Abstract
Purpose
This study clarified the bacterial pathogens currently causing acute infectious enterocolitis (AIE) in children and evaluated the clinical characteristics and ultrasonographic findings according to the different pathogens.
Methods
Medical records regarding age, sex, clinical symptoms, laboratory data, identified enteropathogens, ultrasonographic findings, treatment, and outcome of 34 patients who were diagnosed with AIE via stool examination using multiplex polymerase chain reaction (PCR) or culture, were retrospectively reviewed.
Results
Twenty-four patients (70.6%) were male. The mean age of the patients was 8.5±6.2 (range, 1.1–17.1) years. Six bacterial pathogens were isolated: Salmonella species (spp.) (32.4%), Campylobacter spp. (20.6%), verotoxin-producing Escherichia coli (14.7%), Staphylococcus aureus (11.8%), Clostridium difficile (8.8%), and Shigella spp. (2.9%). Abdominal pain occurred in all patients regardless of pathogen. The patients infected with Salmonella were older than those infected with verotoxin-producing E. coli (p<0.05). C-reactive protein levels were higher in patients with Salmonella and Campylobacter infections than in those with verotoxin-producing E. coli infection (p<0.05), the other clinical and laboratory data were indistinguishable between pathogens. Ultrasonography demonstrated diverse involvement of bowel segments according to pathogen. Wall thickening of both the ileum and the entire colon was the most common lesion site regardless of pathogen.
Conclusion
Various bacterial agents cause AIE and the symptoms are diverse symptoms, however, all most children recovered spontaneously. Use of multiplex PCR on stool samples warrants improvement of its sensitivity for diagnosis of enteropathogenic bacteria. Ultrasonographic examination is useful for diagnosis of AIE; it can also detect the disease extent and severity.
Keywords: Enterocolitis, Child, Ultrasonography
INTRODUCTION
Acute infectious enterocolitis (AIE) in children remains prevalent worldwide. The etiology and clinical course of AIE are diverse. Although the symptoms depend on the causative pathogen, they commonly include fever, abdominal cramps, nausea or vomiting, and watery or bloody diarrhea. However, these symptoms are nonspecific and may mimic a surgical condition such as appendicitis. Thus, accurate diagnosis of AIE is essential to ensure adequate management.
The causative bacterial agents of infectious diarrheal disease were traditionally diagnosed via stool culture, which has a low sensitivity and relatively long processing time. The causative agents remain unidentified in the majority of patients. Recently, more rapid approaches for the direct identification of diarrheagenic bacteria in stool specimens have been developed using polymerase chain reaction (PCR)-based methods. Multiplex PCR has a high sensitivity to rapidly and simultaneously detect multiple enteropathogens [1,2].
Ultrasonography (US) is usually the first imaging examination performed in children with abdominal pain and/or gastrointestinal symptoms. US can be used to evaluate the severity of wall thickening and the extent, distribution and extramural extension of the colitis, as well as the presence of small bowel involvement [3]. However, imaging is not required for diagnosis of AIE. When the sonographic pattern and the anatomic distribution of the colonic disease are correlated with clinical findings, physicians can obtain a correct diagnosis and avoid invasive procedures such as computed tomography or endoscopy [4].
However, only a few reports have described the causative pathogens and US findings in children with AIE. This study used multiplex PCR or culture to identify the bacterial pathogens currently causing acute gastroenteritis in children, assessed the clinical characteristics of AIE according to pathogen, and evaluated ultrasonographic findings in AIE according to pathogen.
MATERIALS AND METHODS
Patients
From January 2013 to July 2014, we investigated 34 patients who were admitted to Pusan National University Children's Hospital for acute abdominal pain and who were diagnosed with infectious enterocolitis by multiplex PCR or culture of stool samples, and by abdominal US. Patients with non-specific enteral co-infection were excluded from this study.
Clinical and laboratory data
Data were collected retrospectively by reviewing patient medical records. Clinical data regarding age, sex, clinical symptoms, laboratory data, identified enteropathogens, US findings, treatment, and outcome were investigated. Laboratory data regarding white blood cell count, percentage of segmented neutrophils, platelet count, and C-reactive protein (CRP) levels were also collected.
Methods for enteropathogenic evaluation
Stool specimens weighing at least 2 g were stored at 4℃ immediately after collection. All of the stool examinations except for Clostridium difficile were conducted in the Division of Microbiology and Epidemiology, at the Gyeongnam Research Institute of Health & Environment in Korea. The specimens were delivered by a cooler bag to the laboratory, where tests were performed once weekly.
The examinations were performed using a standard protocol from The Practice Guideline of Diagnosis of the Waterborne and Food Related Diseases (2013) [5]. The test techniques were as follows. For bacterial examinations, the collected feces were diluted in 1 mL of phosphate-buffered saline (pH 7.4). Each 100 µL of diluted specimen was incubated on 2–5 mL of selected enrichment media for enrichment culture. All cultured specimens underwent PCR analysis. Secondary biochemical PCR analysis was performed to identify toxins isolated from the following enteropathogens: Salmonella, Shigella, Vibrio, enteropathogenic E. coli, Bacillus, Listeria, Clostridium perfringens, Yersinia, S. aureus, and Campylobacter. Together with the culture study, bacterial pathogens were also identified using the Seeplex® Diarrhea ACE Detection kit (Seegene, Seoul, Korea) by standard protocol in our institute. Positive results of culture or PCR tests were considered to be the causative pathogens of the enterocolitis.
US examination
The initial US was performed within the first two days of presentation of symptoms in all patients. The examinations were conducted by one pediatric gastroenterologist and ultrasound specialist. Intestinal wall thickness was examined by real-time US (Sequoia 512 US system; Acuson, Mountain View, CA, USA) using a high frequency probe (10-MHz linear transducers) and graded compression.
Wall thicknesses >2 mm measured in a transverse section were considered abnormal [6]. The extent and anatomic distribution of colonic involvement were described as follows: diffuse, right-sided (cecum, ascending colon, and/or proximal transverse colon) or left-sided (distal transverse, descending and/or sigmoid colon) [4]. Small bowel involvement, including the terminal ileum and cecum, was systematically examined.
Statistical analysis
Clinical symptoms and laboratory parameters were compared according to pathogen. Differences in clinical symptoms were analyzed using χ2 and Fisher's exact tests, while laboratory parameters were analyzed using t-tests. The three groups were compared using one-way analysis of variance (ANOVA). All statistical tests were performed using IBM SPSS Statistics for Windows ver. 21.0 (IBM Co., Armonk, NY, USA). p-values less than 0.05 were considered statistically significant.
RESULTS
Patient demographic, clinical, and laboratory data
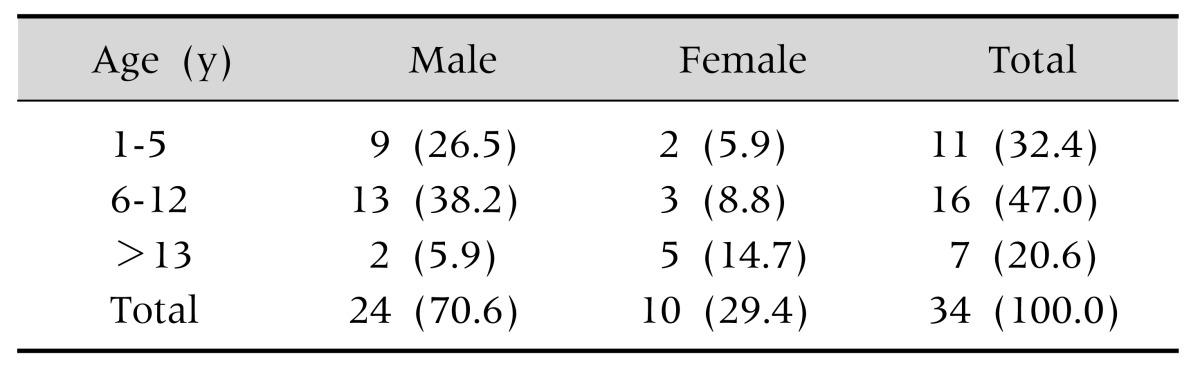
A total 34 patients were included in this study. Of them, 24 (70.6%) patients were male. The mean age of the patients was 8.5±6.2 (range, 1.1–17.1) years. There were 11 (32.4%) and 16 (47.1%) patients in early and late childhood, respectively (Table 1).
Table 1. Distribution of Patients Age and Sex.
Values are presented as number (%).
Mean age (y): 8.5±6.2 (range, 1.1-17.1).
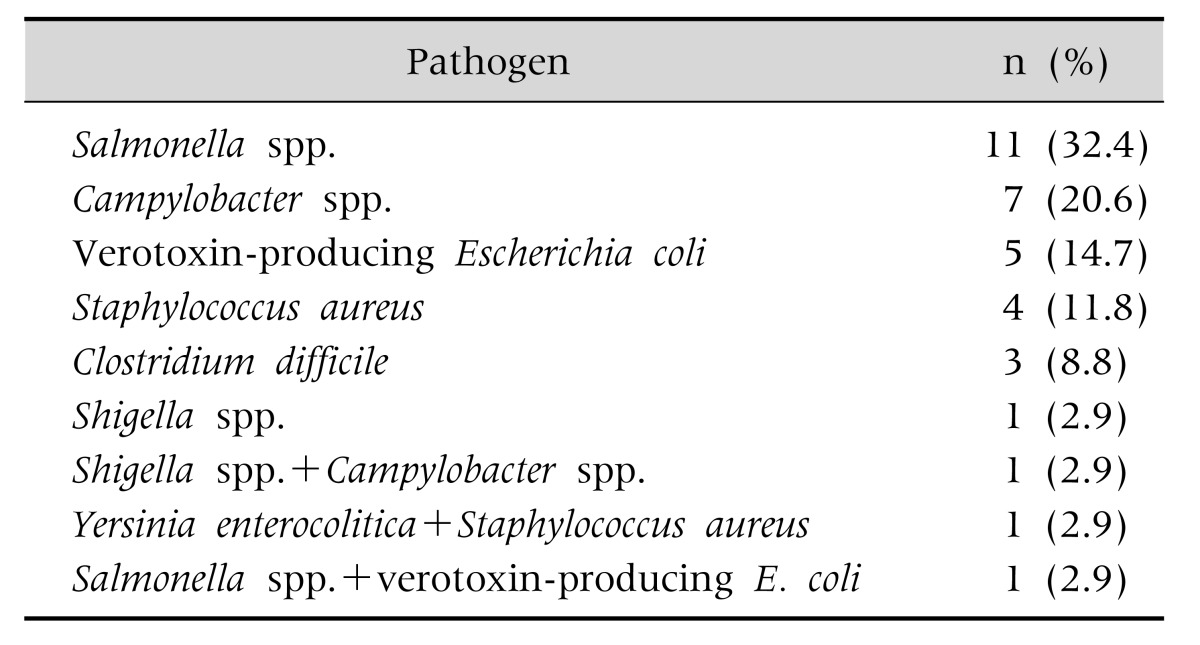
Six bacterial pathogens were isolated: Salmonella species (spp.) in 11 (32.4%) of the patients, Campylobacter spp. in 7 (20.6%), verotoxin-producing E. coli in 5 (14.7%), S. aureus in 4 (11.8%), C. difficile in 3 (8.8%), and Shigella spp. in 1 (2.9%). Two kinds of pathogens were isolated in stool from three patients (Table 2).
Table 2. Causative Pathogens in Acute Bacterial Enterocolitis in 34 Children.
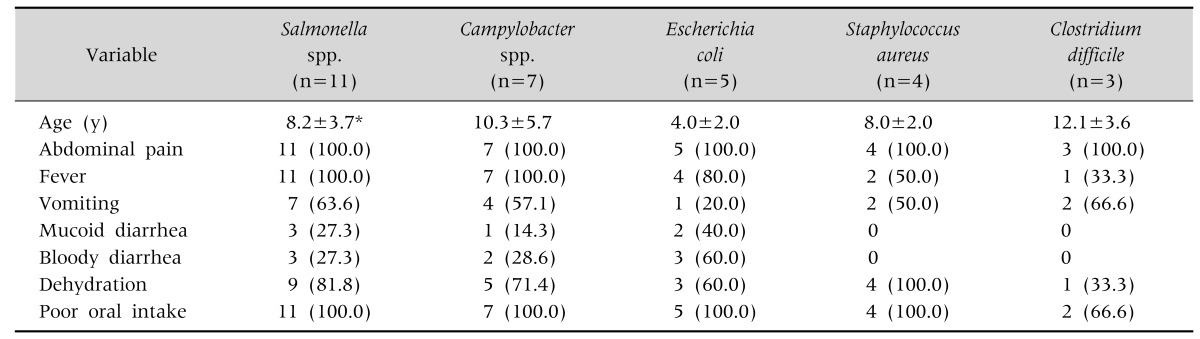
Various clinical symptoms such as abdominal pain, fever, vomiting, mucoid diarrhea, bloody diarrhea, dehydration, and poor oral intake developed according to pathogen. Abdominal pain occurred in all patients regardless of pathogen. All patients with Salmonella and Campylobacter infections had fever. Almost all patients had anorexia. However, the symptomatic data did not differ significantly between pathogens. Patients infected with E. coli were younger than those infected with Salmonella (p<0.05; Table 3).
Table 3. Demographics and Clinical Symptoms according to Pathogen.
Values are presented as mean±standard deviation or number (%).
E. coli: verotoxin-producing E. coli.
*p<0.05, Salmonella spp. vs. E. coli.
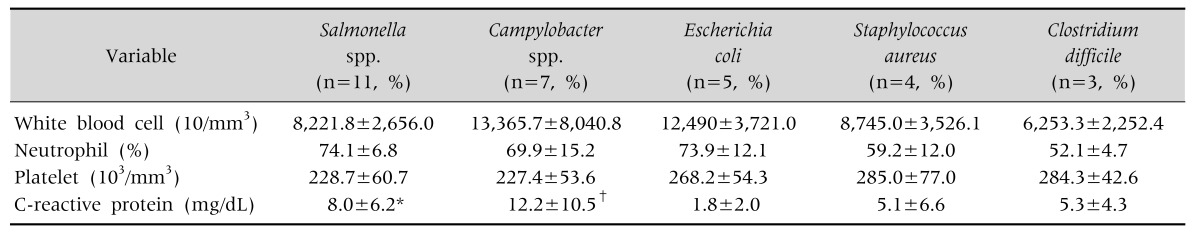
Laboratory data showed mild leukocytosis, increased percentage of segmented neutrophils, and normal platelet counts in patients with Campylobacter and E. coli infections; however, the difference was not statistically significant. CRP levels were higher in patients with Salmonella and Campylobacter infections compared to those with verotoxin-producing E. coli infections (p<0.05), the other clinical and laboratory data were indistinguishable between pathogens (Table 4).
Table 4. Laboratory Data according to Pathogen.
Values are presented as mean±standard deviation.
E. coli: verotoxin-producing E. coli.
*p<0.05, Salmonella spp. vs. E. coli, †p<0.05, Campylobacter spp. vs. E. coli.
Patient US findings
US demonstrated diverse involvement of bowel segments according to the causative pathogen. The most common lesion site of AIE regardless of pathogen was ileocolic, especially in patients with Campylobacter and E. coli infections. However, ileocecal involvement accounted for a large proportion of patients with S. aureus infections. No statistical differences in the distribution of bowel involvement between pathogens were observed (Table 5).
Table 5. Distribution of Intestinal Wall Thickening on Ultrasonographic Examination according to Pathogen.
Values are presented as number (%).
Ileocecal: terminal ileum and/or cecum, Right-sided: cecum, ascending colon, and/or proximal transverse colon, Diffuse: total colon, Left-sided: distal transverse, descending and/or sigmoid colon, Ileocolic: terminal ileum and total colon.
Treatments and outcomes
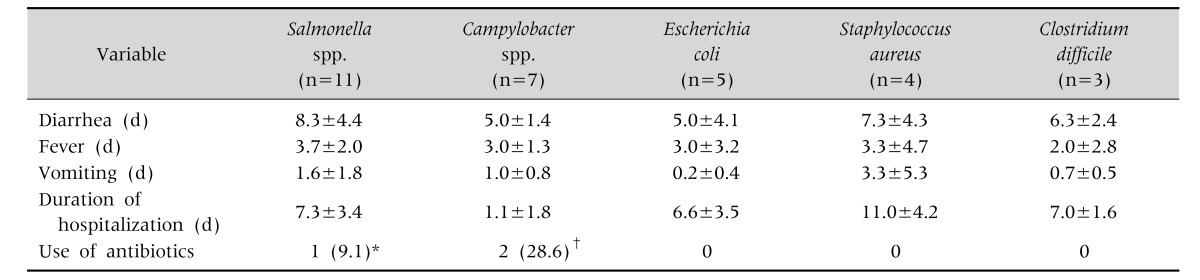
The mean durations of diarrhea and fever in patients with AIE were 5–8 and 2–4 days, respectively, which did not differ significantly difference between pathogens. Vomiting persisted longer in patients with S. aureus infections. Duration of hospitalization was shorter in patients with Campylobacter infections. Antibiotics were prescribed to two patients with Campylobacter and one with Salmonella infections. Two patients, one with Campylobacter and one with Salmonella infections, also had sepsis (Table 6).
Table 6. Treatments and Outcomes according to Pathogen.
Values are presented as mean±standard deviation or number (%).
E. coli: verotoxin-producing E. coli.
*Sepsis developed in one patient. †Septic shock developedin one patient.
DISCUSSION
In this study, six bacterial pathogens were isolated from 34 pediatric patients with AIE. Salmonella was the most frequently identified pathogen in 32.4% of cases, followed by Campylobacter (20.6%), verotoxin-producing E. coli (14.7%), S. aureus (11.8%), C. difficile (8.8%), and Shigella (2.9%). The incidence of AIE showed a male predominance (2.4:1) and no difference between age groups.
According to the 2013 Korea Centers for Disease Control and Prevention reports of acute diarrheal disease in a surveillance study of age groups, the prevalences of S. aureus, E. coli, and Salmonella were 4.77%, 4.23%, and 2.38%, respectively (http://www.cdc.go.kr). Cho et al. [2] studied 173 specimens from Korean pediatric and adult patients with infectious diarrheal disease using multiplex PCR, and detected Salmonella (n=5), Campylobacter (n=15), Vibrio (n=1), Clostridium difficile toxin B (n=4), Clostridium perfringens (n=5), Yersinia enterocolitica (n=1), Aeromonas (n=5), and verotoxin producing E. coli (n=2). Campylobacter was the most common causative bacterial agent in both children and adults.
Ivanova et al. [7] reported the results of surveillance and outbreak of campylobacteriosis and other bacterial gastrointestinal diseases in Sofia, Bulgaria in 1987-2008. Salmonella was the most frequently identified pathogen (32%), followed by Shigella (30%), Campylobacter (22%), and diarrheagenic E. coli (16%). Children between 0 and 4 years of age were most affected by Campylobacter infections (52%).
Discrepancies the incidence of enteropathogens between reports may result from the regional and socioeconomic status of the enrolled patients, as well as the methods used to identify pathogens.
Although the incidence of AIE is high, it remains an underestimated diagnosis in most institutions because identifying the causative organism is difficult and identification of the lesion site is difficult based on clinical suspicion alone. For some important pathogens such as diarrheagenic E. coli, Campylobacter, and Yersinia, testing is not readily available in all institutions. Therefore, there has been interest in developing multiplex platforms that can simultaneously detect a range of enteropathogens. We used a multiplex PCR system that simultaneously detects 10 pathogens as well as a conventional culture system as well. Cho et al. [2] reported that multiplex PCR and cultures identified enteropathogens in 36 (20.8%) and 8 specimens (4.8%) of 173 specimens, respectively.
Salmonella, Campylobacter, and enterohemorrhagic E. coli outbreaks continue to occur with concerning regularity [8]. Campylobacter enterocolitis is the most frequent form of acute bacterial diarrhea affecting humans, particularly children and young adults [9]. According to the World Health Organization, Campylobacter is one of the most frequently isolated bacteria from stools of infants with diarrhea in developing countries [10]. Campylobacter jejuni accounts for more than 90% of infections [9] and was isolated in 56% of patients with colitis in a large study from Sweden [11]. The symptoms of Campylobacter jejuni/coli enterocolitis are not so distinctive as to differentiate from enterocolitis caused by other organisms [10]. It has a generally good prognosis, although postinfectious sequelae are possible and Guillain-Barre syndrome is the most serious secondary complication [12]. Campylobacter enterocolitis does not routinely require antimicrobial treatment, but antibiotics are indicated in immunocompromised and other high-risk patients or in severe forms of the disease [9].
In this study, two kinds of pathogens were isolated simultaneously in stool samples from three patients. The possible explanations include co-infection of two pathogens, secondary nosocomial infection, or laboratory error. Antibiotic treatment of AIE is usually not necessary except in complicated cases such as septic condition and immune compromised state. We prescribed antibiotics to two patients with Campylobacter and one with Salmonella infections. Two case, one each with Campylobacter and Salmonella infections, were accompanied by sepsis.
Clinical symptoms such as abdominal pain, fever, vomiting, mucoid diarrhea, bloody diarrhea, dehydration, and poor oral intake developed according to pathogen. Although almost all patients had abdominal pain, fever, and anorexia regardless of the causative pathogen, symptomatic data did not differ significantly between pathogens. Patients infected with E. coli were significantly younger than those infected with Salmonella. CRP levels were significantly higher in patient with Salmonella and Campylobacter infections compared to those with verotoxin-producing E. coli, the other clinical and laboratory data were indistinguishable between pathogens. Therefore, clinical and laboratory data were insufficient to distinguish the causative pathogens. Differences in clinical and laboratory findings may be attributed to the pathogen type and loading dose as well as host factors.
US is a conventional, non-invasive, and relatively low-cost means of detecting GI lesions. Despite its limitation due to intraluminal gas, US with graded compression has been shown to be a reliable technique for the diagnosis of bowel inflammation. The diagnostic accuracy of US is greatly influenced by sonologist experience. In the current study, US assessment of the entire abdomen was performed by a physician who had performed more than 5,000 bowel examinations. US was helpful in detecting the affected bowel segments based on wall thickening in patients with enterocolitis; US can also be used to evaluate the severity of wall thickening and the extent, distribution, and extramural extension of the colitis, as well as the presence of small bowel involvement [3,4,13].
The sonographic hallmark of AIE is symmetric mural thickening of the terminal ileum and the major part of the colon. The sonographic wall thickening is confined to mucosa and submucosa without involvement of the muscularis, serosa, or surrounding fatty tissue, which is different from the sonographic findings of acute appendicitis or Crohn's disease [3]. In our study, while enteropathogens causing AIE had different patterns of ileocolic affection, there was considerable overlap. Namely, the lesion sites of AIE were not confined to certain segments of the intestine and may have depended on the severity of the AIE. The most common lesion sites of AIE were the ileum and the entire colon, regardless of pathogen, especially in patients with Campylobacter and E. coli infections. Ileocecal involvement accounted for a large proportion of patients with S. aureus infections. Patients with severe abdominal pain had more severe wall thickening.
Ueda et al. [14] reported that 40% of children with Salmonella enterocolitis had mural thickening of the colon, and the colonic wall thickness was significantly correlated with CRP and stool blood levels. Puylaert et al. [3] reported that wall thickening of the cecum and ascending colon was more prominent in Salmonella and Campylobacter ileocecitis.
This study has several limitations. First, US is an operator-dependent procedure in which procedural mistakes are possible. Second, measurement of the intestinal wall with US is not easy in obese patients. Third, since this is a single-center study with a small number of patients, some selection bias is likely. Fourth, this study is insufficient to make conclusions regarding the relationship between clinical severity and degree of intestinal wall thickening.
In conclusion, AIE is a common disorder and may cause problems due to its ability to mimic an acute surgical abdomen. Multiplex PCR may be useful to identify enteropathogenic bacteria causing AIE. US is the best tool to rapidly and easily differentiate AIE from other diseases and to estimate the disease extent, localization, and severity. However, additional larger studies are necessary to investigate the characteristics of US findings according to different pathogens.
ACKNOWLEDGEMENTS
We specially thank Dr. Young Gee Kim and Pharm D. Eun Kyung Lee for their financial support on this work.
References
- 1.Iijima Y, Asako NT, Aihara M, Hayashi K. Improvement in the detection rate of diarrhoeagenic bacteria in human stool specimens by a rapid real-time PCR assay. J Med Microbiol. 2004;53:617–622. doi: 10.1099/jmm.0.45607-0. [DOI] [PubMed] [Google Scholar]
- 2.Cho MC, Noh SA, Kim MN, Kim KM. Direct application of multiplex PCR on stool specimens for detection of enteropathogenic bacteria. Korean J Clin Microbiol. 2010;13:162–168. [Google Scholar]
- 3.Puylaert JB, Van der Zant FM, Mutsaers JA. Infectious ileocecitis caused by Yersinia, Campylobacter, and Salmonella: clinical, radiological and US findings. Eur Radiol. 1997;7:3–9. doi: 10.1007/s003300050098. [DOI] [PubMed] [Google Scholar]
- 4.Baud C, Saguintaah M, Veyrac C, Couture A, Ferran JL, Barnéon G, et al. Sonographic diagnosis of colitis in children. Eur Radiol. 2004;14:2105–2119. doi: 10.1007/s00330-004-2358-5. [DOI] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention, Korea National Institute of Health, The Korean Society of Clinical Microbiology. The practice guideline of diagnosis of the waterborne and food related diseases. Cheongju: Korea Center for Disease Control and Prevention; 2013. pp. 1–127. [Google Scholar]
- 6.Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis. 2009;27:482–493. doi: 10.1159/000233287. [DOI] [PubMed] [Google Scholar]
- 7.Ivanova K, Marina M, Petrov P, Kantardjiev T. Campylobacteriosis and other bacterial gastrointestinal diseases in Sofia, Bulgaria for the period 1987-2008. Euro Surveill. 2010;15:19474. [PubMed] [Google Scholar]
- 8.Navaneethan U, Giannella RA. Infectious colitis. Curr Opin Gastroenterol. 2011;27:66–71. doi: 10.1097/MOG.0b013e3283400755. [DOI] [PubMed] [Google Scholar]
- 9.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Campylobacter. 2000. Fact Sheet No.255. Geneva: (WHO); 2000. Nov, Available from: http://www.who.int/mediacentre/factsheets/fs255/en/ [Google Scholar]
- 11.Ternhag A, Törner A, Svensson A, Ekdahl K, Giesecke J. Short- and long-term effects of bacterial gastrointestinal infections. Emerg Infect Dis. 2008;14:143–148. doi: 10.3201/eid1401.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang RS. The relationship of Campylobacter jejuni infection and the development of Guillain-Barré syndrome. Curr Opin Infect Dis. 2002;15:221–228. doi: 10.1097/00001432-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Hong YR, Yeon GM, Lee JW, Park JH. Clinical features of infectious ileocecitis in children. Korean J Pediatr Gastroenterol Nutr. 2010;13:30–35. [Google Scholar]
- 14.Ueda D, Sato T, Yoshida M. Ultrasonographic assessment of Salmonella enterocolitis in children. Pediatr Radiol. 1999;29:469–471. doi: 10.1007/s002470050620. [DOI] [PubMed] [Google Scholar]