Abstract
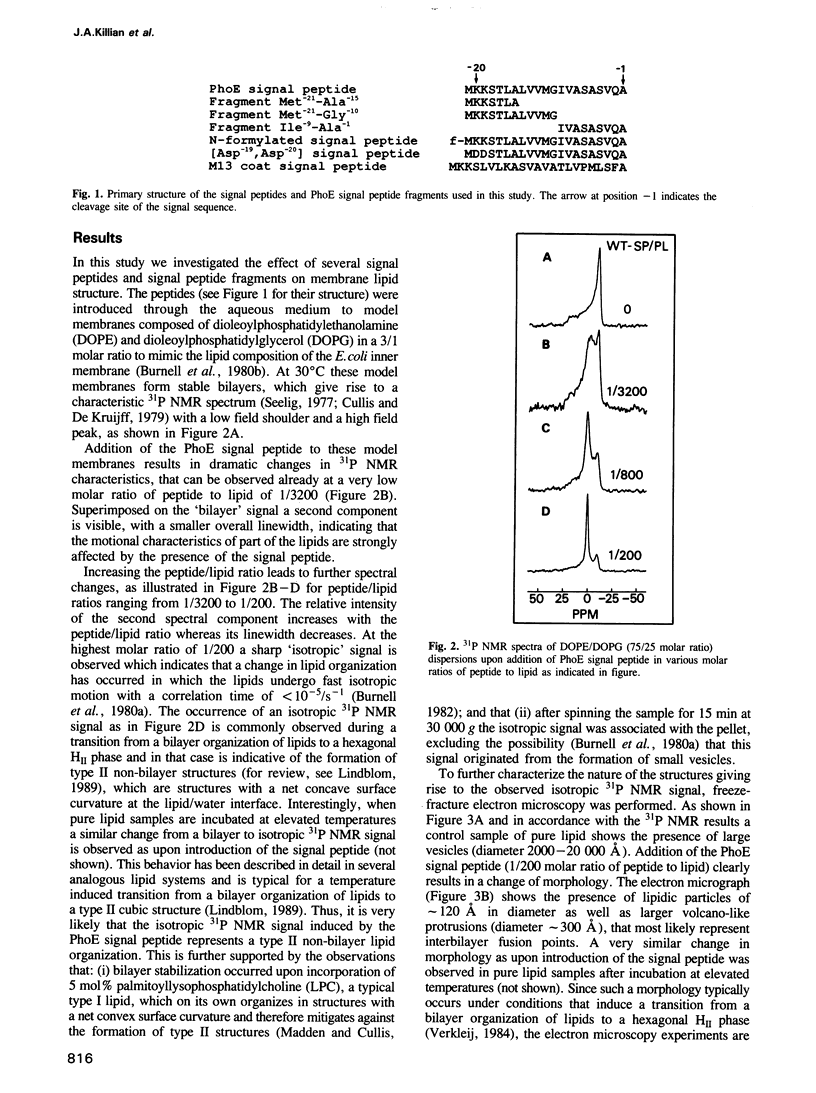
Using 31P NMR and freeze-fracture electron microscopy we investigated the effect of several synthetic signal peptides on lipid structure in model membranes mimicking the lipid composition of the Escherichia coli inner membrane. It is demonstrated that the signal peptide of the E. coli outer membrane protein PhoE, as well as that of the M13 phage coat protein, strongly promote the formation of non-bilayer lipid structures. This effect appears to be correlated to in vivo translocation efficiency, since a less functional analogue of the PhoE signal peptide was found to be less active in destabilizing the bilayer. It is proposed that signal sequences can induce local changes in lipid structure that are involved in protein translocation across the membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K., Mackman N., Holland I. B. Genetics and biochemistry of the assembly of proteins into the outer membrane of E. coli. Prog Biophys Mol Biol. 1987;49(2-3):89–115. doi: 10.1016/0079-6107(87)90010-1. [DOI] [PubMed] [Google Scholar]
- Batenburg A. M., Brasseur R., Ruysschaert J. M., van Scharrenburg G. J., Slotboom A. J., Demel R. A., de Kruijff B. Characterization of the interfacial behavior and structure of the signal sequence of Escherichia coli outer membrane pore protein PhoE. J Biol Chem. 1988 Mar 25;263(9):4202–4207. [PubMed] [Google Scholar]
- Batenburg A. M., Demel R. A., Verkleij A. J., de Kruijff B. Penetration of the signal sequence of Escherichia coli PhoE protein into phospholipid model membranes leads to lipid-specific changes in signal peptide structure and alterations of lipid organization. Biochemistry. 1988 Jul 26;27(15):5678–5685. doi: 10.1021/bi00415a043. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D., de Boer P., Bitter W., Tommassen J. The role of the positively charged N-terminus of the signal sequence of E. coli outer membrane protein PhoE in export. Biochim Biophys Acta. 1989 Feb 13;979(1):69–76. doi: 10.1016/0005-2736(89)90524-5. [DOI] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Exploring the conformational roles of signal sequences: synthesis and conformational analysis of lambda receptor protein wild-type and mutant signal peptides. Biochemistry. 1984 Jul 3;23(14):3111–3114. doi: 10.1021/bi00309a001. [DOI] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Bruch M. D., McKnight C. J., Gierasch L. M. Helix formation and stability in a signal sequence. Biochemistry. 1989 Oct 17;28(21):8554–8561. doi: 10.1021/bi00447a043. [DOI] [PubMed] [Google Scholar]
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Burnell E., van Alphen L., Verkleij A., de Kruijff B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli. I. Cytoplasmic membrane and total phospholipids. Biochim Biophys Acta. 1980 Apr 24;597(3):492–501. doi: 10.1016/0005-2736(80)90222-9. [DOI] [PubMed] [Google Scholar]
- Chupin V., Killian J. A., de Kruijff B. 2H-nuclear magnetic resonance investigations on phospholipid acyl chain order and dynamics in the gramicidin-induced hexagonal HII phase. Biophys J. 1987 Mar;51(3):395–405. doi: 10.1016/S0006-3495(87)83361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell D. G., Dluhy R. A., Briggs M. S., McKnight C. J., Gierasch L. M. Conformations and orientations of a signal peptide interacting with phospholipid monolayers. Biochemistry. 1989 Apr 4;28(7):2789–2797. doi: 10.1021/bi00433a008. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Importance of secondary structure in the signal sequence for protein secretion. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4599–4603. doi: 10.1073/pnas.80.15.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
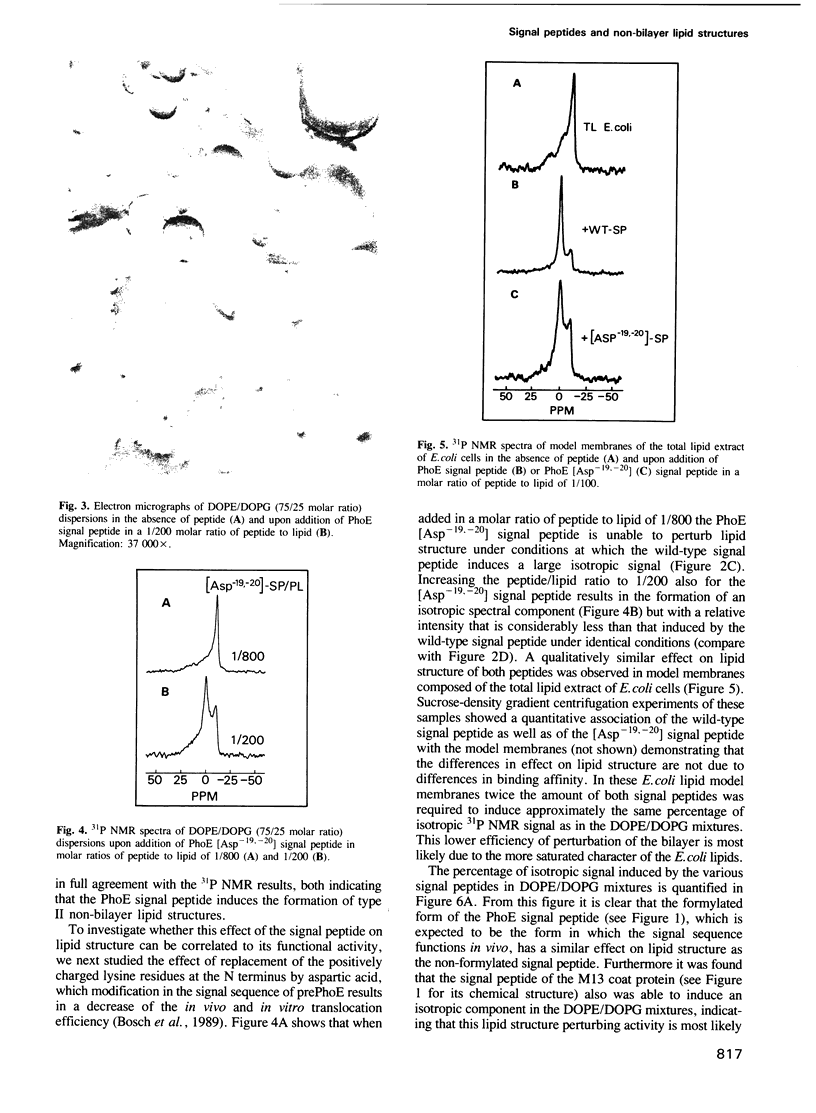
- Geller B. L., Wickner W. M13 procoat inserts into liposomes in the absence of other membrane proteins. J Biol Chem. 1985 Oct 25;260(24):13281–13285. [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Killian J. A., de Kruijff B. The influence of proteins and peptides on the phase properties of lipids. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):259–284. doi: 10.1016/0009-3084(86)90073-3. [DOI] [PubMed] [Google Scholar]
- Killian J. A., van den Berg C. W., Tournois H., Keur S., Slotboom A. J., van Scharrenburg G. J., de Kruijff B. Gramicidin-induced hexagonal HII phase formation in negatively charged phospholipids and the effect of N- and C-terminal modification of gramicidin on its interaction with zwitterionic phospholipids. Biochim Biophys Acta. 1986 May 9;857(1):13–27. doi: 10.1016/0005-2736(86)90094-5. [DOI] [PubMed] [Google Scholar]
- Kusters R., de Vrije T., Breukink E., de Kruijff B. SecB protein stabilizes a translocation-competent state of purified prePhoE protein. J Biol Chem. 1989 Dec 15;264(35):20827–20830. [PubMed] [Google Scholar]
- Madden T. D., Cullis P. R. Stabilization of bilayer structure for unsaturated phosphatidylethanolamines by detergents. Biochim Biophys Acta. 1982 Jan 4;684(1):149–153. doi: 10.1016/0005-2736(82)90061-x. [DOI] [PubMed] [Google Scholar]
- Nagaraj R., Joseph M., Reddy G. L. Perturbation of the lipid bilayer of model membranes by synthetic signal peptides. Biochim Biophys Acta. 1987 Oct 16;903(3):465–472. doi: 10.1016/0005-2736(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Nesmeyanova M. A. On the possible participation of acid phospholipids in the translocation of secreted proteins through the bacterial cytoplasmic membrane. FEBS Lett. 1982 Jun 7;142(2):189–193. doi: 10.1016/0014-5793(82)80131-2. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Wickner W. Reconstitution of rapid and asymmetric assembly of M13 procoat protein into liposomes which have bacterial leader peptidase. J Biol Chem. 1983 Feb 10;258(3):1895–1900. [PubMed] [Google Scholar]
- Rapoport T. A. Protein translocation across and integration into membranes. CRC Crit Rev Biochem. 1986;20(1):73–137. doi: 10.3109/10409238609115901. [DOI] [PubMed] [Google Scholar]
- Reddy G. L., Nagara R. Circular dichroism studies on synthetic signal peptides indicate beta-conformation as a common structural feature in highly hydrophobic environment. J Biol Chem. 1989 Oct 5;264(28):16591–16597. [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., Rubin E. Effects of ethanol on the chemical and structural properties of biologic membranes. Lab Invest. 1985 Feb;52(2):120–131. [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. Site-specific antibodies against the PrlA (secY) protein of Escherichia coli inhibit protein export by interfering with plasma membrane binding of preproteins. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1895–1899. doi: 10.1073/pnas.86.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Mechanisms of membrane assembly: general lessons from the study of M13 coat protein and Escherichia coli leader peptidase. Biochemistry. 1988 Feb 23;27(4):1081–1086. doi: 10.1021/bi00404a001. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- de Kruijff B. Polymorphic regulation of membrane lipid composition. Nature. 1987 Oct 15;329(6140):587–588. doi: 10.1038/329587a0. [DOI] [PubMed] [Google Scholar]
- de Vrije T., de Swart R. L., Dowhan W., Tommassen J., de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988 Jul 14;334(6178):173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]




