Abstract
In this report, a novel fluorescent sensing platform using nitrogen-doped carbon dots (N-CDs) as probes for fluorescence signal transmission has been designed for the detection of significant biomolecules pyrophosphate (PPi) and alkaline phosphatase (ALP). The high fluorescent N-CDs could be selectively quenched by Cu2+, and recovered by the addition of PPi because PPi preferentially binds to Cu2+. Once ALP was introduced into the system, ALP can specifically hydrolyze PPi into Pi, the intense fluorescence of N-CDs could be quenched again due to the recombination of the as-released Cu2+ with N-CDs. So, fluorescence of N-CDs is regulated by an ALP-triggered reaction. Based on this strategy, we demonstrated that N-CDs could serve as a very effective fluorescent sensing platform for label-free, sensitive and selective detection of PPi and ALP with low detection limit of 0.16 μM and 0.4 U/L for PPi and ALP, respectively. Moreover, the assay time is just around 0.5 min for PPi and 30 min for ALP. This developed strategy shows remarkable advantages including sensitive, rapid, simple, convenient, and low-cost and so forth. Furthermore, this method was also successfully applied to monitor ALP in human serum, which indicates its great potential for practical applications in biological and clinical diagnosis.
Introduction
Pyrophosphate (PPi), formed by a condensation reaction of two inorganic phosphate units, plays a key role in energy transduction and several major metabolic processes1, 2. For example, PPi concentration can indicate pivotal information such as DNA replication, which can be used in cancer diagnosis by monitoring telomerase elongation process3. Alkaline phosphatase (ALP), as a membrane-bound enzyme, is one of the most commonly used hydrolase enzyme found in various sources of mammals (bone, liver, placental, and intestinal), which has been widely utilized as an important biomarker for clinical diagnostics4, 5. The abnormal level of ALP in the human body is a signal for a variety of diseases states, particularly involving in the liver, prostate and the bone6–9. Moreover, ALP is also used as a marker reagent for biological studies. Therefore, the accurate determination of ALP and PPi is essential in biochemical study and clinic diagnosis.
Up to date, many techniques have been developed for the detection of PPi and ALP including colorimetric10, 11, fluorometric12, 13, surface-enhanced Raman scattering14 and electrochemical methods15, 16. Among these assays, fluorometric methods have attracted considerable interest for their rapid response, easy operation and high sensitivity17–19. Traditional fluorescent methods are mainly based on organic dyes20, 21, fluorescent polymers22, 23 and metal nanoclusters or nanoparticles24–26. Whereas, most of them have poor photostability, laborious synthetic procedures, and complexed labeling processes. For instance, an organic fluorescent probe HCAP has been reported for ALP activity detection with aggregation-induced emission (AIE)27. However, like most of organic dyes, HCAP has poor photostability and solubility in aqueous system, which limits its further practical application. Jia et al. reported fabrication of β-cyclodextrin-modified CdTe quantum dots for ALP detection via electron transfer28. Nevertheless, the high toxicity and environmental hazards owing to the heavy metal Cd2+ have intrinsically confined its further application. Very recently, a DNA-scaffold silver nanocluster was constructed as the fluorophore to detect PPi and ALP activity with the assistance of copper ion29. However, synthesis of noble metal nanoclusters requires high cost and arduous synthetic procedures; also, they have poor optical and chemical stability in aqueous system. Consequently, there is still an urgent demand for developing a simple, cost effective, sensitive and rapid method for PPi and ALP activity detection.
Fluorescent carbon dots (CDs), as a new class of carbon-based fluorescent nanomaterials with size below 10 nm, have attracted increasing attention due to their superiorities in biocompatibility, photostability, aqueous solubility and tunable fluorescent properties compared with organic dyes and semiconductor quantum dots30–32. In view of the outstanding properties, CDs have been widely used in bioimaging, biosensing, photocatalysis and drug/gene delivery33–38. However, only a few fluorescent assays based on CDs have been reported for PPi or ALP activity detection. Qian’s group39 synthesized CDs for ALP detection based on aggregation/disaggregation of CDs. Although the fluorescence displayed reasonable sensitivity, lengthy preparation process and extremely low fluorescence quantum yield (2.2%) of the CDs limited its further biomedical application. Recently, studies have shown that heteroatom doping, especially nitrogen doping, could be used to fine-tune or obtain new kinds of high-performance CDs40, which hold great potential for biosensing and bioanalysis studies.
Herein in this report, we present an on-off switch fluorescent assay for PPi and ALP activity detection by using highly fluorescent N-CDs as signal transducer. The N-CDs were prepared by a one-step and green solid phase method using sodium alginate and tryptophan as the precursors. The fluorescence of N-CDs could be quenched by Cu2+, and recovered by the addition of PPi. ALP could specifically hydrolyze PPi into Pi, the intense fluorescence of N-CDs could be quenched again due to the recombination of the as-released Cu2+ and N-CDs. Experimental results demonstrate that this proposed assay has robust ability for quantitative analysis of both PPi and ALP activity with high sensitivity, low cost, good selectivity and rapidity as well as simplicity, which are highly desired for the screening of PPi and ALP in clinical diagnostics and other biomedical applications.
Results
Structural and morphological properties of N-CDs
Transmission electron microscopy (TEM) image in Fig. 1a shows the morphological of N-CDs prepared by solid phase method. The N-CDs are nearly spherical and monodispersed, which have an average diameter about 2.3 nm. In order to investigate the structure and composition of N-CDs, X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FT-IR) and X-ray diffraction (XRD) were used for further characterization. As shown in Fig. 1b, three strong peaks appeared at 285.3, 399.1, and 531.6 eV, which are associated with C1s, N1s, and O1s, respectively. The XPS spectrum of C1s (Supplementary Fig. S1a) exhibits three fitted peaks at 284.0, 285.5 and 287.1 eV. The binding energy at 284.0 eV could be assigned to the graphitic structure (C–C). The peak at around 285.5 eV suggests the presence of C–O and C–N, and the peak about 287.1 eV is associated with C=O group41. The high-resolution spectrum of O1s (Supplementary Fig. S1b) reveals the presence of both C=O group and C–OH/C–O–C group. The deconvolution of the N1s spectrum (Supplementary Fig. S1c) displays two peaks at 398.7 and 399.4 eV, which are associated with N in a pyridnic N and pyrrolic N42. In addition, the N/C atomic ratio was calculated to be 16.1%. In the FT-IR analysis of N-CDs, the following were observed: stretching vibration of O–H at 3408 cm−1 and C–H at 2930 cm−1, stretching vibration and bending vibration of N–H at 3256 cm−1 and 1586 cm−1, bending vibration of the C–O at 1401 cm−1 and the stretching peak of C–O–C at 1111 cm−1, C–N stretching vibration at 1246 cm−1 (Fig. 1d)43–45. These results indicate that there are abundant of hydroxyl, amino and carboxyl group on the surface of the N-CDs. The XRD spectrum (Supplementary Fig. S2) exhibits a broad peak at around 23°, corresponding to a disordered carbon atoms46. The above facts suggest that the as-prepared CDs are N-doped and exhibit abundance hydrophilic groups on the surface.
Figure 1.

(a) TEM image of N-CDs. Inset: Corresponding size distribution histograms of N-CDs. (b) XPS spectrum of N-CDs. (c) FT-IR spectrum of N-CDs. (d) Absorption and fluorescence emission spectra of N-CDs. Inset: Fluorescence image of N-CDs solutions with UV irradiation.
Optical properties of N-CDs
In order to investigate the optical properties of the N-CDs, UV-vis absorption and fluorescence spectra (Fig. 1d) were recorded, respectively. As shown in Fig. 1d, the as-prepared N-CDs show an narrow absorption peak at 272 nm and a weak shoulder at around 370 nm (black curve), which are attributed to the π-π* transition of aromatic sp2 domains and n-π* transition of C=O bond, respectively47, 48. Meanwhile, the peak emission of N-CDs occurs at 440 nm with the maximum excitation at 272 nm (red curve). A strong blue fluorescence could be observed when N-CDs aqueous solution was placed under a hand-held UV lamp (inset in Fig. 1d). As shown in Supplementary Fig. S3, the emission wavelength shows nearly no shift when the excitation wavelength is changed from 250 nm to 410 nm, indicating that the N-CDs exhibit excitation-independent PL behavior, which is considered to be related to less surface defects and more uniform size. The absolute fluorescence quantum yield (QY) of the as-prepared N-CDs was detected to be 43.2% by using a FLS 980 fluorometer equipped with an integrating sphere (IS)-based absolute QY measurement system. Also, the N-CDs are very stable, whether stand several months at room temperature (Supplementary Fig. S4a) or under 272 nm light illumination for 1 hour (Supplementary Fig. S4b), the fluorescence signal almost remains constant, which facilitates their further application. Moreover, the fluorescence lifetime of N-CDs was measured to be 14.10 ns according to time-correlated single-photon counting technique, and the result was shown in Supplementary Fig. S5.
Design and construction of assay
As illustrated in Fig. 2, the designed strategy for PPi and ALP activity assay is based on N-doped carbon dots (N-CDs). Typically, the as-prepared N-CDs with strong blue fluorescence can be selectively and sufficiently quenched by Cu2+, and recovered immediately after the addition of PPi due to much higher stability constant between PPi and Cu2+. Once ALP is introduced into the sensing system, PPi would be hydrolyzed into Pi fragments. Consequently, the fluorescence of N-CDs is quenched again because of the reintegration of N-CDs with the as-released free Cu2+. Based on this concept, quantitative evaluation of PPi as well as ALP activity could be realized very easily by using a single sensing system.
Figure 2.
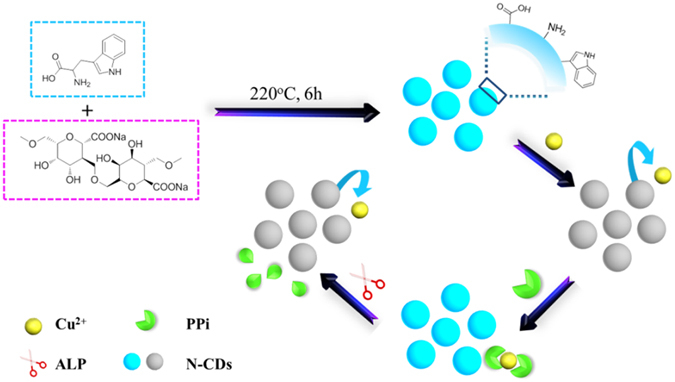
Schematic illustration of the detection strategy for PPi and ALP activity by using N-CDs.
The corresponding responses of this sensing system at different stages were shown in Fig. 3. In the presence of Cu2+, the as-prepared N-CDs with strong fluorescence (curve a) were efficiently quenched (curve b), suggesting that an effective electron or energy transfer process have happened between Cu2+ and N-CDs49, 50. This transfer process was mainly caused by the chelation of Cu2+ with N-CDs, which could be ascribed to the quite high thermodynamics affinity of Cu2+ for typical N,O-chelate ligands on the surface of the N-CDs and rapid metal-to-ligand binding kinetics51, 52. In order to investigate the driving force between Cu2+ and CDs, Zeta potential measurement has been carried out. As shown in Supplementary Fig. S6, the Zeta potential of the N-CDs and copper ions is −27.85 ± 1.5 mV and 0.49 ± 0.5 mV, respectively. When Cu2+ is added into the CDs solution, the Zeta potential becomes to be −13.88 ± 1.2 mV, suggesting that the driving force between CDs and Cu2+ is electrostatic adsorption. Once PPi was added into the N-CDs/Cu2+ solution, a clear recovery of the fluorescence (curve c) of N-CDs was observed because PPi preferentially bound to Cu2+ with very high stability constant of PPi-Cu2+ complex (stability constant log K PPi-Cu = 12.45)53. After ALP was introduced into the system, PPi was hydrolyzed to Pi and the fluorescence of N-CDs was quenched again (curve d) as a result of the reintegration of N-CDs and the as-released Cu2+. The results indicate the great possibility of this N-CDs-based sensing platform for sensitive PPi and ALP detections.
Figure 3.
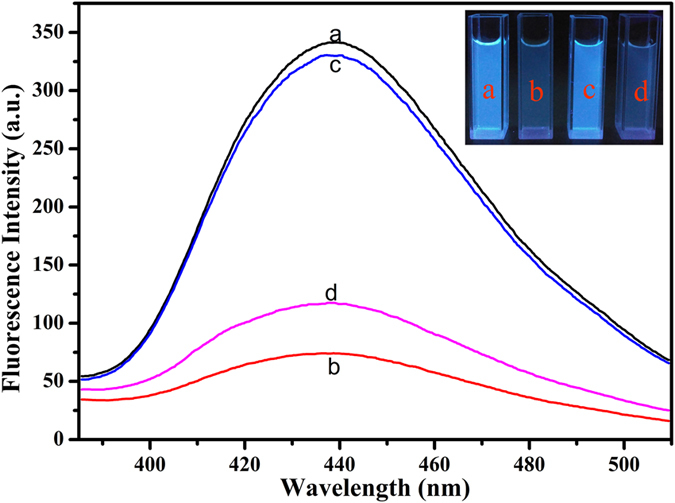
Fluorescence spectra and image of N-CDs in the presence of different composition (a) mere N-CDs; (b) in the presence of 5 μM Cu2+; (c) in the presence of 5 μM Cu2+ and 50 μM PPi; (d) in the presence of 5 μM Cu2+, 50 μM PPi and 1 U/mL ALP.
Detection of PPi
We firstly investigated the effect of Cu2+ concentration in this sensing system. As shown in Supplementary Fig. S7, the fluorescence intensity of N-CDs decreases gradually with increasing Cu2+ concentrations. When the concentration of Cu2+ is higher than 5 μM, the quenching efficiency slows down and then reaches to equilibrium. Thus, 5 μM was selected as the optimal concentration for the following experiments. Meanwhile, the quenching time and selectivity were also studied. As shown in Supplementary Fig. S8a, with only 5 μM Cu2+, the efficiency of fluorescence quenching was up to about 80% in just 30 seconds. And as shown in Supplementary Fig. S8b, Cu2+ can selectively quench the fluorescence of N-CDs.
The quenched fluorescence of N-CDs by Cu2+ could be rapidly recovered once PPi was introduced into the system. As can be seen from Supplementary Fig. S9a, the fluorescence intensity of the mixture recovers quickly to almost 100% when the PPi concentration is 50 μM after an incubation time of only 30 seconds, which indicates that this assay is a very rapid method for PPi detection. The effect of reaction pH values was also optimized, and the results revealed that fluorescence intensity has a slight difference in a buffer solution of pH 6.5–8.5 (Supplementary Fig. S10). These results indicate that this proposed strategy could be used for the detection of PPi when the pH values range from 6.5 to 8.5 and pH 7.4 was used as the experimental condition due to the relatively higher fluorescence recovery. Under the above optimized experimental conditions, the fluorescence intensity of N-CDs was collected after adding different concentrations of PPi from 0 to 200 μM into the N-CDs/Cu2+ mixture for PPi sensing in 10 mM Tris-HCl buffer (pH 7.4) at room temperature. As shown in Fig. 4a, the fluorescence intensity of the N-CDs gradually recovers with the increasing PPi concentrations, and then reaches to maximum when the concentration of PPi is 200 μM. A good linear relationship between the fluorescence intensity and PPi concentration was obtained over the range from 1 to 20 μM (Fig. 4b inset). The linear regression equation is y = 8.111 x + 79.15 (y is the fluorescence intensity of N-CDs/Cu2+ mixture in the presence of different concentrations of PPi and x is the concentration of PPi), R2 = 0.9992, and the detection limit of the developed assay for PPi is 0.16 μM according to the 3σ rule. Moreover, we further compared the sensitivity and assay time of this approach with other fluorescent methods as shown in Table 1, which are more sensitive or comparable with that obtained by the previous reported strategies. To validate the selectivity of our method for PPi sensing, we further prepared different anions as potential interferents. As shown in Supplementary Fig. S9b, the possible interferents including F−, Cl−, Br−, I−, SO4 2−, NO3 −, HCO3 −, Ac−, and PO4 3− cannot affect this competitive assay. The fluorescence responses of N-CDs/Cu2+ mixture were also investigated in the presence of sulphur compounds including Cys, GSH, HS− and HSO3 −. As can be seen from Supplementary Fig. S9c, HSO3 − has little effect on the fluorescence intensity of N-CDs/Cu2+ mixture. Because Cys, GSH, and HS− can also bind with Cu2+, the fluorescence is enhanced after the addition of Cys, GSH and HS−. According to the previous paper, the interference of Cys, GSH and HS− could be eliminated by adding N-ethylmaleimide (NEM, a RSS blocking agent)54. So, after incubation of Cys, GSH or HS− with NEM, the addition of N-CDs/Cu2+ mixture gives a negligible signal enhancement, which indicates that this proposed strategy could be successfully used for the detection of PPi with the introduction of NEM. These results indicate that this N-CDs-based assay is very fast, highly sensitive and selective for PPi detection.
Figure 4.
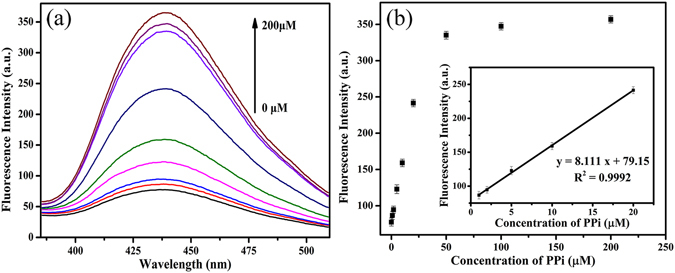
(a) Fluorescence spectra of the mixture containing N-CDs, Cu2+ and increasing amount of PPi (0, 1, 2, 5, 10, 20, 50, 100, 200 μM). (b) The fluorescence intensity of the mixture versus the concentration of PPi. Inset: The fitting curve between fluorescence intensity and PPi concentration.
Table 1.
Comparison of sensitivity and assay time of our assay with other fluorescent methods for PPi and ALP activity detection.
| Material | Analyte | LOD | Assay time | Reference |
|---|---|---|---|---|
| Carbon quantum dot | PPi | 2.56 μM | 2 min | 12 |
| Silver nanocluster | PPi | 0.11 μM | 65 min | 29 |
| Spiropyran compound | PPi | 0.4 μM | 5 min | 13 |
| Gold nanocluster | PPi | 2 μM | 2 min | 25 |
| Carbon quantum dot | PPi | 0.3 μM | 3 min | 9 |
| AuNR@SiO2@TCPP | PPi | 0.82 μM | 10 min | 1 |
| N-CDs | PPi | 0.16 μM | 0.5 min | This work |
| CdTe quantum dots | ALP | 10 U/L | 30 min | 28 |
| Copper nanoparticles | ALP | 0.3 U/L | 60 min | 26 |
| Carbon quantum dot | ALP | 1.4 U/L | 60 min | 39 |
| Silver nanocluster | ALP | 5 U/L | 130 min | 29 |
| Carbon quantum dot | ALP | 1.1 U/L | 30 min | 9 |
| Gold nanocluster | ALP | 0.1 U/L | 90 min | 25 |
| N-CDs | ALP | 0.4 U/L | 30 min | This work |
Detection of ALP
The activity of ALP was monitored by coupling with the N-CDs/Cu2+-PPi system in 10 mM Tris-HCl buffer (pH 7.4). We first investigated the effect of the incubation time on the ALP assay. As shown in Fig. 5a, the fluorescence intensity of the mixture gradually decreases with increasing reaction time. Considering the quenching efficiency and time consumption, 30 min was selected as the appropriate time for the following experiments. The fluorescence spectra of the system after incubating with different concentrations of ALP (0–1000 U/L) was shown in Fig. 5b. Meanwhile, a good linear relationship between the fluorescence intensity and ALP activity was obtained over the range from 2.5 to 45 U/L (Fig. 5c inset). The linear regression equation is y = −3.417 x + 324.6 (y is the fluorescence intensity of N-CDs/Cu2+-PPi system in the presence of different concentrations of ALP and x is the concentration of ALP), R2 = 0.9913. The detection limit of 0.4 U/L was achieved according to the 3σ rule, which is sensitive enough for ALP activity assay in biological samples (46–190 U/L for adults)55. The sensitivity and assay time of this approach were also compared with other fluorescent methods. As can be seen from Table 1, our method shows either comparable or even better response. To explore the specificity of this proposed approach for ALP activity detection, we prepared several different possible interferents including adenosine triphosphate (ATP), bovine serum albumin (BSA), T4 Polynucleotide Kinase (T4PNK), glucose oxidase (GOx), thrombin, exonuclease III (Exo III) and NDP (ADP, GDP, CDP and UDP). As shown in Fig. 5d, only ALP leads to an obvious decrease of fluorescence while the others showed little interference on the response of this bioassay to ALP. These results clearly demonstrate that this proposed assay is highly sensitive and selective for ALP activity detection.
Figure 5.
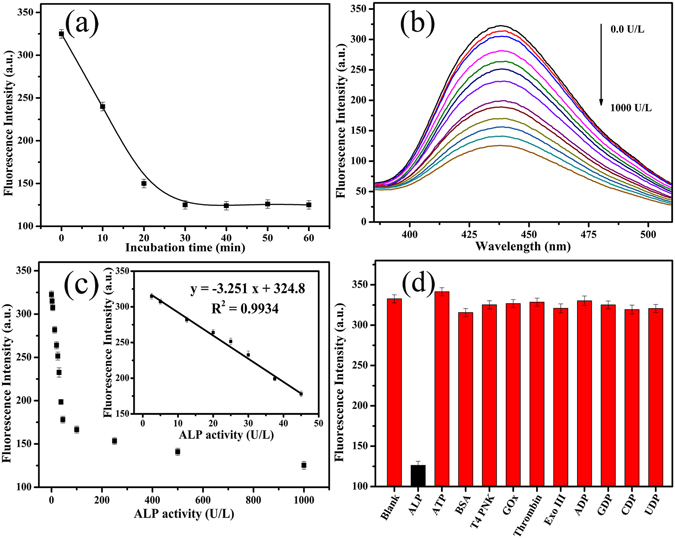
(a) The fluorescence intensity of the mixture containing N-CDs, Cu2+ (5 μM), PPi (50 μM) and ALP (1 U/mL) versus the incubation time. (b) The fluorescence emission spectra of the mixture containing N-CDs, Cu2+ (5 μM) and PPi (50 μM) as increasing ALP concentration (0, 2.5, 5, 12.5, 20, 25, 30, 37.5, 45, 100, 250, 500 and 1000 U/L). (c) The fluorescence intensity of the mixture versus ALP concentration. Inset: The fitting curve between fluorescence intensity and ALP concentration. (d) Selectivity investigation of the proposed assay for ALP activity detection. The concentration is 0.15 μg/mL for ALP and 10 μg/mL for other targets.
Detection of ALP in human serum samples
To demonstrate the feasibility of this procedure in real serum samples, we investigated the analytical performance of this assay for ALP sensing in diluted human serum (1%). Under optimized experimental conditions, we added varying concentrations of ALP into the N-CDs/Cu2+-PPi system in Tris-HCl buffer solution (pH 7.4) containing 1% diluted human serum, and the fluorescence signals were collected after 30 min incubation at 37 °C. As shown in Supplementary Fig. S11, a good work linear equation for ALP activity sensing in serum was obtained over the range from 5 to 100 U/L. With the regression equation in serum, three serum samples with the addition of different concentrations of ALP were measured. As shown in Supplementary Table S1, satisfactory recoveries between 96.0% and 104.0% are achieved with relative standard deviation (RSD) below 1.9%. Successfully detecting the human serum samples displays the promise of the present method for ALP analysis with good accuracy and reliability in various clinical applications and biological studies.
In conclusion, we have developed a rapid N-CDs mediated fluorescent on-off assay for highly sensitive and selective PPi and ALP activity detection. The N-CDs were prepared facilely by a one-step and green solid phase method. The assay relies on the principle that the fluorescence of N-CDs could be efficiently quenched by Cu2+, and recovered by the addition of PPi, which removes Cu2+ from the surface of N-CDs. Once ALP was introduced into the system, PPi was hydrolyzed into Pi and the fluorescence of N-CDs could be quenched again due to the recombination of N-CDs and Cu2+. Under the optimized experimental conditions, the assay exhibits high selectivity and sensitivity for PPi and ALP activity detection with very low detection limit of 0.16 μM and 0.4 U/L, respectively. Moreover, we have also applied this assay to monitor ALP activity in human serum and achieved satisfactory results successfully. Taking full advantages of N-CDs, this assay exhibits significantly properties including good sensitivity and selectivity, short assay time, simple design, convenient operation, low cost and environmental friendliness, which promise its great prospect for practical application in biological and clinical diagnosis.
Methods
Materials
Sodium alginate, tryptophan, and pyrophosphate (PPi) were obtained from Aladdin reagent Co., Ltd. (Shanghai, China). Alkaline phosphatse (ALP) from calf intestinal mucosa, T4 Polynucleotide Kinase (T4 PNK), and exonuclease III (Exo III) were purchased from New England biolabs (Ipswich, MA). Adenosine triphosphate (ATP), bovine serum albumin (BSA), glucose oxidase (GOx), and thrombin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents of analytical grade were directly used without additional purification. The reaction buffer solution employed in this work was 10 mM Tris-HCl (pH 7.4). All solutions were prepared by using ultrapure water which was obtained through a Millipore Milli-Q water purification system (Billerica, MA) with an electric resistance ≥18.2 MΩ.
Synthesis of N-CDs
The N-CDs were prepared by a green and facile solid phase method according to a previously reported method with minor modification43. Briefly, tryptophan (10 mmol, 2.04 g) and sodium alginate (1.08 g) were mixed and ground to a uniform powder in an agate mortar. The prepared mixture was transferred into a Teflon-lined stainless steel autoclave (25 mL) and heated at 220 °C for 6 h. After being cooled down to room temperature, the obtained dark brown product (yield ca. 58%) was dissolved in 20 mL ethanol, and then centrifuged at 11000 rpm for 10 min to remove any precipitations. The supernatant was collected, mixed with 60 mL toluene and centrifuged at 15000 rpm for 10 min. The precipitate was collected, and then dried at 60 °C to obtain the light brownish products of N-CDs.
Instrumentation
The morphology of N-CDs was characterized by transmission electron microscopy (TEM) (FEI Tecnai G2, USA) at an accelerating voltage of 200 kV. A drop of sample solution was placed on a copper grid that was left to dry before being transferred into the TEM sample chamber. Fluorescence experiments and ultraviolet-visible (UV-vis) measurements were carried on F-4600 spectrophotometer (Hitachi, Japan) and Agilent 8453 UV-vis spectrophotometer (USA), respectively. The fluorescence emission spectra of N-CDs were collected from 385 nm to 510 nm at room temperature with an excitation wavelength at 272 nm. The X-ray photoelectron spectroscopy (XPS) analysis was conducted by an ESCALAB 250Xi instrument (Thermo Fisher Scientific, USA) equipped with Al Kα (1486.6 eV). Fourier transform infrared (FT-IR) spectrum was recorded in the range of 4000–400 cm−1 by using a NEXUS-470 spectrometer (Nicolet, USA) with KBr pellets. The Quantum Yield and lifetime of N-CDs were determined by using a FLS 980 fluorescence spectrophotometer (Edinburgh, UK). The Zeta potential measurements were carried out by using a zeta/nano particle analyzer (Nano Plus, Micromeritics Instruments) at room temperature.
PPi detection procedure
In a typical experiment for PPi detection, the mixture of 20 μL Cu2+ (final concentration of 5 μM) and 20 μL PPi of different concentrations were added into Tris-HCl buffer solution (pH 7.4) containing N-CDs (final concentration of 10 μg/mL) in 200 μL centrifuge tube. After incubation for 1 min at room temperature, the fluorescence spectrum was recorded at the excitation wavelength of 272 nm. To examine whether other anions could interfere with the detection of PPi, the specificity assay was performed. The concentration of all other anions was 500 μM in the N-CDs/ Cu2+ system, which is 10-fold than that of PPi. All reaction had been performed in triplicate to ensure reproducibility.
ALP detection procedure
A typical experimental procedure was carried out as follows: different concentrations of 20 μL ALP were added to the Tris-HCl buffer solution (pH 7.4) containing 20 μL N-CDs (final concentration of 10 μg mL−1), 20 μL Cu2+ (final concentration of 5 μM), 20 μL PPi (final concentration of 50 μM). The fluorescence signal of the mixture was recorded after an incubation time of 30 min at 37 °C. For the kinetic assay, the fluorescence signals were collected after different incubation time with a certain amount of ALP concentration (1 U/mL).
ALP activity detection in human serum samples
For ALP activity detection in real sample, ALP solutions of different final concentrations were added into the N-CDs/Cu2+-PPi system in Tris-HCl buffer solution (pH 7.4) containing 1% diluted human serum. The serum from volunteer was collected by the first affiliated hospital of Zhengzhou University and informed consent was obtained for the use of human serum. All experiments were performed in compliance with the relevant laws and institutional guidelines and approved by Life-Science Ethics Review Committee of Zhengzhou University. NEM was added to the samples to eliminate the RSS in real samples. The following detection procedure was the same as shown in the aforementioned experiment for ALP activity detection in clean Tris-HCl buffer solution.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21205108, 21505122), the Startup Research Fund of Zhengzhou University (1511316004), and the Outstanding Young Talent Research Fund of Zhengzhou University (1421316038, 1521316003).
Author Contributions
Z.H.L. and J.G. designed research; Y.L.H. carried out the experiments and collected the data; Y.L.H., X.G., L.Z. and Z.M.H. analyzed the data; Y.L.H., J.G. and Z.H.L. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06356-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia Ge, Email: jiage0630@hnu.edu.cn.
Zhaohui Li, Email: zhaohui.li@zzu.edu.cn.
References
- 1.Wang L, Song QW, Liu QL, He DC, Ouyang J. Plasmon-enhanced fluorescence-based core–shell gold nanorods as a near-IR fluorescent turn-on sensor for the highly sensitive detection of pyrophosphate in aqueous solution. Adv. Funct. Mater. 2015;25:7017–7027. doi: 10.1002/adfm.201503326. [DOI] [Google Scholar]
- 2.Ronaghi M, Uhlén M, Nyrén PA. Sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 3.Bhowmik S, Ghosh BN, Marjomäki V, Rissanen K. Nanomolar pyrophosphate detection in water and in a self-assembled hydrogel of a simple terpyridine-Zn2+ complex. J. Am. Chem. Soc. 2014;136:5543–5546. doi: 10.1021/ja4128949. [DOI] [PubMed] [Google Scholar]
- 4.Zhu YH, Wang GF, Jiang H, Chen L, Zhang XJ. One-step ultrasonic synthesis of graphene quantum dots with high quantum yield and their application in sensing alkaline phosphatase. Chem. Commun. 2015;51:948–951. doi: 10.1039/C4CC07449A. [DOI] [PubMed] [Google Scholar]
- 5.Millán JL. Alkaline phosphatases. Purinerg. Signal. 2006;2:335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, et al. An association between time-varying serum alkaline phosphatase concentrations and mortality rate in patients undergoing peritoneal dialysis: a five-year cohort study. Sci. Rep. 2017;7:43314. doi: 10.1038/srep43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombatto P, et al. Hepatitis G virus RNA in the serum of patients with elevated gamma glutamyltranspeptidase and alkaline phosphatase: a specific liver disease. J. Viral Hepat. 1996;3:301–306. doi: 10.1111/j.1365-2893.1996.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 8.Ooi K, Shiraki K, Morishita Y, Nobori T. High-molecular intestinal alkaline phosphatase in chronic liver diseases. J. Clin. Lab. Anal. 2007;21:133–139. doi: 10.1002/jcla.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian ZS, et al. A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens. Bioelectron. 2015;68:675–680. doi: 10.1016/j.bios.2015.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Deng JJ, Yu P, Yang LF, Mao LQ. Competitive coordination of Cu2+ between cysteine and pyrophosphate ion: toward sensitive and selective sensing of pyrophosphate ion in synovial fluid of arthritis patients. Anal. Chem. 2013;85:2516–2522. doi: 10.1021/ac303698p. [DOI] [PubMed] [Google Scholar]
- 11.Li CM, Zhen SJ, Wang J, Li YF, Huang CZ. A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens. Bioelectron. 2013;43:366–371. doi: 10.1016/j.bios.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Qian ZS, et al. Reversible fluorescent nanoswitch based on carbon quantum dots nanoassembly for real-time acid phosphatase activity monitoring. Anal. Chem. 2015;87:7332–7339. doi: 10.1021/acs.analchem.5b01488. [DOI] [PubMed] [Google Scholar]
- 13.Shao N, Wang H, Gao X, Yang RH, Chan WH. Spiropyran-based fluorescent anion probe and its application for urinary pyrophosphate detection. Anal. Chem. 2010;82:4628–4636. doi: 10.1021/ac1008089. [DOI] [PubMed] [Google Scholar]
- 14.Ruan CM, Wang W, Gu BH. Detection of alkaline phosphatase using surface-enhanced raman spectroscopy. Anal. Chem. 2006;78:3379–3384. doi: 10.1021/ac0522106. [DOI] [PubMed] [Google Scholar]
- 15.Shen CC, et al. A single electrochemical biosensor for detecting the activity and inhibition of both protein kinase and alkaline phosphatase based on phosphate ions induced deposition of redox precipitates. Biosens. Bioelectron. 2016;85:220–225. doi: 10.1016/j.bios.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Ino K, et al. Novel electrochemical methodology for activity estimation of alkaline phosphatase based on solubility difference. Anal. Chem. 2012;84:7593–7598. doi: 10.1021/ac301429n. [DOI] [PubMed] [Google Scholar]
- 17.Lu LH, et al. A novel dinucleariridium(III) complex as a G-quadruplex-selective probe for the luminescent switch-on detection of transcription factor HIF-1α. Sci. Rep. 2016;6:22458. doi: 10.1038/srep22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng RR, Xie XJ, Vendrell M, Chang YT, Liu XG. Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles. J. Am. Chem. Soc. 2011;133:20168–20171. doi: 10.1021/ja2100774. [DOI] [PubMed] [Google Scholar]
- 19.Li N, et al. A highly selective and instantaneous nanoprobe for detection and imaging of ascorbic acid in living cells and in vivo. Anal. Chem. 2014;86:3924–3930. doi: 10.1021/ac5000587. [DOI] [PubMed] [Google Scholar]
- 20.Zhou LY, et al. Molecular engineering of a TBET-based two-photon fluorescent probe for ratiometric imaging of living cells and tissues. J. Am. Chem. Soc. 2014;136:9838–9841. doi: 10.1021/ja504015t. [DOI] [PubMed] [Google Scholar]
- 21.Dong L, Miao QQ, Hai ZJ, Yuan Y, Liang GL. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015;87:6475–6478. doi: 10.1021/acs.analchem.5b01657. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Li Y, Wang XY, Su XG. A label-free conjugated polymer-based fluorescence assay for the determination of adenosine triphosphate and alkaline phosphatase. New J. Chem. 2014;38:4574–4579. doi: 10.1039/C4NJ00935E. [DOI] [Google Scholar]
- 23.Liu Y, Schanze KS. Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal. Chem. 2008;80:8605–8612. doi: 10.1021/ac801508y. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Yang F, Zhao D, Yang XR. Highly sensitive real-time assay of inorganic pyrophosphatase activity based on the fluorescent gold nanoclusters. Anal. Chem. 2014;86:7883–7889. doi: 10.1021/ac501814u. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, et al. Cysteine-directed fluorescent gold nanoclusters for the sensing of pyrophosphate and alkaline phosphatase. J. Mater. Chem. C. 2014;2:4080–4085. doi: 10.1039/c4tc00173g. [DOI] [Google Scholar]
- 26.Zhang LL, et al. Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal. Chem. 2013;85:3797–3801. doi: 10.1021/ac4001942. [DOI] [PubMed] [Google Scholar]
- 27.Song ZG, et al. ACS Appl. Mater. Inter. 2014. A ratiometric fluorescent probe based on ESIPT and AIE processes for alkaline phosphatase activity assay and visualization in living cells; pp. 17245–17254. [DOI] [PubMed] [Google Scholar]
- 28.Jia L, et al. Fluorescence detection of alkaline phosphatase activity with β-cyclodextrin-modified quantum dots. Chem. Commun. 2010;46:7166–7168. doi: 10.1039/c0cc01244k. [DOI] [PubMed] [Google Scholar]
- 29.Ma JL, Yin BC, Wu X, Ye BC. Copper-mediated DNA-scaffolded silver nanocluster on–off switch for detection of pyrophosphate and alkaline phosphatase. Anal. Chem. 2016;88:9219–9225. doi: 10.1021/acs.analchem.6b02465. [DOI] [PubMed] [Google Scholar]
- 30.Sun YP, et al. Host-guest carbon dots for enhanced optical properties and beyond. Sci. Rep. 2015;5:12354. doi: 10.1038/srep12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shangguan JF, et al. Label-free carbon-dots-based ratiometric fluorescence pH nanoprobes for intracellular pH sensing. Anal. Chem. 2016;88:7837–7843. doi: 10.1021/acs.analchem.6b01932. [DOI] [PubMed] [Google Scholar]
- 32.Dong YQ, et al. Natural carbon-based dots from humic substances. Sci. Rep. 2015;5:10037. doi: 10.1038/srep10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, et al. A facile large-scale microwave synthesis of highly fluorescent carbon dots from benzenediol isomers. J. Mater. Chem. C. 2014;2:5028–5035. doi: 10.1039/C3TC32131B. [DOI] [Google Scholar]
- 34.Choi H, et al. Versatile surface plasmon resonance of carbon-dot-supported silver nanoparticles in polymer optoelectronic devices. Nat. Photon. 2013;7:732–738. doi: 10.1038/nphoton.2013.181. [DOI] [Google Scholar]
- 35.Hong GS, Diao S, Antaris AL, Dai HJ. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015;115:10816–10906. doi: 10.1021/acs.chemrev.5b00008. [DOI] [PubMed] [Google Scholar]
- 36.Gowthaman NSK, Sinduja B, Karthikeyan R, Rubini K, Abraham John S. Fabrication of nitrogen-doped carbon dots for screening the purine metabolic disorder in human fluids. Biosens. Bioelectron. 2017;94:30–38. doi: 10.1016/j.bios.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, et al. Preparation of yellow-green-emissive carbon dots and their application in constructing a fluorescent turn-on nanoprobe for imaging of selenol in living cells. Anal. Chem. 2017;89:1734–1741. doi: 10.1021/acs.analchem.6b03983. [DOI] [PubMed] [Google Scholar]
- 38.Zhang HY, et al. Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens. Bioelectron. 2017;87:46–52. doi: 10.1016/j.bios.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Qian ZS, et al. Carbon quantum dots-based recyclable real-time fluorescence assay for alkaline phosphatase with adenosine triphosphate as substrate. Anal. Chem. 2015;87:2966–2973. doi: 10.1021/ac504519b. [DOI] [PubMed] [Google Scholar]
- 40.Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small. 2015;11:1620–1636. doi: 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- 41.Dong YQ, et al. Carbon-based dots Co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Inter. Ed. 2013;52:7800–7804. doi: 10.1002/anie.201301114. [DOI] [PubMed] [Google Scholar]
- 42.Zhang YQ, et al. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012;22:16714–16718. doi: 10.1039/c2jm32973e. [DOI] [Google Scholar]
- 43.Zhu XH, Zhao TB, Nie Z, Liu Y, Yao SZ. Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal. Chem. 2015;87:8524–8530. doi: 10.1021/acs.analchem.5b02167. [DOI] [PubMed] [Google Scholar]
- 44.Kwon W, Do S, Kim J, SeokJeong M, Rhee S. Control of photoluminescence of carbon nanodots via surface functionalization using para-substituted anilines. Sci. Rep. 2015;5:12604. doi: 10.1038/srep12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai QY, et al. A rapid fluorescence “switch-on” assay for glutathione detection by using carbon dots–MnO2 nanocomposites. Biosens. Bioelectron. 2015;72:31–36. doi: 10.1016/j.bios.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 46.Hu YP, Yang J, Tian JW, Yu JS. How do nitrogen-doped carbon dots generate from molecular precursors? An investigation of the formation mechanism and a solution-based large-scale synthesis. J. Mater. Chem. B. 2015;3:5608–5614. doi: 10.1039/C5TB01005E. [DOI] [PubMed] [Google Scholar]
- 47.Liang ZC, Kang M, Payne GF, Wang XH, Sun RC. ACS Appl. Mater. Inter. 2016. Probing energy and electron transfer mechanisms in fluorescence quenching of biomass carbon quantum dots; pp. 17478–17488. [DOI] [PubMed] [Google Scholar]
- 48.Hola K, et al. Carbon dots—emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today. 2014;9:590–603. doi: 10.1016/j.nantod.2014.09.004. [DOI] [Google Scholar]
- 49.Zhu AW, Qu Q, Shao XL, Kong B, Tian Y. Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew. Chem. Intern. Ed. 2012;51:7185–7189. doi: 10.1002/anie.201109089. [DOI] [PubMed] [Google Scholar]
- 50.Zhu XH, et al. Nitrogen-doped carbon nanoparticle modulated turn-on fluorescent probes for histidine detection and its imaging in living cells. Nanoscale. 2016;8:2205–2211. doi: 10.1039/C5NR07826A. [DOI] [PubMed] [Google Scholar]
- 51.Krämer R. Fluorescent chemosensors for Cu2+ ions: fast, selective, and highly sensitive. Angew. Chem. Intern. Ed. 1998;37:772–773. doi: 10.1002/(SICI)1521-3773(19980403)37:6<772::AID-ANIE772>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Hu C, et al. Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu(II) detection. Small. 2014;10:4926–4933. doi: 10.1002/smll.201401328. [DOI] [PubMed] [Google Scholar]
- 53.English JB, Martell AE, Motekaitis RJ, Murase I. Molecular interaction of pyrophosphate with 1, 13-dioxa-4,7,10,16,20,24-hexaazacyclohexacosane (OBISDIPEN) and its mononuclear and dinuclear copper(II) complexes. Inorg. Chim. Acta. 1997;258:183–192. doi: 10.1016/S0020-1693(96)05500-4. [DOI] [Google Scholar]
- 54.Han Q, et al. Highly selective and sensitive one- and two-photon ratiometric fluorescent probe for intracellular hydrogen polysulfide sensing. Anal. Chem. 2016;88:7206–7212. doi: 10.1021/acs.analchem.6b01391. [DOI] [PubMed] [Google Scholar]
- 55.Hausamen TU, Helger R, Rick W, Gross W. Optimal conditions for the determination of serum alkaline phosphatase by a new kinetic method. Clin. Chim. Acta. 1967;15:241–245. doi: 10.1016/0009-8981(67)90060-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


