Abstract
Purpose
The purpose of this paper is to evaluate the efficacy of a lactose- reduced synbiotic partial whey hydrolysate in formula fed infants presenting with colic and the impact of this dietary intervention in mean crying time and quality of life.
Methods
Forty infants with infantile colic were treated during one month with parental reassurance and the intervention formula (partial whey hydrolysate, reduced lactose, Bifidobacterium lactis BB12 and galacto-oligosaccharides) and were compared to a control group of 20 infants with infantile colic treated with parental reassurance and a standard infant formula. Parents completed a quality of life (QoL) questionnaire assessing the burden of infantile colic. Wilcoxon test, t-test and Mann-Whitney test were used to compare QoL scores before and after intervention as well as between the intervention and control group.
Results
At inclusion, duration of crying did not differ between both groups. Crying duration decreased with 2.7 hours (from 3.2 to 0.5 hours) in the intervention group while duration of crying decreased only with 1.2 hours in the control group (p<0.001). Stool composition became looser in the intervention group, but defecation frequency did not change. The median scores of the QoL questionnaire improved significantly in the intervention group for all parameters. In the control group, parameters improved significantly also but not for the parent-child and social interaction. The score changes were significantly greater in the intervention than in the control group.
Conclusion
The intervention formula (partial whey hydrolysate, synbiotic, reduced lactose) significantly reduced the duration of crying and improved QoL of the parents and infants.
Keywords: Colic, Lactose, Partial hydrolysate, Prebiotics, Probiotics, Galacto-oligosaccharide, Bifidobacterium lactis
INTRODUCTION
Infantile colic (IC) is a frequent and distressing functional disorder which is defined as unsoothable crying for longer than three hours per day, for three or more days per week during at least one week without failure to thrive or any obvious organic cause [1]. Although IC is considered to be a limiting and benign condition, it raises anxiousness and concern for parents and is a frequent reason for parents to seek medical help [2,3,4,5].
The prevalence of IC was reported by Canivet et al. [6] to be within a range of 9% and 60%, while the estimation of an expert group was 17.7% [7]. The pathogenesis of IC remains unknown, but exposure to tobacco smoke is recognized as a potential risk factor [8]. It is hypothesized that immaturity of the nervous or digestive system of the infants is interfere as a risk factor.
Dietary changes may be of some benefit for relief of crying in IC [9,10]. The aim of this study was to evaluate the efficacy of a formula containing a partially hydrolyzed whey protein (pWH), reduced lactose content, the probiotic Bifidobacterium lactis BB12 and the prebiotic galacto-oligosaccharide (GOS) in formula fed infants suffering from IC. Data from Savino et al. suggested with a comparable formula that this kind of dietary intervention may be of benefit [11]. The formula in the study by Savino and the intervention formula in this trial both contain a pWH, reduced lactose and GOS as prebiotic. The formula in the study by Savino was in addition thickened with cereals, contained fructo-oligosaccharides (FOS), and did not contain probiotics as did the intervention formula in this study.
MATERIALS AND METHODS
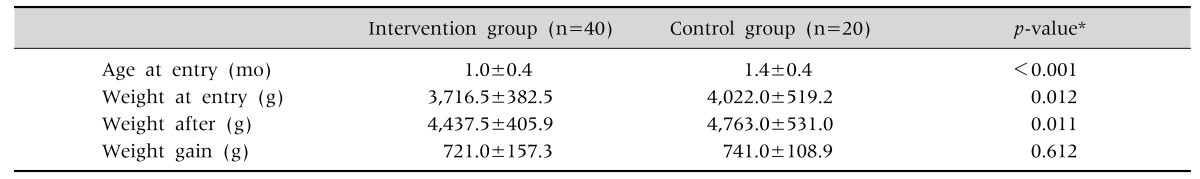
This open study was conducted at the 3rd Pediatric Department of the Hippocration Hospital, Aristotle University, Thessaloniki, from September 2014 to May 2016. The local ethical committee of Aristotle University approved the study protocol (346/20-12-2016). IC was defined as episodes of unsoothable crying for more than three hours a day for at least three days a week since at least one week. Forty full-term infants with an age range of 20 to 60 days suffering from IC without clinical evidence for an organic cause for the crying such as otitis, urine tract infection, constipation, anal fissure, esophagitis, etc. were included. The characteristics of the infants are listed in Table 1. Exclusion criteria were besides an obvious organic cause for the crying, insufficient weight gain (<150 g/week) and/or presenting with blood in the stools.
Table 1. Age at Inclusion and Weight Evolution of Infants.
Values are presented as mean±standard deviation.
*t-test.
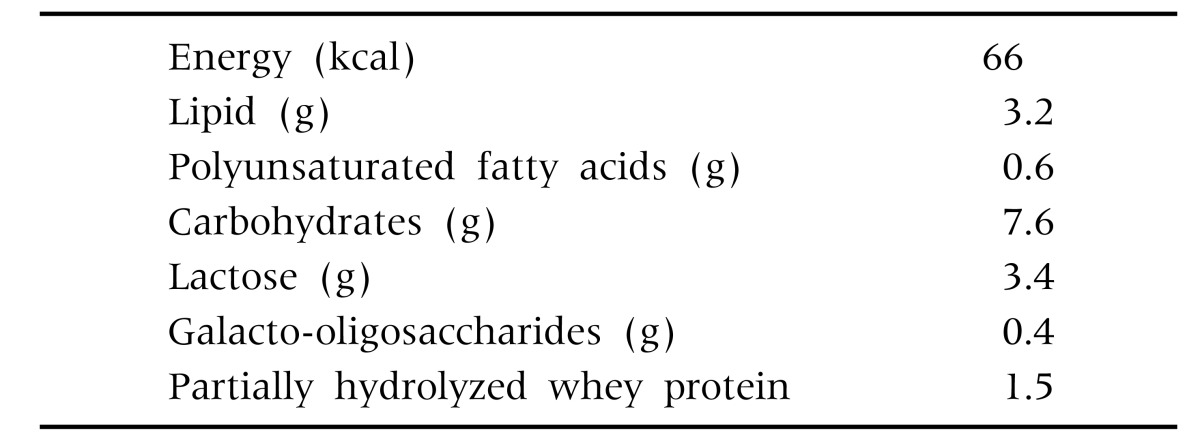
When an infant met the inclusion criteria, parents were invited to participate in the study and had to give their written consent before the infant could be included. Infants were included consecutively: for every two infants receiving the intervention formula, one infant was included in the control group, according to the recommendations, reassurance and anticipatory guidance [11]. The intervention formula (Table 2: composition on the intervention formula) needed for one month was given to the parents. Feeding in the control group consisted of any regular starter infant formula available on the Greek market. The control group received no medication, food supplement or probiotic.
Table 2. Composition of the Intervention Formula (per 100 mL).
Parents were asked to assess their infants at inclusion and at the end of the one month intervention. Duration of crying was the primary outcome (Table 3). Each assessment at the start and end of the study was done over a period of seven consecutive days: before the intervention formula was given, or the standard starter formula was continued, and during the fourth week after the start of dietary intervention.
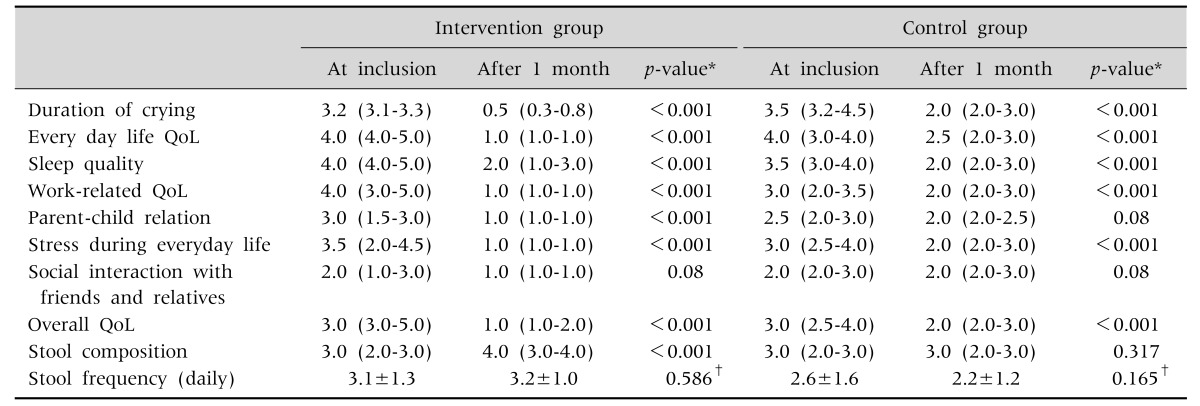
Table 3. Median Scores of the Quality of Life (QoL) Variables in the Intervention and the Control Group, Stool Composition and Frequency at Inclusion and after One Month Intervention.
Values are presented as median (interquartile range, IQR) or mean±standard deviation.
*Wilcoxon test, †t-test.
All parents were asked to complete the parent form of a quality of life (QoL) questionnaire consisting of seven items regarding symptom duration and frequency, emotions, activities and different aspects related to the management of an infant suffering IC. The scale assesses the burden of IC on a scale ranging from 1 to 5 (very low burden=1 to extremely heavy burden=5). More specifically, the questions addressed the following domains: i) every day QoL, ii) sleep quality, iii) work-related QoL, iv) parent-child relationship, v) stress during everyday life, vi) QoL regarding social interaction with friends and relatives, and vii) overall QoL (Table 3). In addition, parents had to record information regarding defecation frequency and composition (Tables 3 and 4). Stool composition was assessed by using a scale from 1 to 5 (hard=1; tight=2, creamy=3, loose=4, watery= 5). During the follow-up visit after one month the parents were asked to rate the evolution during the intervention by selecting one of the following options: a) very important benefit=1, b) important benefit= 2, c) moderate benefit=3, d) very small benefit= 4, and e) no benefit=5.
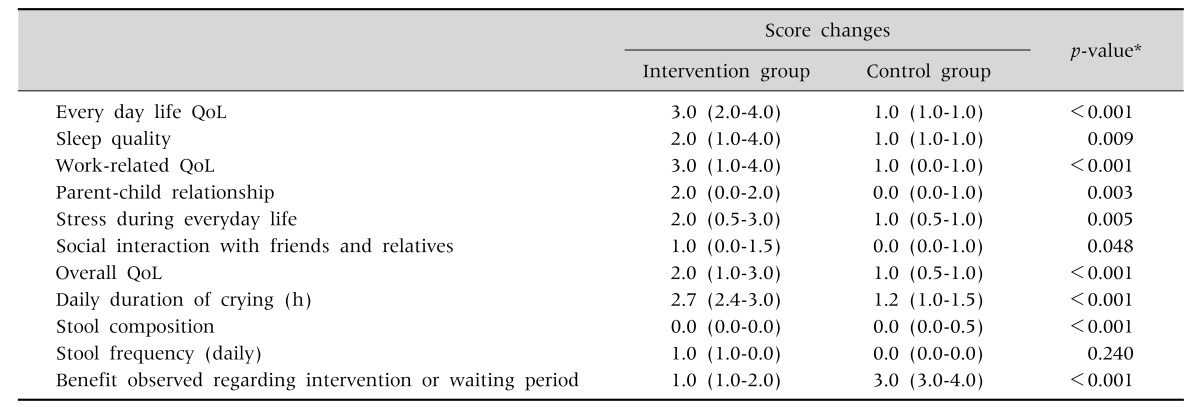
Table 4. The Median Score Changes for All the Quality of Life (QoL) Variables Stool Composition and Frequency between the First and Second Month following the Intervention.
Values are presented as median (interquartile range, IQR).
*Mann-Whitney test.
The non-parametric Wilcoxon test was used to investigate statistically significance of differences in scores between infants in the intervention and control groups at two time points, at inclusion and after one month intervention. t-test (paired and two-sample) was used in order to compare continuous variables between infants in the intervention and control groups, when normality assumption was not violated. Score changes between the intervention and the control group were compared. The non-parametric Mann-Whitney test was used in order to compare score changes between two independent samples based on the variable type. All tests were two-sided and the level of significance was set at 0.05. IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis of data.
RESULTS
The infants in the control group were slightly older than these in the intervention group (Table 1). AT inclusion, the duration of crying between both groups did not differ. Weight gain in the intervention and control group was comparable. Crying time decreased in the intervention group from 3.2 hours (interquartile range [IQR], 3.1–3.3) to 0.5 hours (IQR, 0.3–0.8) (Table 3). Although the decrease in crying time was also significant in the control group, the difference in decrease of crying time between the intervention and control group was statistically significant (1.5 hours difference) (Table 4).
Median scores of the QoL questionnaire obtained at inclusion decreased after one month in both the intervention and the control group for the majority of the variables indicating improvement. More specifically, in the intervention group median scores improved significantly for all parameters. In the control group, all parameters improved as well except for the parent-child interaction and QoL related social interaction with friends and relatives.
Stool composition became looser only in the intervention group. Defecation frequency showed no statistically significant change in either group (Table 4).
The median score changes for all the 7 QoL questionnaire variables between inclusion and after one month was significantly greater in the intervention compared to the control group (Table 4). The median score regarding the benefit of intervention versus no intervention was also statistically significantly lower, i.e., better, in the intervention group (Tables 3, 4).
DISCUSSION
A formula containing a pWH, reduced lactose and supplemented with B. lactis BB12 and GOS improved significantly QoL and decreased duration of crying time in infants with IC. The literature suggesting benefit of pain-relieving agents in the treatment of IC is sparse and mod tog the time negative [12]. Although simethicone is popular, there are no data suggesting benefit in IC [12]. Also data on the efficacy of herbal agents, sugar, dicyclomine and cimetropium bromide in IC are either limited (as for some herbal agents) or not existing [12]. Cimetropium bromide and simethicone were less effective than a thickened pWH with reduced lactose and prebiotics [13].
The role of lactose in the pathophysiology of IC is debated. The absence of lactose in infant formula does not affect normal growth and may result in less regurgitation and softer stools [14]. A transient low lactase activity has been proposed to trigger excessive crying in infants [10,15]. Lactose-free or soy based infant formulae were not consistently reported as beneficial in IC. Lactase or placebo was administered to a small group of infants (n=12) within 5 minutes of breastfeeding in a double-blind, placebo-controlled crossover trial [16]. Compared to placebo, lactase had no significant effect on the duration of crying and fussing [16]. In another double-blind, placebo-controlled crossover trial in 13 infants, lactase reduced crying time with 1.14 hours per day [17]. Selection of patients is likely to be a major bias in most studies. After some initial enthusiasm negative reports resulted a minor role for lactase in IC [10]. In prevention, a formula containing a stable lactase as the result of a fermentation process indicated a decreased incidence of infant crying at the age of four weeks [18]. Although the NICE guidelines (UK-recommendation) suggest a one-week trial of lactase drops in breastfed and formula-fed infants, the evidence for this recommendation is limited [19].
Hydrolyzed protein may also be beneficial in the management of IC. An extensive casein hydrolysate reduced the duration of crying in 15/22 infants [20]. A double-blind challenge test with placebo or capsules containing whey milk powder was positive in 11/15 infants [20]. Similar results, a decrease of crying time with 63 minutes, have been reported with an extensive whey hydrolysate [21]. Crying duration deceased with 45% in a small group of infants with IC (n=6) fed an amino acid based formula [22]. A challenge with 75 mg of bovine immunoglobulin G resulted in an increase of crying and fussing behavior [22].
Several studies have assessed the effect of probiotics in the management of IC. Different studies provided sufficient evidence to conclude in meta-analyses that Lactobacillus reuteri DSM 17938 at a dose of 108 colony-forming units per day in breastfed infants decreased IC and was well tolerated and safe [23,24], resulting in a reduction of parental discomfort and the number of consultations [13]. One study in mainly formula fed infants with the same L. reuteri strain yielded a negative result [25]. At least two trials with L. rhamnosus in infants with IC were negative [26,27]. Infants fed B. lactis and Streptococcus thermophilus reported a significantly lower incidence of IC or irritability than the placebo group [28]. However, there was no significant difference in episodes of fever, loose stools and vomiting, or discomfort passing bowel movements and daycare absenteeism [28]. However, antibiotics were significantly less used in both probiotic groups than in the placebo group [29]. The duration of crying in our study in the intervention group receiving a formula supplemented with the probiotic B. lactis decreased from over 3 hours to less than one hour daily. The reduction of the duration of crying was also noted in the control group, but the effect in the probiotic group was much more pronounced.
A few studies evaluated the efficacy of prebiotics in the management of IC. A pWH supplemented with FOS and GOS resulted in a reduction of crying episodes in infants with IC after 1 and 2 weeks in comparison with a standard formula and simethicone [30]. The same formula was previously evaluated in an open study and did show a reduction of colic and of feeding problems such as regurgitation and defecation problems [29]. A GOS-supplemented formula mimicked the effect of human milk on the gastro-intestinal microbiome promoting Bifidobacterium and Lactobacillus growth and inhibiting Clostridium growth, resulting in a significantly lower prevalence of IC [31].
A major shortcoming is that the present study is an open-labeled trial in which the control group differed at baseline from in the intervention group. The control group was 0.4 months older than the intervention group. However, the difference favors the control group as IC tends to decrease spontaneously over time. Since the difference in median age of inclusion is only twee weeks, with a median age of 1 month in the intervention group and 1.4 months in the control group, it is unlikely that the control group suffered more severe symptoms as the age at which spontaneous decrease starts is 3 months. Moreover, it is virtually impossible to blind studies with infant formula as the formulas that are compared often have differences in texture, smell and taste. Also, this was not a classically randomized trial. Although by applying a consecutive allocation sequence of “two infants intervention formula-one infant standard infant formula” a bias-risk induced by selection of patients was minimized. Another limitation of the present clinical trial is that the data rely on subjective observations made by the parents. Future clinical trials should benefit from more objective observations, such as computer recording of crying episodes, and therefore provide a more precise and reliable way of evaluating IC. Moreover, in this study -as in other studies evaluating a formula change- several interventions are evaluated concomitantly. As a consequence, the effectiveness of each intervention on itself is not known. Furthermore, the bias risk of the study is high as the study was open.
In conclusion, this clinical trial supports the beneficial effects of a formula with a partial whey hydrolysate, reduced lactose, supplemented with a probiotic (B. lactis) and prebiotic (GOS) for IC in formula-fed infants. These data need to be confirmed by a double-blind, prospective, randomized trial.
ACKNOWLEDGEMENTS
This study has been supported by Rontis, providing free formula in the intervention group for the study period. Yvan Vandenplas has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Aspen, Biogaia, Biocodex, Danone, Hero, Kabrita, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Merck, Olygose, Orafti, Phacobel, Rontis, Sari Husada, United Pharmaceuticals, Wyeth and Yakult. The co-authors did not report any potential COI.
References
- 1.Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Savino F, Tarasco V. New treatments for infant colic. Curr Opin Pediatr. 2010;22:791–797. doi: 10.1097/MOP.0b013e32833fac24. [DOI] [PubMed] [Google Scholar]
- 3.Radesky JS, Zuckerman B, Silverstein M, Rivara FP, Barr M, Taylor JA, et al. Inconsolable infant crying and maternal postpartum depressive symptoms. Pediatrics. 2013;131:e1857–e1864. doi: 10.1542/peds.2012-3316. [DOI] [PubMed] [Google Scholar]
- 4.Morris S, James-Roberts IS, Sleep J, Gillham P. Economic evaluation of strategies for managing crying and sleeping problems. Arch Dis Child. 2001;84:15–19. doi: 10.1136/adc.84.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M, Heine RG, Jordan B. Health and well-being in school-age children following persistent crying in infancy. J Paediatr Child Health. 2009;45:254–262. doi: 10.1111/j.1440-1754.2009.01487.x. [DOI] [PubMed] [Google Scholar]
- 6.Canivet C, Hagander B, Jakobsson I, Lanke J. Infantile colic--less common than previously estimated. Acta Paediatr. 1996;85:454–458. doi: 10.1111/j.1651-2227.1996.tb14060.x. [DOI] [PubMed] [Google Scholar]
- 7.Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015;61:531–537. doi: 10.1097/MPG.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shenassa ED, Brown MJ. Maternal smoking and infantile gastrointestinal dysregulation: the case of colic. Pediatrics. 2004;114:e497–e505. doi: 10.1542/peds.2004-1036. [DOI] [PubMed] [Google Scholar]
- 9.Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics. 2000;106:184–190. [PubMed] [Google Scholar]
- 10.Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. J Paediatr Child Health. 2012;48:128–137. doi: 10.1111/j.1440-1754.2011.02061.x. [DOI] [PubMed] [Google Scholar]
- 11.Vandenplas Y, Benninga M, Broekaert I, Falconer J, Gottrand F, Guarino A, et al. Functional gastro-intestinal disorder algorithms focus on early recognition, parental reassurance and nutritional strategies. Acta Paediatr. 2016;105:244–252. doi: 10.1111/apa.13270. [DOI] [PubMed] [Google Scholar]
- 12.Biagioli E, Tarasco V, Lingua C, Moja L, Savino F. Pain-relieving agents for infantile colic. Cochrane Database Syst Rev. 2016;9:CD009999. doi: 10.1002/14651858.CD009999.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasekan JB, Jacobs J, Reisinger KS, Montalto MB, Frantz MP, Blatter MM. Lactose-free milk protein-based infant formula: impact on growth and gastrointestinal tolerance in infants. Clin Pediatr (Phila) 2011;50:330–337. doi: 10.1177/0009922810390511. [DOI] [PubMed] [Google Scholar]
- 14.Savino F, Ceratto S, Poggi E, Cartosio ME, Cordero di Montezemolo L, et al. Preventive effects of oral probiotic on infantile colic: a prospective, randomised, blinded, controlled trial using Lactobacillus reuteri DSM 17938. Benef Microbes. 2015;6:245–251. doi: 10.3920/BM2014.0090. [DOI] [PubMed] [Google Scholar]
- 15.Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. J Hum Nutr Diet. 2001;14:359–363. doi: 10.1046/j.1365-277x.2001.00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller JJ, McVeagh P, Fleet GH, Petocz P, Brand JC. Effect of yeast lactase enzyme on "colic" in infants fed human milk. J Pediatr. 1990;117:261–263. doi: 10.1016/s0022-3476(05)80542-6. [DOI] [PubMed] [Google Scholar]
- 17.Kearney PJ, Malone AJ, Hayes T, Cole M, Hyland M. A trial of lactase in the management of infantile colic. J Hum Nutr Diet. 1998;11:281–285. [Google Scholar]
- 18.Vandenplas Y, Ludwig T, Bouritius H, Hourihane J, Huet F. O-174 The combination of scGOS/lcFOS with fermented infant formula reduces the incidence of colic in 4 week old infants. Arch Dis Child. 2014;99(Suppl 2):A91–A92. [Google Scholar]
- 19.Infantile Colic. National Institute for Health Care and Excellence, Clinical Knowledge Summaries [Internet] Available from: http://cks.nice.org.uk/colic-infantile. (website only available in UK)
- 20.Jakobsson I, Lothe L, Ley D, Borschel MW. Effectiveness of casein hydrolysate feedings in infants with colic. Acta Paediatr. 2000;89:18–21. doi: 10.1080/080352500750028997. [DOI] [PubMed] [Google Scholar]
- 21.Lucassen PL, Assendelft WJ, Gubbels JW, van Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate: a double-blind, randomized, placebo-controlled trial. Pediatrics. 2000;106:1349–1354. doi: 10.1542/peds.106.6.1349. [DOI] [PubMed] [Google Scholar]
- 22.Estep DC, Kulczycki A., Jr Treatment of infant colic with amino acid-based infant formula: a preliminary study. Acta Paediatr. 2000;89:22–27. doi: 10.1080/080352500750029004. [DOI] [PubMed] [Google Scholar]
- 23.Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526–e533. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- 24.Harb T, Matsuyama M, David M, Hill RJ. Infant colic-what works: a systematic review of interventions for breast-fed infants. J Pediatr Gastroenterol Nutr. 2016;62:668–686. doi: 10.1097/MPG.0000000000001075. [DOI] [PubMed] [Google Scholar]
- 25.Sung V, Hiscock H, Tang ML, Mensah FK, Nation ML, Satzke C, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107. doi: 10.1136/bmj.g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res. 2015;78:470–475. doi: 10.1038/pr.2015.127. [DOI] [PubMed] [Google Scholar]
- 27.Fatheree NY, Liu Y, Ferris M, Van Arsdall M, McMurtry V, Zozaya M, et al. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: A pilot study of recruitment, retention, and fecal biomarkers. World J Gastrointest Pathophysiol. 2016;7:160–170. doi: 10.4291/wjgp.v7.i1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79:261–267. doi: 10.1093/ajcn/79.2.261. [DOI] [PubMed] [Google Scholar]
- 29.Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl. 2003;91:86–90. doi: 10.1111/j.1651-2227.2003.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 30.Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr. 2006;60:1304–1310. doi: 10.1038/sj.ejcn.1602457. [DOI] [PubMed] [Google Scholar]
- 31.Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, et al. Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: randomized multicenter trial. J Am Coll Nutr. 2014;33:385–393. doi: 10.1080/07315724.2013.878232. [DOI] [PubMed] [Google Scholar]