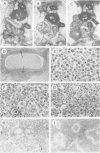
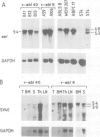
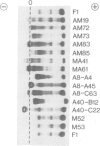
Abstract
To clarify how the v-abl oncogene of Abelson murine leukemia virus contributes to lymphoid tumorigenesis, we introduced the gene linked to an immunoglobulin heavy chain enhancer (E mu) into the mouse germline. Although lymphoid development was not detectably affected in young E mu-v-abl mice, three transgenic lines shared a high predisposition to develop clonal plasmacytomas that secreted IgA or IgG. The unexpected absence of pre-B lymphomas suggests that Abelson virus generates such tumors by infecting an early lymphoid progenitor cell that has not yet activated the heavy chain enhancer. Most plasmacytomas bore a rearranged c-myc gene, apparently as a result of spontaneous translocation to the Igh locus. Moreover, progeny of a cross with analogous E mu-myc mice rapidly developed oligoclonal plasmacytomas. Thus, the collusion of v-abl with c-myc is stage specific, efficiently transforming plasma cells but not pre-B cells or B cells.
Full text
PDF

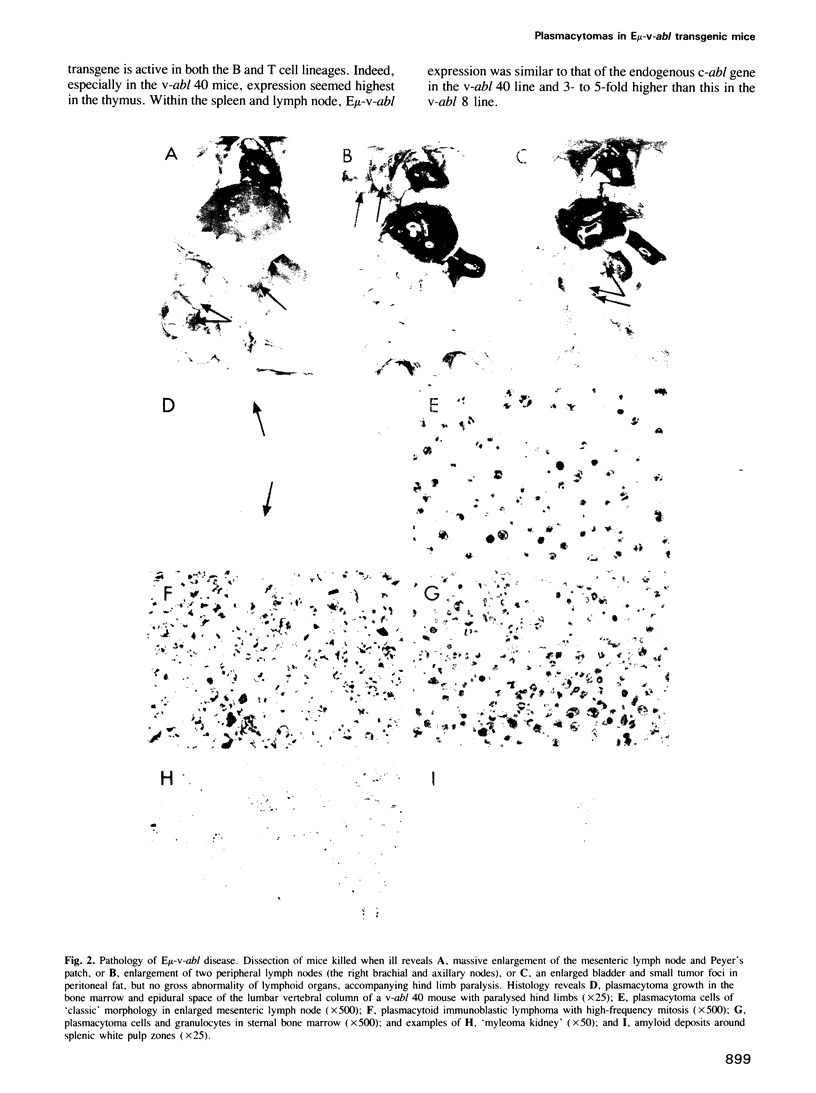





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Alexander W. S., Adams J. M., Cory S. Oncogene cooperation in lymphocyte transformation: malignant conversion of E mu-myc transgenic pre-B cells in vitro is enhanced by v-H-ras or v-raf but not v-abl. Mol Cell Biol. 1989 Jan;9(1):67–73. doi: 10.1128/mcb.9.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R., Wax J., Stanton L. W., Smith-Gill S., Potter M., Marcu K. B. Rapid induction of IgM-secreting murine plasmacytomas by pristane and an immunoglobulin heavy-chain promoter/enhancer-driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S. Activation of cellular oncogenes in hemopoietic cells by chromosome translocation. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Transgenic mice and oncogenesis. Annu Rev Immunol. 1988;6:25–48. doi: 10.1146/annurev.iy.06.040188.000325. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith D., Cory S. Transformation of bone marrow cells from E mu-myc transgenic mice by Abelson murine leukemia virus and Harvey murine sarcoma virus. Oncogene Res. 1988 May;2(4):403–409. [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gould J., Alexanian R., Goodacre A., Pathak S., Hecht B., Barlogie B. Plasma cell karyotype in multiple myeloma. Blood. 1988 Feb;71(2):453–456. [PubMed] [Google Scholar]
- Graf T., von Weizsaecker F., Grieser S., Coll J., Stehelin D., Patschinsky T., Bister K., Bechade C., Calothy G., Leutz A. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell. 1986 May 9;45(3):357–364. doi: 10.1016/0092-8674(86)90321-1. [DOI] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Cell transformation and tumor induction by Abelson murine leukemia virus in the absence of helper virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5932–5936. doi: 10.1073/pnas.84.16.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Clonal dominance and progression in Abelson murine leukemia virus lymphomagenesis. J Virol. 1987 Jul;61(7):2192–2197. doi: 10.1128/jvi.61.7.2192-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Hariharan I. K., Harris A. W., Crawford M., Abud H., Webb E., Cory S., Adams J. M. A bcr-v-abl oncogene induces lymphomas in transgenic mice. Mol Cell Biol. 1989 Jul;9(7):2798–2805. doi: 10.1128/mcb.9.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Content J., Houssiau F., Aarden L., Piechaczyk M., Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989 Feb;73(2):517–526. [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Activation of the abl oncogene in murine and human leukemias. Biochim Biophys Acta. 1985 Nov 12;823(1):1–17. doi: 10.1016/0304-419x(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S. Acceleration of B-lymphoid tumorigenesis in E mu-myc transgenic mice by v-H-ras and v-raf but not v-abl. Oncogene Res. 1989;4(4):253–258. [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S. Growth of E mu-myc transgenic B-lymphoid cells in vitro and their evolution toward autonomy. Oncogene Res. 1988;3(3):271–279. [PubMed] [Google Scholar]
- Lugo T. G., Witte O. N. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989 Mar;9(3):1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A., Fahrlander P. D., Yang J. Q., Marcu K. B. Amplification and altered expression of the c-myc oncogene in A-MuLV-transformed fibroblasts. Nature. 1985 Oct 3;317(6036):440–443. doi: 10.1038/317440a0. [DOI] [PubMed] [Google Scholar]
- Nordan R. P., Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986 Aug 1;233(4763):566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- Ohno S., Migita S., Wiener F., Babonits M., Klein G., Mushinski J. F., Potter M. Chromosomal translocations activating myc sequences and transduction of v-abl are critical events in the rapid induction of plasmacytomas by pristane and abelson virus. J Exp Med. 1984 Jun 1;159(6):1762–1777. doi: 10.1084/jem.159.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Poirier Y., Kozak C., Jolicoeur P. Identification of a common helper provirus integration site in Abelson murine leukemia virus-induced lymphoma DNA. J Virol. 1988 Nov;62(11):3985–3992. doi: 10.1128/jvi.62.11.3985-3992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Mushinski J. F., Mushinski E. B., Brust S., Wax J. S., Wiener F., Babonits M., Rapp U. R., Morse H. C., 3rd Avian v-myc replaces chromosomal translocation in murine plasmacytomagenesis. Science. 1987 Feb 13;235(4790):787–789. doi: 10.1126/science.3810165. [DOI] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. The pathogenesis of Abelson virus lymphomas of the mouse. Biochim Biophys Acta. 1982 Jun 28;651(4):213–244. doi: 10.1016/0304-419x(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Rosenbaum H., Webb E., Adams J. M., Cory S., Harris A. W. N-myc transgene promotes B lymphoid proliferation, elicits lymphomas and reveals cross-regulation with c-myc. EMBO J. 1989 Mar;8(3):749–755. doi: 10.1002/j.1460-2075.1989.tb03435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard P., DesGroseillers L., Rassart E., Poirier Y., Jolicoeur P. Important role of the long terminal repeat of the helper Moloney murine leukemia virus in Abelson virus-induced lymphoma. J Virol. 1987 Oct;61(10):3266–3275. doi: 10.1128/jvi.61.10.3266-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seremetis S., Inghirami G., Ferrero D., Newcomb E. W., Knowles D. M., Dotto G. P., Dalla-Favera R. Transformation and plasmacytoid differentiation of EBV-infected human B lymphoblasts by ras oncogenes. Science. 1989 Feb 3;243(4891):660–663. doi: 10.1126/science.2536954. [DOI] [PubMed] [Google Scholar]
- Serunian L. A., Rosenberg N. Abelson virus potentiates long-term growth of mature B lymphocytes. Mol Cell Biol. 1986 Jan;6(1):183–194. doi: 10.1128/mcb.6.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: molecular cloning of infectious integrated proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2077–2081. doi: 10.1073/pnas.78.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sümegi J., Hedberg T., Björkholm M., Godal T., Mellstedt H., Nilsson M. G., Perlman C., Klein G. Amplification of the c-myc oncogene in human plasma-cell leukemia. Int J Cancer. 1985 Sep 15;36(3):367–371. [PubMed] [Google Scholar]
- Tidmarsh G. F., Heimfeld S., Whitlock C. A., Weissman I. L., Müller-Sieburg C. E. Identification of a novel bone marrow-derived B-cell progenitor population that coexpresses B220 and Thy-1 and is highly enriched for Abelson leukemia virus targets. Mol Cell Biol. 1989 Jun;9(6):2665–2671. doi: 10.1128/mcb.9.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Troppmair J., Potter M., Wax J. S., Rapp U. R. An altered v-raf is required in addition to v-myc in J3V1 virus for acceleration of murine plasmacytomagenesis. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9941–9945. doi: 10.1073/pnas.86.24.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Szikora J. P., Renauld J. C., Van Roost E., Boon T., Simpson R. J. cDNA cloning of murine interleukin-HP1: homology with human interleukin 6. Eur J Immunol. 1988 Feb;18(2):193–197. doi: 10.1002/eji.1830180202. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. The complexity of virus--cell interactions in Abelson virus infection of lymphoid and other hematopoietic cells. Adv Immunol. 1985;37:73–98. doi: 10.1016/s0065-2776(08)60338-7. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Treiman L. J., Stafford J. I., Witte O. N. Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell. 1983 Mar;32(3):903–911. doi: 10.1016/0092-8674(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Witte O. N. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol Cell Biol. 1983 Apr;3(4):596–604. doi: 10.1128/mcb.3.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Krimpenfort P., Domen J., Saris C., Radaszkiewicz T., Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989 Feb 24;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]