Abstract
Subclinical narcissism is a personality trait with two faces: According to social-cognitive theories it is associated with grandiosity and feelings of superiority, whereas psychodynamic theories emphasize vulnerable aspects like fluctuating self-esteem and emotional conflicts. The psychodynamic view, however, is commonly not supported by self-report studies on subclinical narcissism. Personality neuroscience might help to better understand the phenomenon of narcissism beyond the limits of self-report research. While social-cognitive theory would predict that self-relevant processing should be accompanied by brain activity in reward-related areas in narcissistic individuals, psychodynamic theory would suggest that it should be accompanied by activation in regions pointing to negative affect or emotional conflict. In this study, extreme groups of high and low narcissistic individuals performed a visual self-recognition paradigm during fMRI. Viewing one’s own face (as compared to faces of friends and strangers) was accompanied by greater activation of the dorsal and ventral anterior cingulate cortex (ACC) in highly narcissistic men. These results suggest that highly narcissistic men experience greater negative affect or emotional conflict during self-relevant processing and point to vulnerable aspects of subclinical narcissism that might not be apparent in self-report research.
Introduction
The ancient myth of narcissus comes in several different versions. In Ovid’s classic version, the beautiful young hunter Narcissus, who rejects the love of the nymph Echo, is deemed by the gods to fall in love with his mirror image. Fully entranced by his own reflection in a pool of water, Narcissus eventually realizes that his love cannot be reciprocated, which leads him to commit suicide. In another prominent version by Pausanias, the myth has a different ending: Narcissus is gazing at himself, when suddenly a leaf falls into the water and distorts the image. Narcissus is shocked by the ugliness of his mirror image, which ultimately leads him to death.
These two versions of this ancient myth relate to the “two faces” of narcissism, namely grandiose and vulnerable narcissism1. Subclinical manifestations of narcissism are commonly conceptualized in terms of grandiose narcissism, which circumscribes feelings of superiority, exaggerated self-worth, and entitlement2. In contrast, narcissistic vulnerability is expressed in hypersensitivity, anxiety, and pronounced self-monitoring3. Psychodynamic theory posits that narcissistic grandiosity is always accompanied by vulnerable aspects, which appears to be supported by clinical research (e.g. ref. 4). In nonclinical personality research, however, measures of grandiose and vulnerable narcissism display small5 or zero correlation1, 3. Grandiose narcissism is associated with higher explicit6 and implicit self-esteem7, which might be the cause of higher self-reported psychological well-being8. However, narcissism is also associated with instability of self-esteem in terms of greater dependence upon everyday interpersonal events9. This might relate to emotion regulation difficulties and interpersonal conflicts associated with narcissism, particularly in men (cf. refs 10 and 11).
Developmental theories of narcissism also emphasize either its grandiose or vulnerable aspects. Social-cognitive theory posits that, for the development of subclinical narcissism, no “deficits deep down” are needed, but narcissism rather emerges from (unjustified) parental overvaluation12, 13. Consequently, social-cognitive theorists emphasize the view that narcissism is neither a facade to mask latent deficits nor a defense to protect the (fragile) self, but reflects a truly inflated sense of self-worth. This view is supported by studies demonstrating an increase of narcissism in younger birth cohorts due to cultural changes (e.g., ref. 14). In contrast, psychodynamic theory posits that narcissism emerges primarily from unreliable, cold, and not sufficiently empathic parents who exhibit indifference or even aggression towards their children15, 16. Empirical tests of both theories show that parental overvaluation is the primary driving force of subclinical narcissism, but parental coldness also plays a major role12, 17.
According to behavioral research, subclinical narcissism seems to be associated with psychological health rather than developmental deficits, although there is some evidence for emotion regulation difficulties; specifically in men10, 11. Grandiose narcissism is largely unrelated to vulnerable aspects in self-report studies, which points to the independence of the “two faces” within subclinical personality variation. Personality neuroscience might help to unveil aspects of narcissism that might not be subject to self-report studies. Recent research shows, for instance, that male grandiose narcissists display increased activity in the “social pain network” (anterior insula, subgenual anterior cingulate, and dorsal anterior cingulate) following social rejection, though they do not report experiencing higher levels of social rejection in self-report measures18.
This study set out to explore the neural correlates of narcissistic “self-admiration”, the probably most striking feature of grandiose narcissism. We had extreme groups of high and low narcissistic individuals perform a visual self-recognition paradigm during functional imaging, thus “transferring” the myth of Narcissus into the scanner. This experimental procedure was not only chosen because it nicely resembles the ancient myth, but also because visual-self recognition triggers various aspects of self-referential emotional processing that might not be accessible to self-report. As this is the first study to directly address the neural correlates of visual self-recognition associated with narcissism, it is largely explorative in nature, and no exact regions of interest can be defined a-priori. However, relevant psychological processes and their respective neural substrates can be inferred from social-cognitive vs. psychodynamic theories of narcissism: If narcissism reflects a truly exaggerated sense of self-worth in terms of self-admiration, visual self-recognition should be accompanied by brain activation reflecting reward and/or liking, as it is the case for high self-esteem19. Following this “self-reward” - hypothesis, one might expect activation in the dopaminergic system, specifically in subcortical regions20. If narcissists display latent deficits in self-esteem and emotion regulation as psychodynamic theory suggests, visual self-recognition should go along with neural activity indicating negative affect or conflicting emotional processing (anterior cingulate18). Of course, both aspects could also hold true simultaneously. This study hence undertakes an empirical test of opposing assumptions regarding the psychological mechanisms underlying narcissism as posited by social-cognitive and psychodynamic theories.
Method
Participants and Material
The sample consisted of extreme groups of high (n = 21) and low (n = 22) narcissistic participants selected out of a large pool (N > 600) of individuals who were pre-screened with a German version of the Narcissistic Personality Inventory (NPI; ref. 2). The NPI assesses narcissism as a continuous trait using 40 forced-choice statements such as “I think I am a special person” vs. “I am no better or worse than most people” and is conceived the long-time standard self-report measure of narcissism21. It is based on the diagnostic criteria for clinical narcissism (2; for latest version, see ref. 22) and thus focuses on grandiose aspects of narcissism such as authority, exhibitionism, and superiority23. NPI scores are, in the general population, uncorrelated with measures exclusively designed to assess vulnerable aspects of narcissism3.
Upon arrival at the lab, participants were re-assessed using a Likert-type adaptation of the NPI (see also ref. 24), which allowed for intermixing the items with a Big Five personality scale25 in order to prevent participants from guessing the study aim. Additionally, participants completed the German Multidimensional Self-Esteem Scale26 and other personality measures that are not analyzed here. Only individuals who scored in the lower or upper tertile of the NPI at both measurements (screening and first lab session; timespan of at least one month) were classified as stable non-/narcissists and were invited to take part in this study. To address the possibility that the low-narcissism group might display below-average self-esteem (i.e., possibly pointing in the direction of anxious personalities, which would not be an adequate control group), we compared sample means with the sex-specific norms26. Self-esteem of the low narcissism groups did not deviate from the respective population mean (t 13 women = −1.29, p = 0.220; t 7 men = −1.02; p = 0.341). In the high narcissism groups, women showed higher self-esteem (t 7 women = 4.83; p = 0.002), but men did not (t 12 men = 1.60; p = 0.135). All participants were right-handed, heterosexual, did not report any history of neurological or mental disorders, and gave written informed consent to the study. Sex was counterbalanced across the high and low narcissistic groups. Table 1 displays personality characteristics of the two experimental groups. The experimental protocol was approved by the ethics committee of the University of Graz (GZ. 39/28/63 ex 2013/14) and carried out in accordance with the relevant guidelines and regulations.
Table 1.
Sample Characteristics.
| Women (n = 22) | Low Narcissism (n = 14) | t (df) | d | p | Men (n = 21) | Low Narcissism (n = 8) | t (df) | d | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| High Narcissism (n = 8) | High Narcissism (n = 13) | |||||||||
| Narcissism | 3.13 (0.24) | 2.24 (0.22) | 8.87 (20) | 3.97 | <0.001 | 3.13 (0.16) | 2.11 (0.24) | 11.64 (19) | 5.34 | <0.001 |
| Self-Esteem | 5.63 (0.44) | 4.63 (0.72) | 3.54 (20) | 1.58 | 0.002 | 5.57 (0.85) | 4.82 (1.02) | 1.81 (19) | 0.83 | 0.086 |
| Neuroticism | 1.88 (0.48) | 2.40 (0.58) | −2.14 (20) | −0.96 | 0.045 | 2.04 (0.73) | 2.28 (0.73) | −0.74 (19) | −0.34 | 0.469 |
| Extraversion | 3.75 (0.33) | 2.90 (0.64) | 4.15 (19.88) | 1.86 | 0.001 | 3.50 (0.43) | 2.53 (0.62) | 4.23 (19) | 1.94 | <0.001 |
| Openness | 3.40 (0.44) | 3.40 (0.42) | 0.00 (20) | 0.00 | 1.00 | 3.77 (0.26) | 3.25 (0.59) | 2.35 (8.64) | 1.60 | 0.045 |
| Agreeableness | 1.91 (0.60) | 2.59 (0.45) | −3.06 (20) | −1.37 | 0.006 | 1.92 (0.60) | 2.25 (0.60) | −1.22 (19) | −0.56 | 0.239 |
| Conscientiousness | 3.44 (0.53) | 2.95 (0.75) | 1.62 (20) | 0.72 | 0.121 | 3.33 (0.33) | 2.78 (0.62) | 2.65 (19) | 1.22 | 0.016 |
| Age | 24.25 (4.20) | 23.43 (3.55) | 0.49 (20) | 0.22 | 0.630 | 23.38 (2.60) | 24.63 (3.50) | −0.93 (19) | −0.43 | 0.363 |
| PAextSelf | 4.47 (1.19) | 4.48 (1.08) | −0.03 (20) | −0.01 | 0.979 | 4.04 (0.87) | 3.91 (0.61) | 0.38 (19) | 0.17 | 0.711 |
| PAextFriend | 4.06 (0.88) | 4.16 (1.26) | −0.19 (20) | −0.08 | 0.848 | 3.94 (1.05) | 4.03 (0.69) | −0.21 (19) | −0.10 | 0.834 |
| PAextStranger | 4.47 (1.09) | 4.57 (0.99) | −0.23 (20) | −0.10 | 0.824 | 4.06 (0.82) | 3.88 (0.61) | 0.54 (19) | 0.25 | 0.595 |
| ΔPAextSelf-Friend | 0.41 (1.37) | 0.32 (1.37) | 0.14 (20) | 0.06 | 0.890 | 0.10 (0.99) | −0.13 (0.88) | 0.52 (19) | 0.24 | 0.610 |
| ΔPAextSelf-Stranger | 0.00 (0.30) | −0.09 (0.25) | 0.75 (20) | 0.34 | 0.463 | −0.02 (0.22) | 0.03 (0.09) | −0.63 (19) | −0.29 | 0.539 |
| PApartSelf | 4.13 (1.25) | 3.93 (1.07) | 0.39 (20) | 0.17 | 0.701 | 4.54 (0.88) | 3.75 (1.39) | 1.44 (10.49) | 0.89 | 0.179 |
| PApartFriend | 5.25 (1.67) | 4.79 (1.25) | 0.74 (20) | 0.33 | 0.467 | 4.46 (1.39) | 4.88 (1.55) | −0.63 (19) | −0.29 | 0.534 |
| PApartStranger | 4.13 (2.03) | 4.50 (1.02) | −0.49 (9.06) | −0.33 | 0.637 | 4.46 (0.88) | 4.25 (1.58) | 0.40 (19) | 0.18 | 0.696 |
| ΔPApartSelf-Friend | −1.13 (1.73) | −0.86 (1.35) | −0.41 (20) | −0.18 | 0.690 | 0.08 (1.32) | −1.12 (1.13) | 2.14 (19) | 0.98 | 0.046 |
| ΔPApartSelf-Stranger | 0.00 (1.31) | −0.57 (1.50) | 0.87 (20) | 0.39 | 0.381 | 0.08 (1.19) | −0.50 (1.93) | 0.85 (19) | 0.39 | 0.404 |
| Task Accuracy (%) | 96.53 (00.03) | 97.95 (00.02) | −1.47 (20) | −0.66 | 0.158 | 97.72 (0.02) | 99.42 (0.01) | −2.70 (15.77) | −1.34 | 0.016 |
| Task AccuracySelf (%) | 97.57 (00.05) | 97.62 (00.03) | −0.03 (20) | −0.01 | 0.977 | 98.72 (00.03) | 98.96 (00.02) | −0.19 (19) | −0.09 | 0.850 |
| Task AccuracyFriend (%) | 97.22 (00.03) | 98.61 (00.02) | −1.37 (20) | −0.61 | 0.185 | 97.44 (00.04) | 100.00 (00.00) | −2.52 (12.00) | −1.45 | 0.027 |
| Task AccuracyStranger (%) | 94.79 (00.05) | 97.62 (00.03) | −1.70 (20) | −0.76 | 0.105 | 97.01 (00.02) | 99.31 (00.01) | −2.49 (19) | −1.14 | 0.022 |
Note. PAext = Physical Attractiveness, external ratings; PApart = Physical Attractiveness, participant’s ratings. Corrected df were used in case of unequal variances.
Design and Procedure
Our main aim was to investigate brain activation differences between high and low narcissists during visual self-face recognition. As common in visual self-recognition research (e.g., ref. 27), we sought to control for important confounds by employing three experimental conditions in the fMRI paradigm: Self (own face), Friend (a close same-sex friend’s face), and Stranger (a same-sex stranger’s face matched for physical attractiveness).
Participants were kept blind to the study’s aim by telling a cover story based on visual self-recognition and personality. The experiment encompassed two sessions: At the first session, participants were invited to the University’s photo studio and were asked to bring a close, same-sex friend (cf. ref. 19). In most cases, this person was the participant’s “best friend”, thus controlling social distance in an ipsative manner (i.e., personal minimum of social distance). Both were instructed to come to the lab in their everyday outfit wearing usual makeup etc. They completed the NPI (intermixed with a German Big Five inventory; see above) and were photographed by a professional photographer. Participants were instructed to display a neutral facial expression and not to wear eyeglasses. We took 72 photographs of each individual from 24 different horizontal and three different vertical angles under standardized lighting conditions. Figure 1 shows a sample photo. Photographs were rated for physical attractiveness by six independent raters (α = 0.88). The independent ratings did not show significant differences between Self and Friend or Self and Stranger photographs (PASelf = 4.24 [0.97], PAFriend = 4.05 [1.01], PAStranger = 4.27 [0.92]; t 42 Self-Friend = 1.06, d = 0.19, p = 0.297; t 42 Self-Stanger = −0.84, d = −0.03, p = 0.404). Table 1 provides sex-split external PA ratings and group comparisons within sexes; there were no significant differences between narcissism groups.
Figure 1.

Schematic time course of the experimental paradigm. Participants saw faces of themselves, a friend, or a stranger and were asked to indicate the direction of the face (left or right) when the questionmark sign appeared. The depicted person served as a participant’s friend and did not take part in the experiment; written permission was obtained.
At the second lab session, participants underwent the fMRI experiment. After initial instruction outside the scanner, the fMRI session consisted of three blocks encompassing 36 trials each in which participants were presented twelve Self, Friend, and Stranger photographs (with the latter being matched for physical attractiveness) in different horizontal and vertical angles. Conditions were randomized within each block. Blocks were separated by resting periods of 20 sec. The total duration of the MRI experiment was about 10 min.
Figure 1 depicts the time course of a single trial, which consisted of a fixation period (jittered to an average of two seconds within subjects), followed by a one-second presentation of a photograph (Self/Friend/Stranger), and a two-seconds response period, in which participants were asked to indicate via button press whether the face was oriented left or right. This task was chosen to ensure that participants’ attention was directed to the experimental paradigm without induction of evaluative processes on a conscious level. Self-photographs were mirrored at the vertical axis to preserve the natural feeling of gazing into a mirror (e.g., ref. 28).
After completion of the MRI session, participants were asked to rate the physical attractiveness of Self, Friend, and Stranger photographs on a 7-point scale. Self and Stranger photos were rated as equally attractive by high (t 20 = 0.18, p = 0.86, d = 0.04) and low narcissists (t 21 = −1.57, p = 0.13, d = −0.47), and this difference was equal across groups (see Table 1), thus further validating our matching procedure. We also performed sex-split analyses as sex is known to be an important distinguishing factor in narcissism (cf. refs 10 and 11). These revealed that low narcissistic men viewed themselves as less attractive than their friends; strangers were perceived as equally attractive in both sexes (see Table 1). It is important to note that this differences concerns subjective declarations and/or impressions, while there was no objective difference in the external ratings (see above). Finally, participants were debriefed and asked whether they had guessed the study aim. One participant (high narcissist, male) did so and was thus excluded from all analyses.
fMRI Data Acquisition
Whole brain imaging was performed on a 3 T Siemens Skyra MRI system (Siemens Medical Systems, Erlangen, Germany) using a 32-channel head coil. T2*-weighted functional images were acquired using a single shot gradient echo planar imaging (EPI) sequence (TR = 1850 ms, TE = 30 ms, flip angle = 90°, 32 transversal slices, 3.5 mm isomorphic with distance factor 20%, interleaved slice ordering). Head motion was corrected online. The first two volumes of each scan were discarded in order to allow for T1 equilibration effects. Head motion was restricted using firm padding surrounding participant’s heads. Visual stimuli were presented using the software Presentation (Neurobehavioral Systems, Albany, CA) onto a computer screen and viewed through a mirror attached to the head coil.
Analysis Plan
For the fMRI analyses, our main contrast of interest was the conjunction of Self > Friend & Self > Stranger. This contrast reflects brain activation specific to viewing one’s own face versus someone else’s face while controlling for potential differences in familiarity (Friend) and physical attractiveness (Stranger). To check for the validity of the experimental protocol, we first performed a whole-brain analysis of this contrast in the full sample, which should unveil regions that are known to be implicated in self-viewing29, 30. In the next step, we used them as regions of interest (ROIs), for comparisons of brain activation (i.e., signal change) between high and low narcissism groups. Sex was considered as an additional moderating factor because male and female narcissism are commonly found to differ, particularly when it comes to emotion regulation10, 11. Additionally, we examined the effect of self-viewing in group-specific analyses to test the robustness of effects across narcissism groups.
fMRI Data Analyses
Functional MRI data analysis was performed using SPM 8 software (Wellcome Department of Imaging Neuroscience, London, UK) and the rex toolbox (version 2.1). Preprocessing steps included slice time acquisition correction, realignment, spatial normalization to an averaged EPI template in standard Montreal Neurological Institute (MNI) space, and smoothing with a 10-mm full-width at half-maximum Gaussian kernel.
We specified first level models by regressing voxelwise BOLD on the conditions Self, Friend, and Stranger. Additionally, horizontal and vertical viewing angle and left/right-orientation of the images were included as trial-by-trial regressors. Six motion parameters were specified as additional regressors of no interest. Linear contrasts were used to obtain subject-specific (1st level) estimates for the effects of Self > Friend and Self > Stranger. These contrasts were then entered as within-subjects factors in a 2nd level flexible factorial design, where the main effect of interest represents the conjunction Self > Friend & Self > Stranger. Note that these contrasts are dependent, as Friend and Stranger conditions were subtracted from Self in each contrast. SPM orthogonalizes the respective conjunction analysis in the case of non-orthogonal contrasts (for details, see refs 31 and 32) which was not the case for these contrasts. Narcissism (high/low) was included as a between-subjects factor. Correction for multiple comparisons was performed by means of family-wise error (FWE) – correction at voxel level (p FWE < 0.05) with a minimum of k = 3 contiguous voxels and false discovery rate (FDR) at cluster level (p FDR < 0.05).
Data availability statement
The study data can be obtained via the first author upon request.
Results
We first evaluated the validity of our experimental protocol by a whole-brain analysis of Self-specific effects in the full sample. The findings should resemble meta-analytic effects of visual self-recognition29, 30. The conjunction analysis of Self > Friend & Self > Stranger revealed a pattern of predominantly right-hemispheric clusters. Viewing one’s own face (as compared to faces of friends and strangers) was accompanied by increased brain activation in clusters in the right and left inferior frontal gyri (IFG) and anterior insular cortices, a cluster in the dorsal anterior cingulate cortex (ACC), as well as several mainly right-hemispheric temporo-occipital clusters (extending to the fusiform gyri), and parieto-occipital clusters (see Fig. 2 and Supplementary Table 1). This activation pattern closely resembles meta-analytic findings for visual self-recognition29, 30, thereby supporting the validity of our experimental procedure. We also observed a small cluster in the midbrain (ventral tegmental area; see Supplementary Table 1), which is not commonly implicated in self-face processing, but was previously reported in terms of dopaminergic activity during visual self-face evaluation 19.
Figure 2.
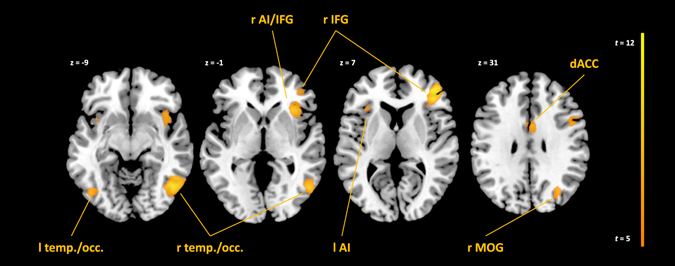
Conjunction Self> Friend & Self > Stranger, full Sample, p FWE < 0.05. l = left, r = right. AI = anterior insula, IFG = inferior frontal gyrus, dACC = dorsal anterior cingulate cortex, MOG = middle occipital gyrus. Plane identifiers are in MNI space.
In a next step, we tested whether brain activation during viewing one’s one face differs between people of high versus low narcissism. We conducted between-subjects analyses for functionally defined ROIs obtained from the analysis in the full sample within clusters that survived error correction by means of FDR (see Supplementary Table 1). Although the midbrain cluster did not meet this conservative criterion, it was also subjected to ROI analyses because of a-priori interest in the dopaminergic system (see above). Individual signal change estimates were subjected to ANOVAs encompassing the within-subjects factor condition (Self > Friend/Self > Stranger) and the between-subject factors narcissism (high/low) as well as sex (female/male). Sex was considered a moderating factor because previous research indicated that female and male narcissists differ at psychological10, 11 and also neurophysiological levels33, 34. Male narcissists displayed higher brain activation in the dorsal ACC (interaction narcissism * sex, p = 0.043, η 2 part. = 0.101), while the main effects of condition, narcissism, and sex were not significant in this cluster (ps = 0.258, 0.366 and 0.915, respectively). A similar finding also emerged in the right temporo-occipital cortex by trend (interaction narcissism * sex, p = 0.096, η 2 part. = 0.069). We observed no significant main effects or interactions of narcissism and sex in the other clusters, including the midbrain (see Supplementary Table 3), confirming the specificity of the experimental procedure.
We observed similar effects for self-viewing in separate analyses for high and low narcissism groups as for the total sample, though the effects were generally stronger in the high narcissistic group (see Fig. 3 and Supplementary Table 2). In the high narcissistic group, there was an additional peak in the ventral ACC that was not evident in the full sample, why we also conducted a functional ROI analysis in this region. We observed a significant main effect of narcissism in the ventral ACC (p = 0.045, η 2 part. = 0.099), which was significantly moderated by the interaction narcissism * sex (p = 0.011, η 2 part = 0.154) in the way that male narcissists exhibited higher brain activation (see Fig. 3 and Supplementary Table 3). In complemental whole brain and ROI analyses, we also checked for effects of self-esteem and reaction time (button press in experimental paradigm), which did not alter the results substantially. Supplementary Table 4 provides ROI analyses statistically corrected for individual differences in self-esteem; the effects in the dACC and vACC remained significant after correction (interaction narcissism*sex, p dACC = 0.050 and p vACC = 0.009, respectively).
Figure 3.
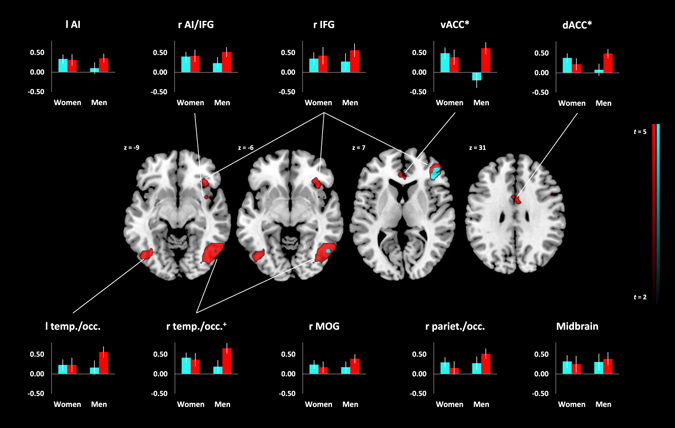
Conjunction Self > Friend & Self > Stranger, whole brain analyses within subsamples (red = high narcissism, cyan = low narcissism.), p FWE < 0.05. Graphs display interaction narcissism*sex in regions of interest, + p < 0.10, *p < 0.05; vertical axes indicate signal change, error bars denote +/−1 SE of the mean. l = left, r = right. AI = anterior insula, IFG = inferior frontal gyrus, v/dACC = ventral/dorsal anterior cingulate cortex, MOG = middle occipital gyrus. Plane identifiers are in MNI space.
Discussion
This is the first published study to examine the neural correlates of narcissistic “self-admiration” using fMRI. We conducted a carefully designed experiment in which extreme groups of high and low narcissism (assessed with the NPI, the long-time standard measure of subclinical narcissism), viewed images of themselves, close friends, and similarly attractive strangers. By these means, we sought to uncover the neural responses of visual self-recognition in narcissistic individuals, which might help to better understand the phenomenon of narcissism beyond the limits of self-reports (cf. ref. 18). Based on social-cognitive and psychodynamic theories of narcissism, we expected that highly narcissistic individuals would either display neural activation indicating self-gratification (subcortical dopaminergic regions; refs 19, 20) or negative affect and conflicting emotional processing (anterior cingulate18).
Our results support the hypothesis of negative affect rather than self-admiration: Highly narcissistic men displayed increased activation in the dorsal and ventral ACC. While anterior midline regions are known to be generally involved in self-referential processing29, 35, interindividual differences in d/vACC activation might be associated with more specific psychological processes: The dorsal ACC is a key region in conflict monitoring36, expectancy violation37, and negative affect38. All of these can be considered relevant to self-referential processing (which implies evaluative processing; see below), but their actual degree of involvement might depend upon interindividual differences. For example, the dorsal ACC has been associated with the experience of social exclusion in individuals with low self-esteem39. Recently, in a similar vein, Cascio and colleagues18 reported that narcissistic individuals display increased activation in the “social pain network” (dorsal ACC, subgenual ACC, and anterior insula) following social exclusion in a cyberball paradigm. Importantly, highly narcissistic individuals did not report elevated feelings of social exclusion in a self-report measure, which lead the authors to conclude that “narcissists’ social pain [is] seen only in the brain” (p. 335). Visual self-recognition may induce similar distress in narcissistic individuals, because narcissists might engage in greater self-monitoring, which is consistent with the adaptive control hypothesis of ACC function38. Interestingly, dACC activation is also associated with self-viewing when being observed by others, which may lead to the experience of embarrassment, in which “the ACC might […] serve as a hub, integrating information about the reflective self that is used in evaluating perceptual self-face images” (ref. 40, p. 570).
The view that narcissistic self-processing is accompanied by negative affect or emotional conflict is further substantiated by the activation in the ventral ACC in highly narcissistic individuals. The ventral ACC is specifically involved in processing negative self-referential material41, particularly when this material is self-relevant 42. This implies that visual self-recognition is a potentially threatening situation to narcissistic individuals, which are known to be overly sensitive to ego-threat43.
Grandiose narcissism thus may encompass vulnerable aspects as well, though these might not be apparent in self-report research. Our results point to subclinical narcissism being not qualitatively but rather quantitatively different from clinical narcissism, for which “many contemporary clinical experts on narcissistic personality disorder now recognize that grandiose self-states oscillate or co-occur with vulnerable self-states and affective dysregulation” (ref. 4, p. 428).
A recent structural diffusion tensor imaging study found that narcissism goes along with weakened frontostriatal connectivity of white matter tracts44. The authors interpret their findings in terms of a neural disconnect between brain regions responsible for self-representation (medial frontal cortex) and reward (ventral striatum). This finding might help to understand why one of our initial hypotheses, the “self-reward-hypothesis”, did not hold true: Narcissists seem to habitually lack an intrinsic system for self-rewarding activity, why they strive for reward from their external (social) environment11. In order to receive reward in terms of positive social feedback, highly narcissistic individuals must be very concerned about their (visual) self-presentation45. Narcissists thus have a pronounced disposition to self-evaluation, which may lead to increased (voluntary or involuntary) self-monitoring46. This explains why highly narcissistic individuals display neural activation that points to negative affect in the course of evaluative processes rather than self-reward and/or liking (which appears to be intrinsically reduced on a brain structural level). Considering the two versions of the ancient myth of Narcissus, our results are in favor of the less prominent version, in which Narcissus is shocked to death by the ugliness of his mirror image when a leaf drops into the water. This myth can be seen to metaphorically reflect the ongoing critical self-monitoring that narcissists display when confronted with self-relevant material, presumably due to a lowered intrinsic coupling between self-representation and self-reward/liking.
Activation differences pointing to expectancy violation and affective dysregulation were apparent only in men, but not in women, in this study. It has long been hypothesized that narcissism might qualitatively differ between men and women, with men displaying more emotionally dysfunctional characteristics (e.g., ref. 11). Recent empirical evidence indicates that male narcissists display lower performance on measures of emotional intelligence, which is not the case in women10. Most strikingly, it was also found that narcissism is accompanied by general elevations in cortisol levels and exaggerated physiological stress responses, but only in men33, 34. Taken together with our fMRI findings, these results underpin the presumption that narcissism is qualitatively different in women and men, with primarily men showing increased sensitivity to potentially threatening situations and maladaptive affective regulation. The question remains, however, why our experiment – like previous ones33, 34 – did not unveil any dissociation between high and low narcissistic women. Reinhard and colleagues34 argued that sex differences in distress indicators associated with narcissism (higher basal cortisol levels in men) might be explained along different narcissistic strategies associated with male and female gender roles: While masculinity is associated with independence and agency, thus promoting individualism over the acceptance of social support, femininity encourages seeking social support. By this means, women might, on average, develop higher resilience towards potentially stressful situations. Future studies could investigate gender roles as potentially mediating variables between sex and physiological outcomes associated with narcissism; it might be the case that women with a more masculine attitude display similar effects as men do. Also, considering gender roles might help to unveil other – probably more subtle – behavioral and neural mechanisms associated specifically with female narcissism.
There are some limitations to this study. Most notably, the sample under study was rather small, but nonetheless carefully selected with respect to large (about 4 SD) and stable differences in narcissism. Using these extreme groups and a two-step fMRI data analysis procedure (whole brain and ROI analyses), it was possible to obtain robust results that satisfy the most conservative statistical criteria. Another limitation can be seen in that this study focused on grandiose narcissism, though increasing efforts are devoted to the study of vulnerable narcissism as an independent trait in narcissism research. However, our results strengthen the notion that grandiose narcissism entails vulnerable aspects when it comes to involuntary neurophysiological reactions, which points to a general mechanism underlying both traits. Future studies could use the experimental paradigm in an extended design encompassing grandiose and vulnerable aspects of narcissism to further elucidate their similarities and differences.
Finally, it shall be acknowledged that ACC activation cannot unambiguously be attributed to any single mental process36. As outlined above, the (d) ACC is generally involved in (visual or non-visual) self-processing29, but has also been associated with conflict monitoring, expectancy violation, pain, and negative affect36–38. Since processing of self-referential information might always involve some degree of self-evaluation, and thus, conflicting emotional processing or negative affect, these functions might not be as different as they might seem in the first place. In line with this, it has been proposed that the dACC might act as a hub integrating information for self-evaluative processing40. Importantly, though the ACC is generally involved in self-processing, the degree of ACC activation might still relate to interindividual differences18. For the interpretation of these interindividual differences, it seems most important that ACC activation is related to processes with negative emotional valence, especially in the context of conflict monitoring38. However, the ACC has not only been associated with self-referential processing and negative affect, but also with reward (particularly the ventral ACC; ref. 36), which could be seen as supporting the self-reward hypothesis rather than the negative affect/emotional conflict hypothesis. However, we did not observe significant group differences in the more unambiguously reward-related midbrain cluster. In complemental analyses, we also investigated other reward-related areas such as the nucleus accumbens and the caudate nucleus, but did not find any significant activation (small volume correction). Finally, the results observed here closely resemble those of Cascio and colleagues18, who related ACC activation in narcissistic individuals to negative affect (social exclusion). Taken together, the data are clearly more in line with the notion of negative affect or emotional conflict than the self-reward hypothesis.
This study set out to explore the neural correlates of narcissistic “self-admiration” within the normal personality variation of narcissism. Contrary to what would be expected on the basis of self-reports, we found that highly narcissistic men display brain activation patterns that point to prevailing negative affect or emotional conflict during visual self-recognition. These results are more in line with psychodynamic than social-cognitive theories on narcissism. While previous social-cognitive research used to focus on voluntary and conscious aspects of narcissism by means of self-report, our neurophysiological results point to latent affective dysregulation in the processing of self-relevant material.
Electronic supplementary material
Acknowledgements
This research was supported by a grant from the Austrian Science Fund (FWF): P23914. We thank Tobias Ehrhardt and the team of the university’s audiovisual lab for their help in conducting the photo sessions. The valuable contributions of Jürgen Pretsch and Andreas Höfler in coding work are gratefully acknowledged. Moreover, we like to thank Philip Brandner and Till Schües for organizing and conducting the lab sessions. Finally, we thank Thomas Zussner for his support in MRI measurement.
Author Contributions
E.J. developed the study concept. E.J., M.B., K.K. and A.C.N. contributed to the study design. Testing and data collection were performed by E.J. and M.B. E.J., G.K. and K.K. performed the data analysis and interpretation under the supervision of A.C.N. E.J. drafted the manuscript, M.B. and G.K. provided critical revisions. All authors approved the final version of the manuscript for submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03935-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wink P. Two faces of narcissism. J. Pers. Soc. Psychol. 1991;61:590–597. doi: 10.1037/0022-3514.61.4.590. [DOI] [PubMed] [Google Scholar]
- 2.Raskin RN, Hall CS. A narcissistic personality inventory. Psychol. Rep. 1979;45:590–590. doi: 10.2466/pr0.1979.45.2.590. [DOI] [PubMed] [Google Scholar]
- 3.Hendin HM, Cheek JM. Assessing hypersensitive narcissism: a reexamination of murray’s narcissism scale. J. Res. Pers. 1997;31:588–599. doi: 10.1006/jrpe.1997.2204. [DOI] [Google Scholar]
- 4.Pincus AL, Lukowitsky MR. Pathological narcissism and narcissistic personality disorder. Ann. Rev. Clin. Psychol. 2010;6(8):1–8.26. doi: 10.1146/annurev.clinpsy.121208.131215. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell K, Donnellan MB, Hopwood CJ, Ackerman RA. The two faces of narcissus? an empirical comparison of the narcissistic personality inventory and the pathological narcissism inventory. Pers. Indiv. Differ. 2011;50:577–582. doi: 10.1016/j.paid.2010.11.031. [DOI] [Google Scholar]
- 6.Campbell, W. K. A meta-analysis of the narcissism–self-esteem link. Unpublished manuscript, University of Georgia (2001).
- 7.Campbell WK, Bosson JK, Goheen TW, Lakey CE, Kernis MH. Do narcissistic dislike themselves “deep down inside”? Psychol. Sci. 2007;18:227–229. doi: 10.1111/j.1467-9280.2007.01880.x. [DOI] [PubMed] [Google Scholar]
- 8.Sedikides C, Rudich EA, Gregg AP, Kumashiro M, Rusbult C. Are normal narcissists psychologically healthy?: self-esteem matters. J. Pers. Soc. Psychol. 2004;87:400–416. doi: 10.1037/0022-3514.87.3.400. [DOI] [PubMed] [Google Scholar]
- 9.Rhodenwalt F, Madrian JC, Cheney S. Narcissism, self-knowledge organization, and emotional reactivity: The effect of daily experiences on self-esteem and affect. Pers. Soc. Psychol. B. 1998;24:75–87. doi: 10.1177/0146167298241006. [DOI] [Google Scholar]
- 10.Jauk E, Freudenthaler HH, Neubauer AC. The dark triad and trait versus ability emotional intelligence. emotional darkness differs between women and men. J. Indiv. Differ. 2016;37:112–118. doi: 10.1027/1614-0001/a000195. [DOI] [Google Scholar]
- 11.Morf CC, Rhodenwalt F. Unraveling the paradoxes of narcissism: a dynamic self-regulatory processing model. Psychol. Inq. 2001;14:177–196. doi: 10.1207/S15327965PLI1204_1. [DOI] [Google Scholar]
- 12.Brummelmann E, et al. Origins of narcissism in children. P. Natl. Acad. Sci. USA. 2015;112:3659–3662. doi: 10.1073/pnas.1507468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millon, T. Modern psychopathology: A biosocial approach to maladaptive learning and functioning. (Saunders, 1969).
- 14.Twenge JM, Foster JD. Birth cohort increases in narcissistic personality traits among american college students, 1982–2009. Soc. Psychol. and Pers. Sci. 2010;1:99–106. doi: 10.1177/1948550609355719. [DOI] [Google Scholar]
- 15.Kernberg, O. F. Borderline conditions and pathological narcissism. (Aronson, 1975).
- 16.Kohut, H. The restoration of the self. (International Universities Press, 1977).
- 17.Otway L, Vignoles VL. Narcissism and childhood recollections: a quantitative test of psychoanalytic predictions. Pers. Soc. Psychol. B. 2006;32:104–116. doi: 10.1177/0146167205279907. [DOI] [PubMed] [Google Scholar]
- 18.Cascio CN, Konrath SH, Falk EB. Narcissists’ social pain seen only in the brain. Soc. Cogn. Affect. Neurosci. 2015;10:335–341. doi: 10.1093/scan/nsu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikawa H, et al. Self-face evaluation and self-esteem in young females: an fMRI study using contrast effect. NeuroImage. 2012;59:3668–3676. doi: 10.1016/j.neuroimage.2011.10.098. [DOI] [PubMed] [Google Scholar]
- 20.Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr. Opin. Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JD, et al. Comparison of the criterion validity of popular measures of narcissism and narcissistic personality disorder via the use of expert ratings. Psychol. Assessment. 2014;26:958–969. doi: 10.1037/a0036613. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). (American Psychiatric Publishing, 2013).
- 23.Raskin R, Terry H. A principal-components analysis of the Narcissistic Personality Inventory and further evidence of its construct validity. J. Pers. Soc. Psychol. 1988;54:890–902. doi: 10.1037/0022-3514.54.5.890. [DOI] [PubMed] [Google Scholar]
- 24.Jauk E, et al. How alluring are dark personalities? The Dark Triad and attractiveness in speed dating. Eur. J. Personality. 2016;30:125–138. doi: 10.1002/per.2040. [DOI] [Google Scholar]
- 25.Rammstedt B, John OP. Kurzversion des Big Five Inventory (BFI-K): entwicklung und validierung eines ökonomischen inventars zur erfassung der fünf faktoren der persönlichkeit. Diagnostica. 2005;51:195–206. doi: 10.1026/0012-1924.51.4.195. [DOI] [Google Scholar]
- 26.Schütz, A. & Sellin, I. Multidimensionale Selbstwertskala. (Hogrefe, 2006).
- 27.Sugiura M, et al. Self-face recognition in social context. Hum. Brain Mapp. 2012;33:1364–1374. doi: 10.1002/hbm.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiura M, et al. Neural mechanism for mirrored self-face recognition. Cereb. Cortex. 2015;25:2806–2814. doi: 10.1093/cercor/bhu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C, et al. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neurosci. Biobehav. R. 2016;61:197–207. doi: 10.1016/j.neubiorev.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Platek SM, Wathne K, Tierney NG, Thomson JW. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Edelstein RS, Yim IS, Quas JA. Narcissism predicts heightened cortisol reactivity to a psychosocial stressor in men. J. Res. Pers. 2010;44:565–572. doi: 10.1016/j.jrp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhard DA, Konrath S, Lopez WD, Cameron HG. Expensive egos: narcissistic males have higher cortisol. PloS One. 2012;7:e30858. doi: 10.1371/journal.pone.0030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 36.De la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartile functional organization. J. Neurosci. 2016;36:6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- 38.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onoda K, et al. Does low self-esteem enhance social pain? the relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc. Cogn. Affect. Neurosci. 2010;5:385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morita T, et al. The anterior insular and anterior cingulate cortices in emotional processing for self-face recognition. Soc. Cogn. Affect. Neurosci. 2014;9:570–579. doi: 10.1093/scan/nst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimura S, et al. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cognition. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cognitive Neurosci. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- 43.Twenge JM, Campbell WK. “Isn’t it fun to get the respect that we’re going to deserve?” narcissism, social rejection, and aggression. Pers. Soc. Psychol. B. 2003;29:261–272. doi: 10.1177/0146167202239051. [DOI] [PubMed] [Google Scholar]
- 44.Chester DS, Lynam DR, Powell DK, DeWall CN. Narcissism is associated with weakened frontostriatal connectivity: a DTI study. Soc. Cogn. Affect. Neurosci. 2015;10:335–341. doi: 10.1093/scan/nsu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazire S, Naumann LP, Rentfrow PJ, Gosling SD. Portrait of a narcissist: Manifestations of narcissism in physical appearance. J. Res. Pers. 2008;42:1436–1447. doi: 10.1016/j.jrp.2008.06.007. [DOI] [Google Scholar]
- 46.Rauthmann JF. Acquisitive or protective self-presentation of dark personalities? associations among the dark triad and self-monitoring. Pers. Indiv. Differ. 2011;51:502–508. doi: 10.1016/j.paid.2011.05.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data can be obtained via the first author upon request.


