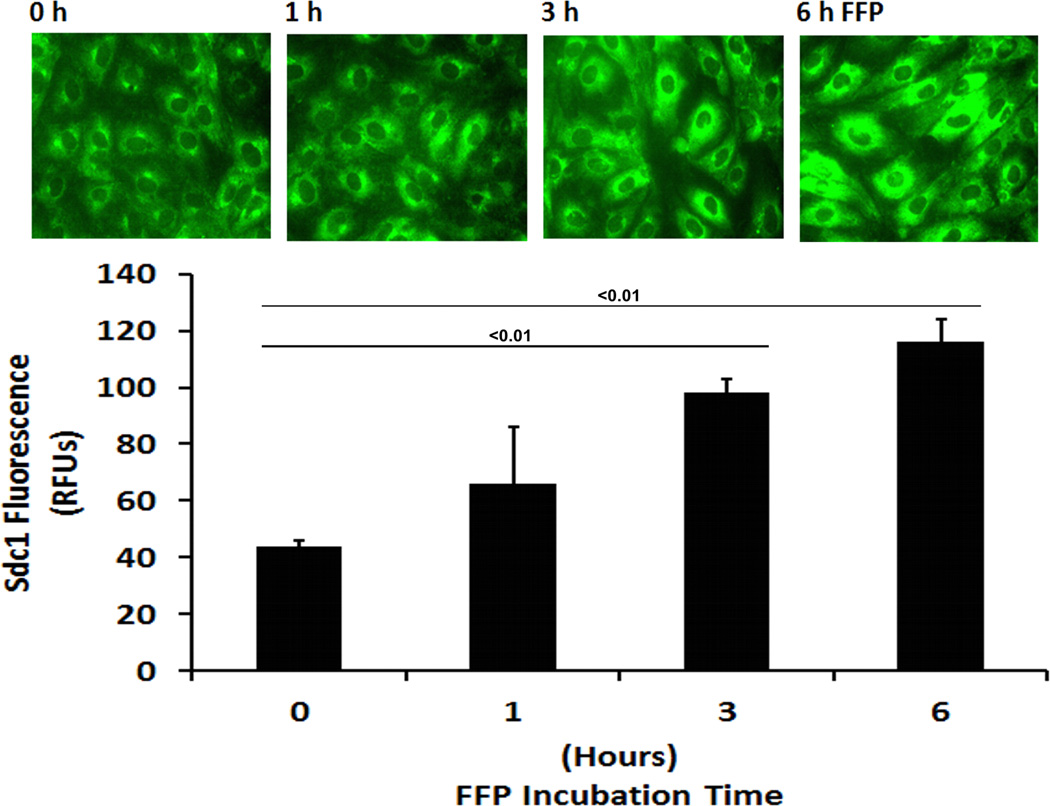
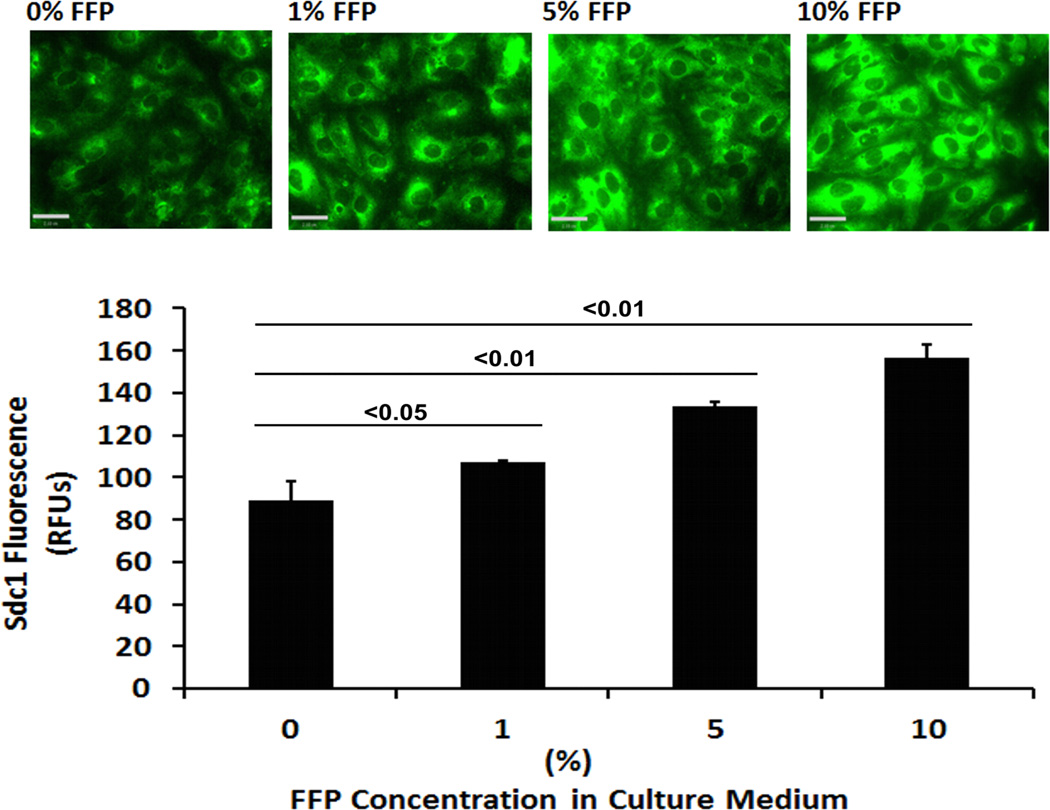
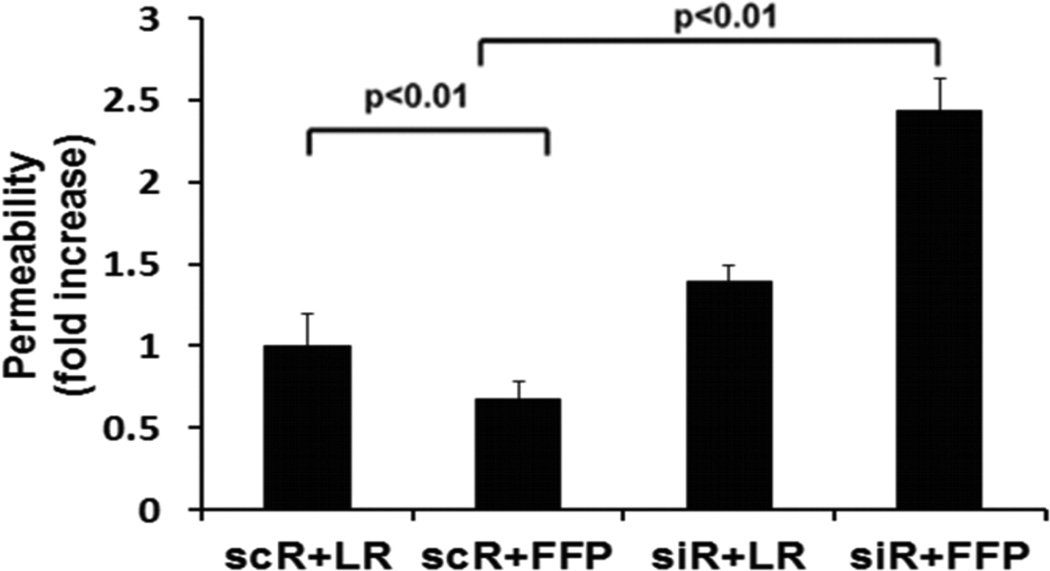
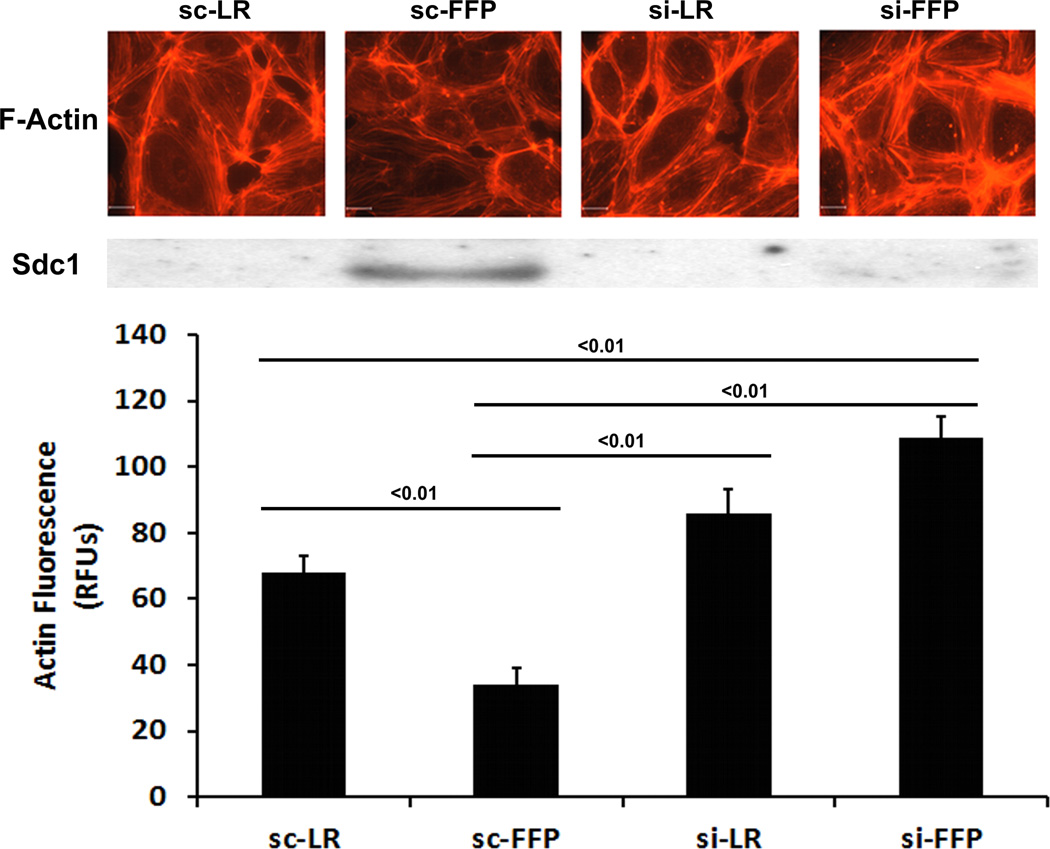
In HLMECs, FFP increased Sdc1 immunostaining in a dose-response and time-dependent fashion (Fig. 1A and 1B). To investigate the contribution of Sdc1 to pulmonary endothelial cell permeability, siRNA was used to silence Sdc1 expression. Under basal conditions, permeability was significantly reduced by FFP compared to LR-treated cells (0.67 ± 0.15 FFP vs 1.0 ± 0.07 LR, p<0.05), similar to our previous findings (Fig 2A).15, 19, 20 After silencing, however, permeability was significantly increased in Sdc1 siRNA transfected cells compared to non-silenced cells (2.43 ± 0.08 si+FFP vs 0.67 ± 0.15 sc+FFP, p<0.01) and to silenced cells treated with LR (1.39 ± 0.24 si-LR, P<0.05). Actin stress fibers are markers of endothelial disruption. Stress fiber staining was consistent with permeability results with stress fibers increased in silenced FFP-treated cells (Fig. 2B).
Fig. 1. FFP increases cell surface Sdc1 in a time- and dose-dependent manner.
Human lung microvascular endothelial cells were cultured in low serum medium (0.5% FBS) for 2 hours and then incubated with serum-free media containing the indicated percentages of FFP. Cells were stained with anti syndecan-1 antibody and images were captured with Nikon E800 fluorescence microscope. Original magnification, × 600. The relative fluorescence intensity was quantified using Quantity One (Bio-RAD) after converting the images to greyscales and reported as relative fluorescence units (RFU). A. Dose dependent increase in sdc1 and B. Time dependent increase in sdc1. Results are presented as means ± SEM, n=3/group.
Fig. 2. FFP increases monolayer permeability and stress fiber formation in Sdc1-deficent endothelial cells.
Human lung microvascular endothelial cells were transfected with Sdc1 siRNA (siR) or scrambled RNA (scR) as control. A. In vitro permeability was assessed by fluoresceine-isothiocyanate cells treated with 10% LR or 10% FFP in serum free medium. B. Stress fiber formation, a marker of endothelial disruption, was examined by staining cells with Texas red-X phalloidin. Top panel: Images were captured with Nikon E800 fluorescence microscope. Original magnification, × 600. Middle panel: Sdc1 protein expression was assessed by Western blot. Bottom panel: the relative fluorescence intensity was quantified using Quantity One (Bio-RAD) after converting the images to greyscales and reported as relative fluorescence units (RFUs). Results are presented as means ± SEM, n=4/group.
In vivo
FFP lessens hemorrhagic shock-induced lung hyperpermeabilty in wild type but not Sdc1−/− mice
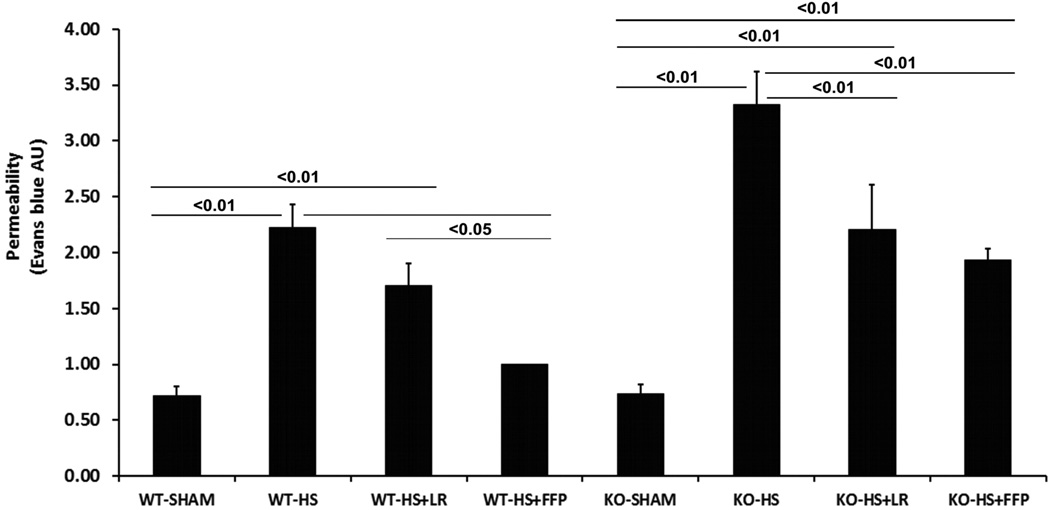
To confirm and expand upon on our in vitro results, wild type and Sdc1 −/− mice were subjected to hemorrhagic shock and resuscitation with either FFP or lactated ringers. As we have previously shown, hemorrhagic shock significantly increased lung permeability compared to shams (2.46 ± 0.27 HS vs 0.72 ± 0.07 Sham, p<0.01), is lessened by LR (1.70 ± 0.20) but further reduced by FFP (1.00 ± 0.08) (Fig. 3).21 Sdc1 −/− mice similarly had enhanced permeability after hemorrhagic shock (3.03 ± 0.31 HS vs 0.74 ± 0.06 Sham, p<0.01) which was also lessened by LR (2.21± 0.18) but with no further benefit from FFP (1.94 ± 0.16).
Fig. 3. Plasma lessens hemorrhagic shock-induced lung hyperpermeabilty.
Wild-type and Sdc1 null mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either LR or FFP. After 3 hours Evans blue dye extravasation was measured in lungs. Data are expressed as mean ± SEM, n=8 per group with significance indicated by lines over the respective groups. FFP, fresh frozen plasma; LR, lactated Ringer’s; WT, wild type; KO, Sdc1 knockout; HS, hemorrhage shock.
FFP reduces pulmonary inflammation in wild type but not Sdc1−/− mice
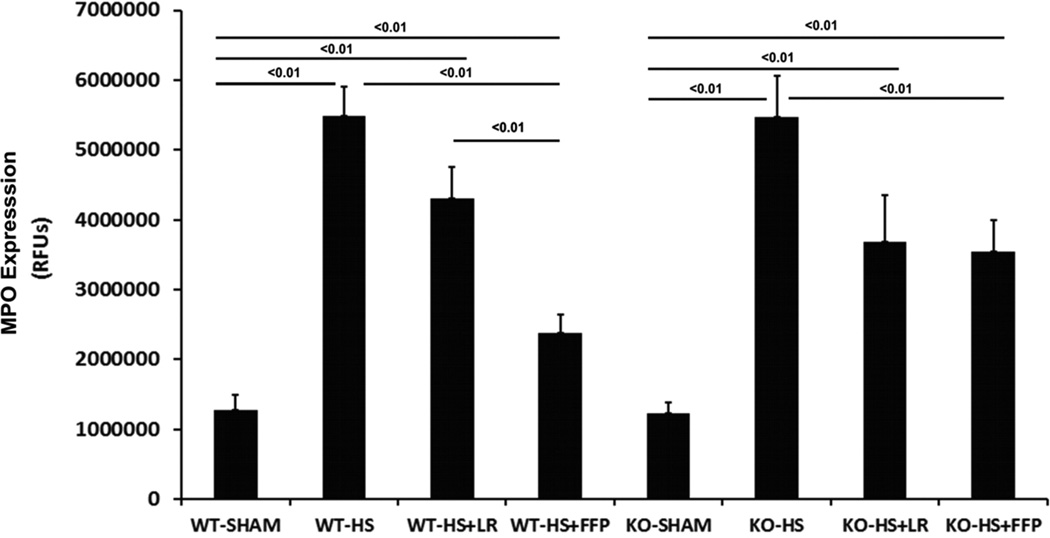
Myeloperoxidase is an indicator of neutrophil infiltration. MPO immunostaining (Fig. 4A) was significantly increased after hemorrhagic shock in WT mice (5,48,4261 ± 424,484 HS vs 1,276,451 ± 212,250 Sham, p<0.01) and LR resuscitated mice (4,299,425 ± 448,282 HS+LR; p<0.01) with a significant decrease in MPO after FFP (2,379,512 ± 258,990) compared to shock alone or LR resuscitated mice. Similarly, shock resulted in an increase in MPO in Sdc1−/− mice (5,464,399 ± 590,065 shock vs 1,232,143 ± 159,153 sham) and a reduction after resuscitation. However, Sdc1−/− mice resuscitated with LR and FFP demonstrated comparable MPO immunostaining. (3544622 ± 444478 HS+FFP vs 3680413 ± 666271 HS+LR, p>0.05)
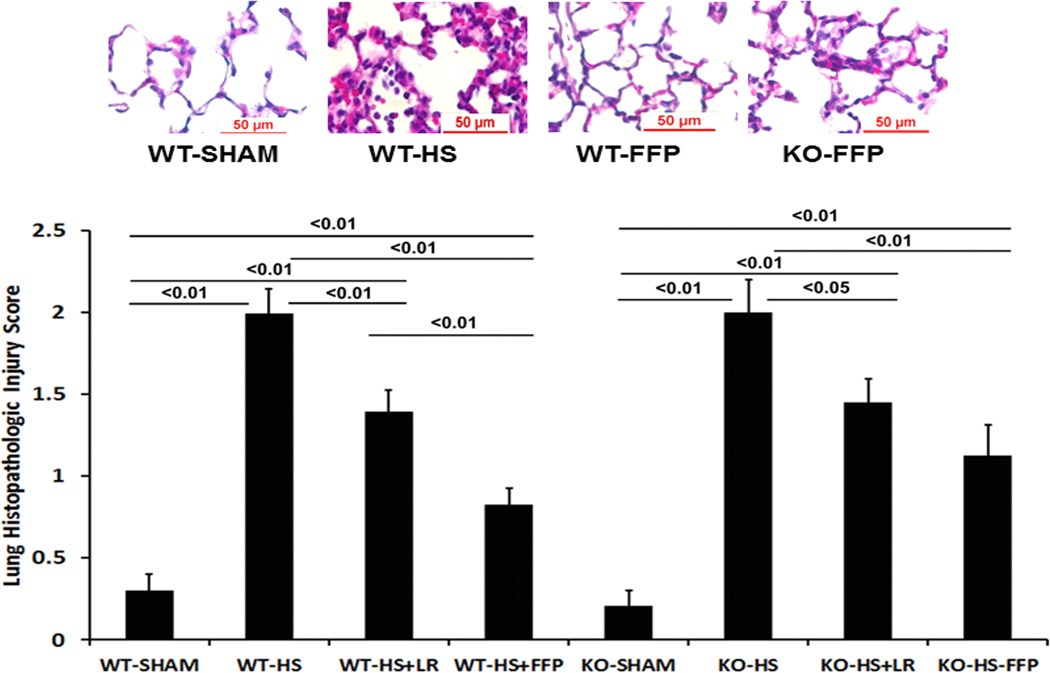
Fig. 4. Plasma mitigates lung inflammation and injury after hemorrhagic shock in wild-type but not syndecan-1 −/− mice.
Wild-type and syndecan-1 null mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either LR or FFP. After 3 hours lungs were harvested for assessment of A. Myeloperoxidase (MPO) enzyme by immunofluorescent staining. Results are reported as relative fluorescence units (RFUs) or B. Lung histopathologic injury. Lungs sections were stained with hematoxylin and eosin and scored on alveolar thickness, capillary congestion, and cellularity. Representative images are shown in the upper panel and injury scores in the lower panel. Data are expressed as mean SEM, n=8 per group with significance indicated by lines over the respective groups. FFP, fresh frozen plasma; LR, lactated Ringer’s; WT, wild type; KO, Sdc1 knockout; HS, hemorrhage shock.
FFP mitigates lung histopathologic injury in wild type but not Sdc1−/− mice
In wild type mice, lung injury was significantly increased after hemorrhagic shock compared to shams (2.0 ± 0.15 HS vs 0.30 ± 0.09 Sham, p<0.01) (Fig. 4B). Resuscitation with LR decreased injury (1.4 ± 0.13) which was further decreased by FFP (0.82 ± 0.10). While Sdc1−/− mice did not demonstrate worsened lung injury compared to WT mice in shams (0.30 ± 0.09 WT vs 0.21 ± 0.09 KO, p>0.05) or in mice subjected to hemorrhagic shock (2.0 ± 0.15 WT vs 2.0 ± 0.20 KO, p>0.05), FFP lost its protective effect and was comparable to LR resuscitated animals (1.13 ± 0.19 FFP vs 1.41 ± 0.14 LR, p>0.05).
Impact of resuscitation on pulmonary syndecan 2 and syndecan 4
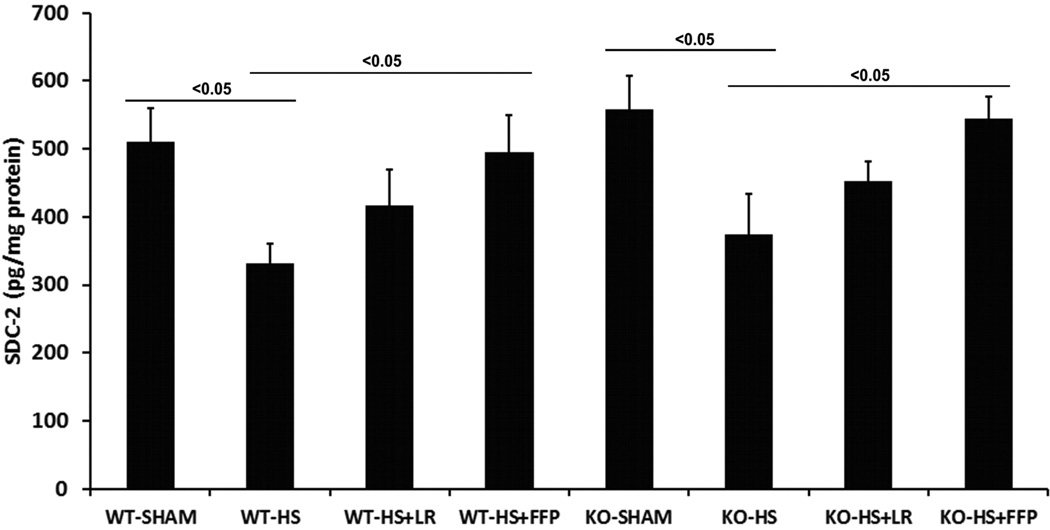
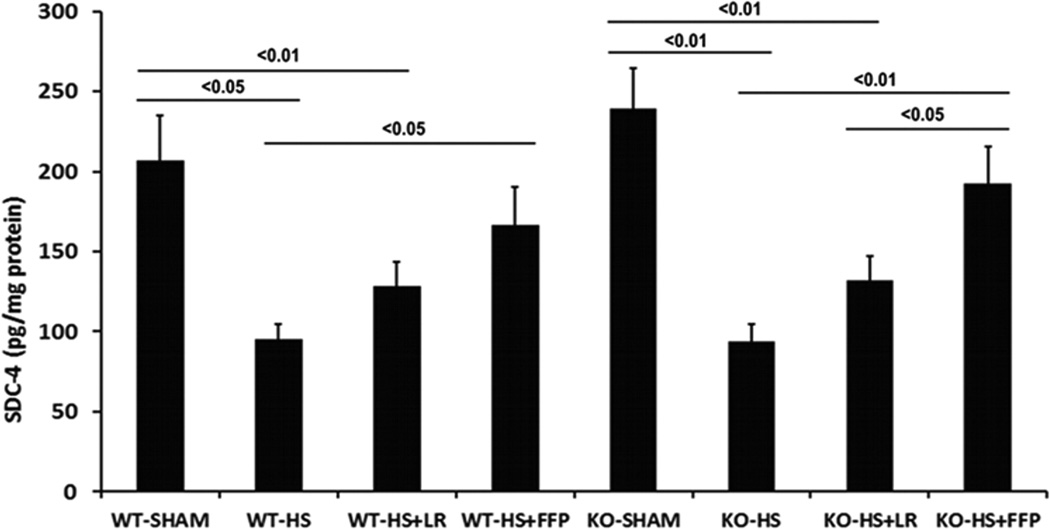
In the next set of experiments, we assessed pulmonary expression of syndecan 2 and syndecan 4, both of which are found in endothelial cells and in the lung24, to evaluate possible compensatory increases in other members of the syndecan family. In WT and in Sdc1−/− mice, pulmonary syndecan 2 and syndecan 4 protein expression showed syndecan 2 was reduced by shock compared to sham mice, increased by FFP compared to shock alone, but with no significant difference in expression between FFP and lactated Ringers resuscitated animals (Fig 5A). Syndecan 4 expression in WT mice was reduced by hemorrhagic shock compared to shams, increased by FFP compared to shock alone, but again with no significant difference in expression between FFP and LR-resuscitated animals (Fig. 5B). However, in Sdc1−/− mice, FFP resuscitation significantly increased pulmonary syndecan-4 expression compared to lactated Ringers resuscitated animals.
Fig. 5. Pulmonary syndecan4, but not syndecan2, expression is increased by FFP in syndecan1 −/− mice.
Wild-type and syndecan-1 −/− mice underwent laparotomy and hemorrhagic shock followed by resuscitation with either LR or FFP. After 3 hours lungs were homogenized for measurement of A. syndecan2 and B. Sdc4 by ELISA. Data are expressed as mean SEM, n=8 per group with significance indicated by lines over the respective groups. FFP, fresh frozen plasma; LR, lactated Ringer’s; WT, wild type; KO, Sdc1 knockout; HS, hemorrhage shock.
DISCUSSION
We demonstrated in vitro that FFP enhanced pulmonary endothelial sdc1 expression in time- and dose-dependent manners. Importantly, expression was increased as early as three hours, the same time that we have shown in vivo partial restoration of the endothelial glycocalyx.18 We hypothesized that if indeed sdc1 was key to FFPs post hemorrhagic shock protection to the endothelium, that the absence of sdc1 would abrogate FFPs protection. When Sdc1 expression in pulmonary vascular endothelial cells was silenced, FFP not only lost its protection compared to LR, but monolayer permeability was actually enhanced. Consistent with this, actin stress fiber formation, a marker of endothelial disruption, was similarly increased in silenced cells exposed to FFP. The worsening of permeability in silenced endothelial cells suggests that FFP interacts with syndecan to exert its protection. In the absence of syndecan, FFP may be in direct contact with the endothelium. Yakovlev et al examined the effects of a recombinant fibrinogen fragment with proven benefit to barrier integrity in animal models of shock. In vitro (no insult) they found that fibrinogen caused internalization of VE-cadherin and loosening of cell-cell junctions.25 It’s possible that FFP may be functioning by a similar mechanism, as fibrinogen is a normal component of plasma. Clinically, cell membranes are likely never completely devoid of cell surface Sdc1, as there appears to be rapid mobilization from presumably intracellular stores, though this has not been well studied.16 Cells do attempt to maintain cell surface expression of heparan sulfate proteoglycans such as syndecan. How this is accomplished is not known. There are several studies suggesting that heparan sulfate proteoglycans can both be internalized via endocytic vesicles and can be recycled to the cell surface26,27.
Our In vivo studies confirmed our in vitro results, demonstrating the key role of Sdc1 in FFPs reduction in pulmonary permeability, inflammation, and injury after hemorrhagic shock. This is important as in vitro studies were in isolated pulmonary endothelial cells but in vivo, intact lungs were used which are comprised of both immune and epithelial cells as well as endothelial cells. Other investigators have also shown that pulmonary sdc1 is important. Brauer et al. reported that Sdc1 knockout increased bronchial epithelial apoptosis and enhanced bronchoaveolar lavage protein extravasation in mice during influenza infection.28 In another study, Sdc1 attenuated allergic lung inflammation, with null mice exhibiting exaggerated airway responsiveness.29
We have previously shown that plasma abrogated gut injury and inflammation after hemorrhagic shock in WT but not in Sdc1−/− mice, similar to our current findings in the lung.22 With an intact Sdc1backbone and endothelial glycocalyx, an important barrier between circulating leukocytes and the endothelial cell surface is established. In the absence of Sdc1, leukocyte transmigration and inflammation is enhanced. Wild type animals demonstrated a significant increase in both systemic TNFα and ADAM-17, a protein that cleaves TNFα, which was further increased in Sdc1−/− mice. Plasma resuscitation lessened both TNFα and ADAM-17 in wild-type but not in Sdc1 −/− mice, highlighting the role of Sdc1 in plasma’s protection. However, the phenotypic shift to a pro-inflammatory phenotype identified in the gut of Sdc−/− mice was not present in the lung. Also, although FFP lost its protective effect in Sdc−/− mice in both the gut and the lung, differences were not as striking in the lung. This led us to hypothesize that other members of the syndecan family may be contributing to pulmonary endothelial protection after hemorrhagic shock. Both syndecan 2 and syndecan 4 are found in endothelial cells and in the lung. In wild type mice, there was a significant increase in both pulmonary syndecan 2 and 4 after FFP compared to shock alone. There was also an increase, though not statistically significant, in both syndecans after FFP compared to LR, implying that these syndecans may be at least contributing to endothelial protection by FFP. However, in Sdc1 −/− mice, syndecan 4 was significantly increased by FFP compared to LR, implying that syndecan 4 is compensating for the loss of syndecan 1. This finding is supported by Savery et al who reported that the endothelial glycocalyx in Sdc1−/− mice was thinner but still detectable, raising the question by these authors if other members of the syndecan family were compensating for the loss of Sdc1.30 Although we did not measure or visualize the glycocalxy in this study, our results are consistent with the hypothesis that syndecan 4 may be contributing to the integrity of the glycocalyx in Sdc1 −/− mice. Additionally, Voyvodic et al recently found that in pulmonary endothelial cells isolated from Sdc1 −/− mice, syndecan 4 was significantly increased compared to WT endothelial cells.31 We used intact lungs and while we did not demonstrate an increase at baseline or after shock between WT and null mice, we did find an increase in syndecan 4 in Sdc1 −/− lungs after FFP administration.
Taken together our results suggest a protective role for Sdc1 after shock with its expression supporting pulmonary protection and generation of a shock protective phenotype that is generated by FFP. In vitro loss of Sdc1 in pulmonary endothelial cells worsened permeability and stress fiber formation by FFP while loss in vivo lead to equivalency between LR and FFP in restoring pulmonary injury, inflammation, and permeability after shock. Lastly, the loss of Sdc1 also lead to an increase in pulmonary syndecan 4 expression specifically after hemorrhagic shock and FFP based resuscitation. We believe this is a compensatory mechanism in Sdc1 −/− mice; despite this response, FFPs loss of pulmonary protection further highlights the key role of Sdc1 in its protection.
Acknowledgments
This grant was funded by the National Institutes of Health grants RO1GM107482 (RAK)
Footnotes
Conflict of Interest: The authors declare no competing conflicts of interest.
REFERENCES
- 1.CDC. Deaths: final data for 2009. US Department of Health and Human Services, CDC, National Center for Health Statistics; 2010. [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Borgman M, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. Blood product replacement affects survival in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB,Wade CE, Michalek JE, Chisholm GB, Zarzabal L, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, Cotton BA, Matijevic N, Muskat P, Myers JG, Phelan HA, White CE, Zhang J, Rahbar MH PROMMTT Study Group. The prospective, observational, multicenter, massive transfusion study, PROMMTT: comparative effectiveness of a time-varying treatment and competing risks. JAMA Surg. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, Depasquale M, Doughty H, Glassberg E, Hervig T, Hooper TJ, Kozar R, Maegele M, Moore EE, Murdock A, Ness PM, Pati S, Rasmussen T, Sailliol A, Schreiber MA, Sunde GA, van de Watering LM, Ward KR, Weiskopf RB, White NJ, Strandenes G, Spinella PC. THOR Position Paper on Remote Damage Control Resuscitation: Definitions, Current Practice and Knowledge Gaps. Shock. 2014;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitsma S, Slaaf DW, Vink H, van Zndvoort M, Oude Egbrink M. The endothelial glycocalyx:composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Bruno R, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 9.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83:715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Ostrowski SR, Haase N, Müller RB, Møller MH, Pott FC, Perner A, Johansson PI. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care. 2015;19:191. doi: 10.1186/s13054-015-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, Russell RT, Kerby JD, Pittet JF, Dull RO. The glycocalyx and trauma: a review. Shock. 2016;45:338–348. doi: 10.1097/SHK.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 13.Darwiche SS, Ruan X, Hoffman MK, Zettel KR, Tracy AP, Schroeder LM, Cai C, Hoffman RA, Scott MJ, Pape HC, et al. Selective roles for toll-like receptors 2, 4, and 9 in systemic inflammation and immune dysfunction following peripheral tissue injury. J Trauma Acute Care Surg. 2013;74:1454–1461. doi: 10.1097/TA.0b013e3182905ed2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang WW, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6:e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, Protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73:60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 18.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter D, Baimukanova G, Peng Z, Gibb S, Keating S, Deng X, Muench M, Fomin M, Spinella P, Kozar R, Pati S. Fresh frozen plasma and spray dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma and Acute Care Surg. 2015;78(6 Suppl 1):S7–S17. doi: 10.1097/TA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 20.Wataha K, Menge T, Deng X, Shah A, Bode A, Holcomb JB, Potter D, Kozar RA, Spinella PC, Pati S. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013;53(Suppl 1):80S–90S. doi: 10.1111/trf.12040. [DOI] [PubMed] [Google Scholar]
- 21.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013;40:195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban K, Peng Z, Pati S, Witkov RB, Park PW, Kozar RA. Plasma-mediated gut protection after hemorrhagic shock is lessened in syndecan-1−/− mice. Shock. 2015;44:452–457. doi: 10.1097/SHK.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia reperfusion injury is lectin complement pathway dependent without involving C1q1. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 24.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns molecular biology of the cell. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakovlev S, Mikhailenko I, Cao C, Zhang L, Strickland DK, Medved L. Identifiction of VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent transendothelial migration of leukocytes. Blood. 2012;119(2):637–644. doi: 10.1182/blood-2011-09-382580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schultz J, N’Kuli F, Courtoy PJ, David G. Syndecan recyling is controlled by syntenin-PIP2 interaction and Arf6. Developmental Cell. 2005 Sep;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Wittrup A, Zhang SH, Ten Dam GB, van Kuppevelt TH, Bengtson P, Johansson M, Welch J, Mörgelin M, Belting M. ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: evidence for heparan sulfate epitope specificity and role of both syndecan and glypican. J Biol Chem. 2009 Nov 20;284(47):32959–32967. doi: 10.1074/jbc.M109.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brauer R, Ge L, Schlesinger SY, Birkland TP, Huang Y, Parimon T, Lee V, McKinney BL, McGuire JK, Parks WC, Chen P. Syndecan-1 Attenuates Lung Injury during Influenza Infection by Potentiating c-Met Signaling to Suppress Epithelial Apoptosis. Am J Respir Crit Care Med. 2016 Aug 1;194(3):333–344. doi: 10.1164/rccm.201509-1878OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous attenuation of allergic lung inflammation by syndecan-1. J Immunol. 2005 May 1;174(9):5758–5765. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]
- 30.Savery MD, Jiang JX, Park PW, Damiano ER. The endothelial glycocalyx in syndecan-1 deficient mice. Microvas Res. 2013;87:83–91. doi: 10.1016/j.mvr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014 Apr 4;289(14):9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]