Abstract
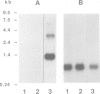
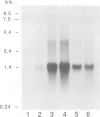
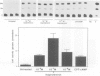
The expression of opioid genes was examined in isolated populations of glial cells in primary culture. Northern blot analysis of purified type I astrocytes, oligodendrocytes and mixed oligodendrocyte-type-2-astrocyte lineage cells derived from cerebral cortex demonstrated robust expression of proenkephalin mRNA exclusively in type I astrocytes. The expression of proenkephalin mRNA was stimulated by the beta-adrenergic agonist isoproterenol, and 8-(4-chlorophenyl thio)adenosine 3'-5'-cyclic monophosphate (cpt-cAMP). Both of these compounds regulated a proenkephalin-chloramphenicol acetyltransferase fusion gene transiently transfected into type I astrocytes. HPLC and immunoassay of the cell culture media revealed significant levels of unprocessed proenkephalin secreted by the cell and this secretion was stimulated by isoproterenol and cpt-cAMP. The relatively high levels of proenkephalin expressed suggest that enhanced expression in astrocytes may be important during neural development, in trauma-induced gliosis and in neuroimmune interactions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Hatfield J. M., Stack J. Post-translational processing of pro-opiomelanocortin (POMC)-derived peptides during fetal monkey pituitary development. I. Adrenocorticotropin (ACTH) and alpha-melanotropins (alpha-MSHs). Dev Biol. 1988 Mar;126(1):156–163. doi: 10.1016/0012-1606(88)90249-7. [DOI] [PubMed] [Google Scholar]
- Allen R. G., Herbert E., Hinman M., Shibuya H., Pert C. B. Coordinate control of corticotropin, beta-lipotropin, and beta-endorphin release in mouse pituitary cell cultures. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4972–4976. doi: 10.1073/pnas.75.10.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chepelinsky A. B., King C. R., Zelenka P. S., Piatigorsky J. Lens-specific expression of the chloramphenicol acetyltransferase gene promoted by 5' flanking sequences of the murine alpha A-crystallin gene in explanted chicken lens epithelia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2334–2338. doi: 10.1073/pnas.82.8.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cholewinski A. J., Wilkin G. P. Peptide receptors on astrocytes: evidence for regional heterogeneity. Biochem Soc Trans. 1988 Aug;16(4):429–432. doi: 10.1042/bst0160429. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Fedoroff S., McAuley W. A., Houle J. D., Devon R. M. Astrocyte cell lineage. V. Similarity of astrocytes that form in the presence of dBcAMP in cultures to reactive astrocytes in vivo. J Neurosci Res. 1984;12(1):14–27. doi: 10.1002/jnr.490120103. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W. The endothelium--astrocyte immune control system of the brain. Springer Semin Immunopathol. 1985;8(1-2):57–70. doi: 10.1007/BF00197247. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K. F., McLaughlin P. J., Zagon I. S. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987 Jul 21;416(1):157–161. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- Janković B. D., Marić D. Enkephalins modulate in vivo immune reactions through delta- and mu-opioid receptors. Ann N Y Acad Sci. 1988;540:691–693. doi: 10.1111/j.1749-6632.1988.tb27214.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. G., Nielsen C. P., West N. B., Douglass J., Brenner R. M., Maslar I. A., Melner M. H. Proenkephalin gene expression in the primate uterus: regulation by estradiol in the endometrium. Mol Endocrinol. 1989 May;3(5):852–857. doi: 10.1210/mend-3-5-852. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Abney E. R., David S., Ffrench-Constant C., Lindsay R., Patel R., Stone J., Raff M. C. Is reactive gliosis a property of a distinct subpopulation of astrocytes? J Neurosci. 1986 Jan;6(1):22–29. doi: 10.1523/JNEUROSCI.06-01-00022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. G., Page M. J. Use of gene transfer to study expression of steroid-responsive genes. Mol Cell Endocrinol. 1984 Mar;34(3):159–168. doi: 10.1016/0303-7207(84)90172-2. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rataboul P., Faucon Biguet N., Vernier P., De Vitry F., Boularand S., Privat A., Mallet J. Identification of a human glial fibrillary acidic protein cDNA: a tool for the molecular analysis of reactive gliosis in the mammalian central nervous system. J Neurosci Res. 1988;20(2):165–175. doi: 10.1002/jnr.490200204. [DOI] [PubMed] [Google Scholar]
- Saneto R. P., de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985 May;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Kume-Iwaki A., Goldman J. E. Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia. 1988;1(5):346–354. doi: 10.1002/glia.440010507. [DOI] [PubMed] [Google Scholar]
- Sonders M., Weber E. Distribution pattern of metorphamide compared with other opioid peptides from proenkephalin and prodynorphin in the bovine brain. J Neurochem. 1987 Sep;49(3):671–680. doi: 10.1111/j.1471-4159.1987.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Hawelu-Johnson C. L., Guyenet P. G., Lynch K. R. Astrocytes synthesize angiotensinogen in brain. Science. 1988 Dec 9;242(4884):1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Thomas G., Herbert E., Hruby D. E. Expression and cell type--specific processing of human preproenkephalin with a vaccinia recombinant. Science. 1986 Jun 27;232(4758):1641–1643. doi: 10.1126/science.3754979. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Tsang D., Ng S. C., Ho K. P., Ho W. K. Ontogenesis of opiate binding sites and radioimmunoassayable beta-endorphin and enkephalin in regions of rat brain. Brain Res. 1982 Nov;281(3):257–261. doi: 10.1016/0165-3806(82)90124-9. [DOI] [PubMed] [Google Scholar]
- Varon S. S., Somjen G. G. Neuron-glia interactions. Neurosci Res Program Bull. 1979 Feb;17(1):1–239. [PubMed] [Google Scholar]
- Vilijn M. H., Das B., Kessler J. A., Fricker L. D. Cultured astrocytes and neurons synthesize and secrete carboxypeptidase E, a neuropeptide-processing enzyme. J Neurochem. 1989 Nov;53(5):1487–1493. doi: 10.1111/j.1471-4159.1989.tb08542.x. [DOI] [PubMed] [Google Scholar]
- Vilijn M. H., Vaysse P. J., Zukin R. S., Kessler J. A. Expression of preproenkephalin mRNA by cultured astrocytes and neurons. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6551–6555. doi: 10.1073/pnas.85.17.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. K., de Vellis J. Effect of forskolin on primary cultures of astrocytes and oligodendrocytes. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(1):59–67. [PubMed] [Google Scholar]
- Yoshikawa K., Sabol S. L. Glucocorticoids and cyclic AMP synergistically regulate the abundance of preproenkephalin messenger RNA in neuroblastoma-glioma hybrid cells. Biochem Biophys Res Commun. 1986 Aug 29;139(1):1–10. doi: 10.1016/s0006-291x(86)80071-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Young S. L., Nielsen C. P., Lundblad J. R., Roberts J. L., Melner M. H. Gonadotropin regulation of the rat proopiomelanocortin promoter: characterization by transfection of primary ovarian granulosa cells. Mol Endocrinol. 1989 Jan;3(1):15–21. doi: 10.1210/mend-3-1-15. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]