Abstract
Aim: Research shows that subclinical hypothyroidism (SCH) is related to an increased carotid intima –media thickness (CIMT), a surrogate marker of subclinical cardiovascular disease (CVD). It is controversial whether or not SCH should be treated to reduce CVD morbidity and mortality. This meta-analysis aimed to determine whether SCH is associated with an increase in CIMT as compared to Euthyroidism (EU) and whether thyroxin (T4) treatment in SCH can reverse the change in CIMT.
Methods: Two independent reviewers conducted an extensive database research up to December 2016. A total of 12 clinical trials discussed the effect of Thyroxin on CIMT values at pre- and post-treatment in subjects with SCH.
Results: CIMT was significantly higher among SCH (n = 280) as compared to EU controls (n = 263) at baseline; the pooled weighted mean difference (WMD) of CIMT was 0.44 mm [95% confidence interval (CI) 0.14, 0.74], p = 0.004; I2 = 65%. After treatment with thyroxin in subjects with SCH (n = 314), there was a statistically significant decrease in CIMT from pre- to post-treatment; the pooled WMD of CIMT decrease was [WMD −0.32; 95% CI (−0.47, −0.16), p = < 0.0001; I2 = 2%], and it was no longer different from EU controls [WMD 0.13 mm; 95% CI (−0.04, 0.30); p = 0.14; I2 = 27%]. The total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL) were higher in SCH as compared to EU controls and decreased significantly after treatment with thyroxin.
Conclusion: This meta-analysis shows that thyroxin therapy in subjects with SCH significantly decreases CIMT and improves lipid profile, modifiable CVD risk factors. Thyroid hormone replacement in subjects with SCH may play a role in slowing down or preventing the progression of atherosclerosis.
Keywords: Subclinical hypothyroidism, Euthyroid/Euthyroidism, Carotid intima–media thickness, Thyroxin treatment, Dyslipidemia, Clinical trials, Meta-analysis
Introduction
How important is it to treat subclinical hypothyroidism (SCH) ? This question leads to a chain of controversial discussions. SCH is a condition with an elevated thyroid-stimulating hormone (TSH) in the setting of normal free thyroid hormone levels, both FT3 and FT41). In the recent past, the detection of SCH has been increasing with evolving diagnostic tests. The prevalence of SCH is greater than that of type 2 diabetes mellitus (estimated to be 5–10%) and with an increased occurrence among females and the elderly2–4). The prevalence of SCH is almost up to 20% in females older than 60 years2).
SCH has also been considered to increase the risk of atherosclerosis alongside overt hypothyroidism5, 6). The association of overt hypothyroidism with atherosclerotic disease has been well established, and treating those patients with levothyroxine has shown proven benefits in reducing cardiovascular risk7). There is evidence that SCH had effects on some important cardiovascular risk factors, such as high blood pressure, dyslipidemia, and altered coagulability8, 9). However, how significant a role does SCH play as an independent risk factor for atherosclerosis was debatable, until a recent population-based study proved otherwise10, 11).
Carotid Intima –media thickness (CIMT), measured using carotid ultrasonography, is trusted to be a good marker of atherosclerotic changes in early stages apart from being accepted as a surrogate endpoint for cardiovascular events12, 13). Many studies have shown that SCH is independently related to a significant increase in CIMT in relatively healthy subjects when compared with euthyroid (EU) healthy matched groups14–19). Several studies, including some randomized controlled trials, have shown reduced cardiovascular risk, including a significant reduction in CIMT in subjects with SCH treated with levothyroxin15, 17, 18, 20–28).
The primary focus of this review and meta-analysis is to determine the differences of CIMT between subjects with SCH and EU controls at baseline as well as to demonstrate the effects of thyroxine treatment on CIMT reduction from pre-to-post treatment after a follow-up period. We hypothesized that “thyroxin treatment in subjects with SCH causes a decrease in CIMT values from pre-to-post treatment.”
Methods
We conducted this systematic review and metaanalysis using the PRISMA statement as guideline29).
Eligibility Criteria
In this systematic review and meta-analysis, we included the clinical trials that reported the treatment of subjects with SCH and discussed the effects of thyroxin treatment on CIMT reduction in subjects with SCH from pre- to post-treatment during a follow-up period. Our hypothesis was to analyze “the effects of thyroxin treatment on CIMT reduction in patients with SCH during a follow-up period.” We selected original published clinical trials with no language and regional limitations. According to the hypothesis, we defined a strict inclusion and exclusion criteria as described below.
Inclusion Criteria were as Follows
(1) Studies investigating subjects with SCH (the mean pretreatment basal serum TSH concentration must have been above the upper limit of normal for the assay used in the study, but less than 20 mU/L along with a normal T4 level) and comparing them with subjects with EU; (2) Use of the ultrasound method to measure CIMT both in subjects with SCH and EU at baseline; and (3) Use of the ultrasound method to measure CIMT in subjects with SCH at pre- and post-treatment with thyroxin along a follow-up period. We included studies that discussed demographically, anthropometrically, and metabolically matched SCH and EU control groups to discuss the effect of SCH on CIMT and the role of thyroxin treatment on CIMT reduction in subjects with SCH.
We excluded all studies that discussed subjects with chronic diseases/risk factors that can potentially affect CIMT and thyroid function tests. Exclusion criteria were as follows: (1) Use of overt clinical hypothyroidism/hyperthyroidism subjects; (2) Use of subjects on any medications to treat hypo and hyper functions of thyroid, including thyroid cancer; (3) Use of any subjects with average TSH > 20 (mIU/l); (4) Use of any subjects with established coronary artery disease (CAD), congestive heart failure (CHF), obesity (BMI ≥ 30 kg/m2), chronic liver disease, chronic kidney failure, chronic inflammatory diseases, hypophyseal insufficiency, or any type of cancer; (5) Use of any pregnant, lactating, or menopausal women; (6) Use of any subject using medications that can potentially alter thyroid function tests (e.g., amiodarone, carbamazepine, carbidopa, phenytoin, furosemide, haloperidol, heparin, interferon, levodopa, Lithium, metoclopramide, propranolol, primidone, rifampicin, and valproic acid.) (7) Use of any subject using medications that can potentially affect hormonal changes in the body (e.g., antidiabetics, glucocorticoid therapy, OCP, steroids, GnRH agonists and antagonists, insulin-sensitizing drugs, antiandrogens, and aspirin), as well as affect blood pressure (anti-hypertensives) or lipid levels (anti-hyperlipidemics); and (8) Use of different therapeutic approaches apart from thyroxin/T4 treatment of subjects with SCH.
Information Sources and Search Strategy
An extensive literature search, not limited by language and regions, was performed, which was directed to our hypothesis by two independent reviewers (M.A., Y.K.) via Medline using PubMed and Ovid SP, Web of Science, Cochrane Central Register of Controlled Trials, and EMBASE, up to December 2016. The following medical subject headings [Mesh] were used to select the relevant studies for a final review and meta-analysis: SCH [Mesh] OR SCH [Mesh] AND thyroxin [Mesh] OR 3, 5, 3′, 5′-tetraiodothyronine [Mesh] OR T4 [Mesh] AND CIMT [Mesh] OR CIMT [Mesh] OR carotid atherosclerosis [Mesh] AND (random [Free Item] OR randomized controlled trials [Free Item] OR RCTs [Free Item]). Furthermore, the reference sections of the finally selected studies were screened for additional eligible studies. In rare cases, authors of the relevant studies were contacted when more information or clarification was needed. Fig. 1 shows the PRISMA flow diagram of the effect of thyroxin treatment on the reduction of CIMT values in subjects with SCH at pre- to post-treatment.
Fig. 1.
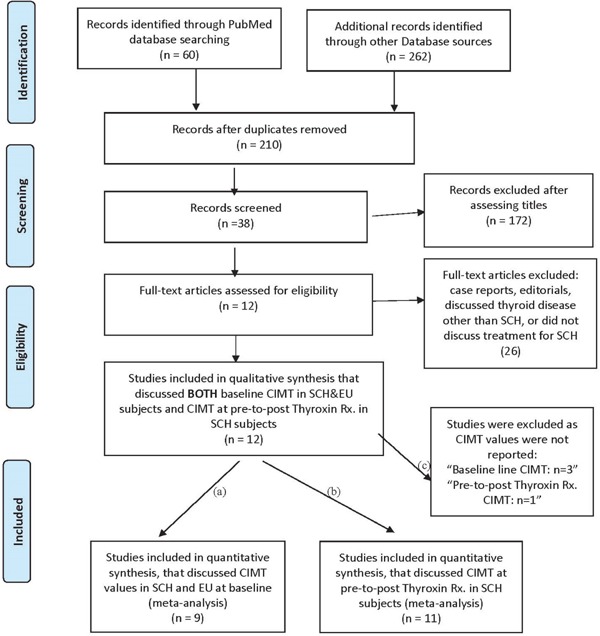
PRISMA search Flow Diagram for CIMT values at baseline in SCH and EU subjects as well as CIMT values at pre-to-post Thyroxin treatment in SCH subjects.
Study Selection
A total of 12 original studies included in the final review had a sample size ranging from 20 to 5615, 17, 18, 20, 21, 23–28, 30). The total sample of subjects with SCH was (n = 380) and that with EU controls was (n = 367). First, we screened 38 studies that discussed CIMT values in subjects with SCH and EU at baseline. Of these 38 studies, only 12 studies were clinical trials that discussed the treatment of SCH with thyroxin and measured CIMT values at pre- and post-treatment with a follow-up period. We excluded all other studies (n = 26) as these were case control, and/or cross-sectional, case reports/editorials, and none of these studies discussed the treatment of subjects with SCH. Among these finally selected 12 studies for review, 11 studies were included in the metaanalysis as the study by Köroglu et al. 201224) did not report any mean CIMT values at pre- and post-treatment with thyroxin.
Data Extraction and Quality Assessment
Three authors (Machavarapu, A; Saxena, A; Nguyen, M) extracted the data on an excel sheet independently from eligible studies related to subjects with SCH, EU controls, as well as the treatment of subjects with SCH. The data extracted included first author last name, publication year, study design, country of origin, study subjects' age, sample size, gender, cut-off TSH value to diagnose SCH, systolic blood pressure (SBP), diastolic blood pressure (DBP), lipid levels [total cholesterol (TC), triglycerides (TG) high-density lipoprotein (HDL), low-density lipoprotein (LDL), TSH], and CIMT in subjects with EU and SCH at baseline as well as at pre- and post-treatment with thyroxin. In different studies selected for a review, different biochemical and hormones assays were used to measure all classes of lipids and TSH levels. All studies used Doppler ultrasound of carotid arteries to measure CIMT. CIMT was assessed as the distance between the lumen–intima interface and the media–adventitia interface. Any type of disagreement in data collection was resolved by discussion with a fourth reviewer (Kandimalla, Y). In all clinical trials included in this meta-analysis, the confounding factors that may affect CIMT, for example, use of recent previous/present thyroid medications or other medications that affect thyroid hormones, any thyroid disease other than SCH, smoking, HTN, DM, CVD, stroke, chronic liver disease, and chronic kidney disease were balanced between subjects with SCH and EU controls by the respective authors of each included study. All studies in this review have clearly mentioned about the research approval of the institutional ethical committee and participants in the studies completed and signed the informed consent form.
Data Synthesis and Analysis
The baseline mean CIMT as well as the standard deviations of CIMT were extracted in subjects with SCH and EU controls. The mean CIMT values and standard deviations were also extracted in subjects with SCH at pre- and post-treatment with thyroxin. We used RevMan 5 free version to conduct the data analysis31). The overall variation among studies termed as heterogeneity was calculated by I2 (Tau2) statistics. The square root of this number is the estimated standard deviation of the underlying effects across studies. The estimate of the between-study variance can be measured via a fixed- or random-effect model. We calculated the weighted mean difference (WMD) or the standardized mean difference (SMD) with a 95% confidence interval (CI) to calculate the pooled effect size using a fixed- or random-effect model as appropriate. Under a fixed-effect model, we assume that there is one true effect size that is shared by all the included studies. It follows that the combined effect is our estimate of this common effect size. In contrast, under a random-effect model, we allow that the true effect could vary from study to study and we try to estimate the mean of a distribution of true effects. Large studies may produce more precise estimates of a true effect than small studies, but each study estimates a different effect size. Therefore, the weights assigned under a random-effect model are more balanced as compared with that in a fixed-effect model. WMD with a 95% CI of CIMT values was measured for subjects with SCH and EU at baseline. WMD with a 95% CI of CIMT was also measured for subjects with SCH at pre- and post-treatment periods with thyroxin. Statistical heterogeneity was tested using Tau2 with p < 0.05 considered as significant.
Two authors (Khan, IM; Aziz, M) used the Cochrane risk of bias tool to determine the risk of a bias graph (Fig. 2) and that of a bias summary (Fig. 3) in individual studies per methodological quality of included clinical trials. The Cochrane risk of bias tool is based on the following items: Random sequence generation, allocation concealment, blinding of participant and personnel, outcome data blinding, incomplete outcome data, selective reporting, and other bias. A subgroup/sensitivity analysis was used to explore the potential sources of between-studies heterogeneity according to the type of studies, study quality by the JADAD score as a low (≤ 2 score) or high (≥ 3 score) range of quality score, BMI < 25 kg/m2, TSH values as ≤10 mIU/l and > 10 mIU/l, and duration of treatment as less than 6 months and more than 6 months. Potential publication bias was assessed and represented graphically with funnel plots of WMD or SMD versus standard error32, 33). The decision to use the results of the Cochrane risk of bias assessment tool and publication bias among studies was taken by discussing with senior authors (Nasir, K; Kandimalla, Y; Veledar, E)
Fig. 2.
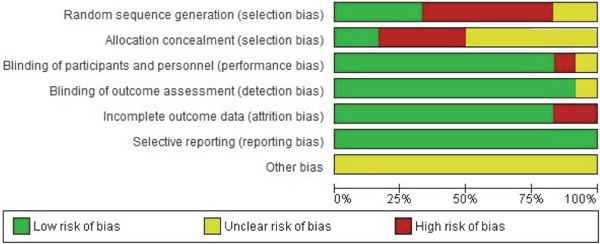
Risk of bias graph for randomized controlled trials using the Cochrane risk of bias tool. Review authors' judgments about each risk of bias item presented as percentages across all included studies.
Fig. 3.
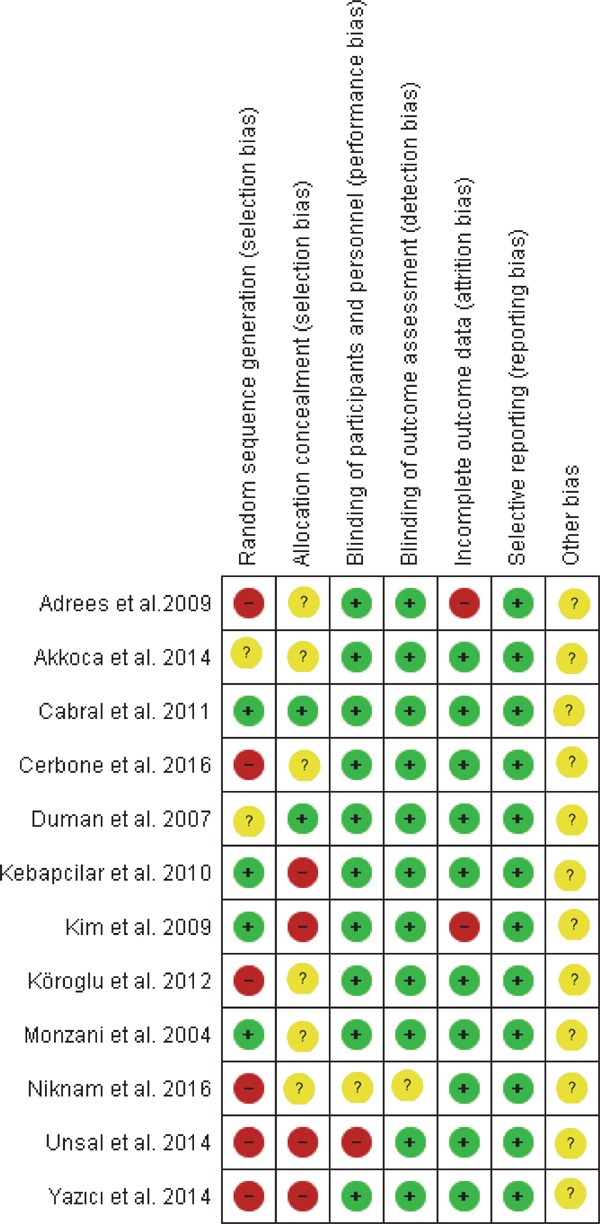
Risk of bias summary: Review authors' judgments about each risk of bias item for each included study.
Results
Study Characteristics
Table 1 shows the characteristics of subjects with SCH and EU controls. All 12 studies discussed the comparison of CIMT values in subjects with SCH and EU at baseline as well as the effect of thyroxin treatment on CIMT reduction in subjects with SCH. However, only 9 out of 12 studies reported the mean CIMT values of subjects with SCH and EU controls at baseline and 11 out of 12 studies reported the effect of thyroxin treatment on CIMT reduction from preto post-treatment. Table 2 shows the JADAD score for the quality of each study, duration of follow-up, TSH and CIMT values at pre- and post-treatment, and change and percentage change in TSH and CIMT values from pre- to post-treatment.
Table 1. Characteristics of study population.
| Author(s), Year Published & Study Type | Country | Sample (SCH/EU) | Age (yr) (SCH/EU); Female % | TSH cutoff value (mIU/l) | TSH (mIU/l) at baseline (SCH/EU) | CIMT (mm) at baseline: SCH/EU | Exclusion criteria used | SCH and EU Groups matched for |
|---|---|---|---|---|---|---|---|---|
| Monzani et al. 200418); Clinical trial | Italy | 45/32 | 37 ± 11/35 ± 10 | > 3.6 | 6.03 ± 8.4/1.19 ± 1.6 | 0.76 ± 0.14/0.63 ± 0.07 | Thyroid Rx., subjects > 55 yr, obese (BMI > 30 kg/m2), smoking, HTN, DM, CVD, CRF, CLD. | sex, age, BMI |
| Duman et al. 200723); Clinical trial | Turkey | 20/20 | 37.0 ± 12.6/37.0 ± 12.6 | > 4.2 | 10.9 ± 5.8/2.3 ± 0.8 | 0.65 ± 0.99/0.54 ± 0.10 | Taking any medication, Obesity (BMI > 30 kg/m2), DM, HTN, CHD, CLD, CRF, FH, PVD, age < 18 years or > 60 years, smoking, menopause, pregnancy. | sex, age. |
| Adrees et al. 200920); Clinical trial | Ireland | 56/56 | 50 ± 9/NR | NR | 13.2 ± 4.5/1.9 ± 1.0 | 0.82 ± 0.2/NR | Hx. of IHD, TIA, HTN, DM, or impaired fasting glycaemia, smoking, coeliac disease, pernicious ane-mia. | age, BMI. |
| Kim et al. 200917); Clinical trial | Korea | 36/32 | 36.0 ± 6.2/36.1 ± 5.4 | > 5.5 | 11.48 ± 4.70/1.60 ± 0.60 | 0.67 ± 0.11/0.57 ± 0.08 | Hx. of thyroid disease or Rx., thyroidectomy, or radioiodine therapy, DM, HTN, serum Creatinine > 1.3 mg/dL, smoking, statin use, previous preg-nancy in the last 1 year, postmenopausal state. | gender, age, BMI |
| Kebapcilar et al. 201015); Clinical trial | Turkey | 38/19 | 49.47 ± 10.04/49.95 ± 8.12 | > 5.0 | 11.26 ± 7.54/1.48 ± 1.12 | 0.64 ± 0.13/0.57 ± 0.08 | DM, CHD, CLD, CRF, other systemic diseases, morbid obesity, FH, cancer. MEDS: anti-hyperlipid-emics, antihypertensives, acetylsalicylic acid, antihis-tamines, corticosteroids, HRT, multivitamins, or excessive alcohol. | age, smoking habit, waist circumference, BMI. |
| Cabral et al. 201130); Clinical trial | Brazil | 32/NR | 47.59 ± 8.4/43.36 ± 9.8 | > 4.0 | 6.79 ± 2.0/6.77 ± 1.9 | 0.66 ± 0.11/NR | Hx. of previous thyroid disease, TSH > 12 mIU/ml, obesity, HTN, DM, CAD, CLD, CRF, alcohol use. MEDS*: amiodarone, corticosteroids, estrogens, lith-ium, anti-lipids, diuretics, anti-diabetics, antihyper-tensive, and drugs to treat obesity. | NR |
| Koroglu et al. 201224); Clinical trial | Turkey | 30/NR | 44.0 ± 11.6/47.9 ± 14.6 | NR | 7.5 ± 1.5/6.8 ± 1.4 | NR/NR | CAD, CLD, CRF, chronic inflammatory disease, hypophyseal insufficiency, Thyroid Rx. in recent 3 months, use of statins, use of HRT. | NR |
| Akkoca et al. 201421); Clinical trial | Turkey | 20/20 | 34.47 ± 1.43/35.25 ± 2.21 | NR | 8.97 ± 1.1/3.50 ± 0.43 | 0.74 ± 0.63/0.39 ± 0.72 | Hx. of thyroid Rx., HTN, DM, CAD, CLD, CRF, cancer, FH, use of lipid lowering drugs, BMI < 20 kg/m2 or > 30 kg/m2, smoking. | Age, height, weight, BMI |
| Unsal et al. 201425); Clinical trial | Turkey | 56/46 | 41.32 ± 14.48/36.07 ± 10.58 | > 4.2 | 6.77 ± 2.902/1.65 ± 0.913 | 0.533 ± 0.112/0.5 ± 0.086 | Hx. of thyroid disease or Rx, thyroidectomy, obesity, DM, HTN, CVD, CLD, CRF, radioiodine therapy, statin use, alcohol use. | NR |
| Yazici et al. 201428); Clinical trial | Turkey | 43/30 | 35.2 ± 10.7/34.5 ± 8.2 | > 4 | 6.0 ± 1.4/2.0 ± 0.3 | 0.51 ± 0.09/0.48 ± 0.04 | DM, HTN, heart failure, IHD, valvular disease, CLD, CRF, rheumatological disease, malignancy. MEDS: antihypertensives, antihyperlipidemics, ace-tylsalicylic acid, HRT. | Sex, age, SBP, DBP, BMI |
| Cerbone et al. 201626); Clinical trial | Italy | 39/39 | 9.18 ± 3.56/9.45 ± 3.62; | > 4.5 | 6.30 ± 1.01/2.92 ± 0.68 | 0.44 ± 0.08/0.44 ± 0.06 | Chronic diseases, chromosomal and genetic syn-dromes, previous or current thyroid diseases, use of drugs that may interfere with thyroid function, pre-vious irradiation in the neck region, detection of SCH at neonatal screening, familial history of genetic lipid disorders or early CVD | age, height, BMI, SBP, DBP, |
| Niknam et al. 201627); Clinical trial | Iran | 25/25 | 35.9 ± 7.6/37.5 ± 7.3 | 7.19 ± 1.29/2.4 ± 0.55 | 0.56 ± 0.09/0.58 ± 0.08 | Rx. of hypothyroidism, CVD, CRF, CLD, malig-nancies, or CVA, HTN, DM, obesity (BMI > 30 kg/m2), smoking, pregnancy, lactating women. | sex, age. |
SCH = Subclinical Hypothyroidism, NR = Not reported, Hx = History of, Rx = Treatment of, HTN = Hypertension, DM = Diabetes Mellitus, ATH Disease = atherosclerotic disease (e.g. CAD, PAD etc.), CAD = Coronary Artery Disease, IHD = Ischemic Heart Disease, CHD = Coronary Heart Disease, CHF = Congestive Heart Failure, CLD = Chronic Liver Disease, CRF = Chronic Renal Failure, CARS = Coronary Artery Revascularization Surgery, TC = Total cholesterol, HCL = Hypercholesterolemia, CAH = congenital adrenal hyperplasia, HPRL = hyperprolactinemia, WC = Waist Circumference, FH = Familial Hypercholesterolemia, CD = Cushing's disease, TIA = Transient Ischemic Attacks, HRT = Hormone Replacement Therapy, MNG = Multinodular Goiter, DBP = Diastolic Blood Pressure, SBP = Systolic Blood pressure. MEDS* use of any drugs that alter Thyroid function test.
Table 2. Characteristics of SCH subjects: Pre-to-Post Treatment (INCLUDED ONLY STUDIES THAT DISCUSSED TREATMNET OF SCH).
| Authors | N (SCH): Pre-Rx/Post-Rx; % Females | JADAD Score | Primary disease | Rx. given | Dose of Thyroxin (µg/day) | Duration of Rx. (Mo) | TSH (mIU/L) in SCH (pre-Rx) | TSH (mIU/L) in SCH (Post-Rx) | Change | % change | CIMT (mm) in SCH (Pre-Rx) | CIMT (mm) in SCH (Post-Rx) | Change | % change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monzani et al. 200418) | 23/23; 82% | 4 | SCH | T4 | 25 | 6 | 6.03 ± 8.4 | 1.32 ± 1.7 | −4.71 | −78% | 0.76 ± 0.14 | 0.67 ± 0.13 | −0.09 | −12% |
| Duman et al. 200723) | 20/20; 100% | 4 | SCH | T4 | 25 | 8 | 10.9 ± 5.8 | 2.0 ± 1.1 | −8.9 | −82% | 0.65 ± 0.99 | 0.55 ± 0.08 | −0.1 | −15% |
| Adrees et al. 200920) | 56/52; 100% | 1 | SCH | T4 | 50 | 18 | 13.2 ± 4.5 | 1.6 ± 1.8 | −11.6 | −88% | 0.82 ± 0.2 | 0.71 ± 0.2 | −0.11 | −13.4% |
| Kim et al. 200917) | 36/28; 86.1% | 3 | SCH | T4 | 67 | 18 | 11.48 ± 4.70 | 1.26 ± 3.30 | −10.22 | −89% | 0.67 ± 0.11 | 0.60 ± 0.10 | −0.07 | −10% |
| Kebapcilar et al. 201015) | 38/38; 82% | 3 | SCH | T4 | 25–50 | 6 | 11.26 ± 7.54 | 2.29 ± 1.43 | −8.97 | −80% | 0.64 ± 0.13 | 0.63 ± 0.12 | −0.01 | −1.6% |
| Cabral et al. 201130) | 14/14; 100% | 4 | SCH | T4 | 44.23 | 12 | 6.79 ± 2.0 | 3.02 ± 0.89 | −3.77 | −56% | 0.66 ± 0.11 | 0.66 ± 0.15 | 0 | 0% |
| Koroglu et al. 201224) | 30/30; 97% | 4 | SCH | T4 | 50 | 6 | 7.5 ± 1.5 | 3.6 ± 0.6 | −3.9 | −52% | NR | NR | NA | NA |
| Akkoca et al. 201421) | 20/20; 75% | 3 | SCH | T4 | NR | 7 | 8.97 ± 1.1 | 2.94 ± 0.43 | −6.03 | −67% | 0.74 ± 0.63 | 0.43 ± 0.32 | −0.31 | −42% |
| Unsal et al. 201425) | 56/56; 91% | 1 | SCH | T4 | 25–50 | 6 | 6.77 ± 2.90 | 2.73 ± 1.17 | −4.04 | −60% | 0.53 ± 0.11 | 0.51 ± 0.13 | −0.02 | 2% |
| Yazıcı et al. 201428) | 23/23; 98% | 4 | SCH | T4 | NR | 6 | 5.9 ± 1.2 | 1.7 ± 0.9 | −4.2 | −71% | 0.51 ± 0.09 | 0.46 ± 0.07 | −0.05 | 10% |
| Cerbone et al. 201626) | 39/39; 51% | 2 | SCH | T4 | 50 | 24 | 6.30 ± 1.01 | 2.82 ± 1.31 | −3.48 | −55% | 0.44 ± 0.08 | 0.46 ± 0.07 | 0.02 | 4.5% |
| Niknam et al. 201627) | 25/25; 60% | 1 | SCH | T4 | 50 | 2 | 7.19 ± 1.29 | 2.56 ± 0.69 | −4.63 | −64% | 0.56 ± 0.09 | 0.57 ± 0.08 | 0.01 | 2% |
The JADAD score/scale or the Oxford quality scoring system, is a procedure to independently assess the methodological quality of a clinical trial. SCH = Subclinical Hypothyroidism, TSH = Thyroid stimulating hormone.
CIMT Values Among Subjects with SCH and EU at Baseline
The 9 clinical trials reported differences of CIMT values among subjects with SCH and EU controls at baseline (SCH, n = 280; EU, n = 263). There was a statistically significant heterogeneity among studies (I2 = 65%; p = 0.004). A random-effect model was used to calculate the pooled WMD and the overall WMD showed significantly higher CIMT values among subjects with SCH as compared to those with EU [WMD 0.44 mm; 95% CI (0.14, 0.74); p = 0.004] (Fig. 4a).
Fig. 4a.
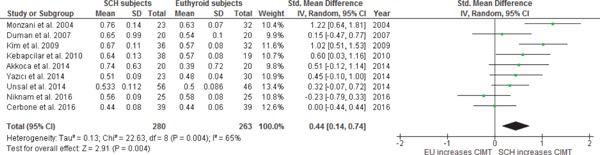
WMD with 95% CI of CIMT in SCH and EU at baseline.
A subgroup analysis was also conducted by considering TSH cut-off values as ≤ 10 mIU/l and > 10 mIU/l in subjects with SCH and comparing their CIMT values with those of EU controls at baseline. Subjects with SCH with TSH ≤ 10.0 mU/l exhibited a near significant increase in CIMT as compared to EU controls at baseline; WMD was 0.36 mm with 95% CI (−0.01, 0.73); p = 0.06 with significant heterogeneity; I2 = 68%; p = 0.009. However, WMD between subjects with SCH with a mean TSH > 10.0 mU/l and EU controls was 0.61 with 95% CI (0.13, 1.10); p < 0.01, and heterogeneity was decreased to I2 = 56%; p = 0.10. This shows that with a decreased between studies heterogeneity, subjects with SCH with TSH > 10.0 mU/l exhibited a significantly higher WMD increase in CIMT as compared to SCH with TSH ≤ 10.0 mU/l when compared to EU controls (WMD 0.61 vs. 0.36). We also conducted a subgroup analysis based on BMI groups. We calculated WMD with 95% CI by excluding all the studies with BMI > 25 kg/m2 (as an increase in BMI is related to an increase in CIMT). We used only those studies that reported a mean BMI of subjects with SCH and EU as < 25 kg/m2. CIMT was still significantly higher in subjects with SCH as compared to EU [WMD 0.51; 95% CI (0.14, 0.89); p = 0.008] with significant heterogeneity, I2 = 70%; p = 0.005. As increasing age, smoking, hypertension, diabetes, non-alcoholic fatty liver disease, dyslipidemia, polycystic ovarian disease, and menopause are related to increased CIMT and increased CVD risks, we removed all studies that discussed subjects with SCH and EU controls with one or multiple of these risk factors. The remaining 8 studies still showed a significant increase in CIMT among subjects with SCH as compared to EU controls [WMD 0.42; 95% CI (0.09, 0.75); p = 0.01)] with significant heterogeneity, I2 = 68%; p = 0.002. The test for the overall effect of CIMT differences in a subgroup analysis was significant with Z = 4.88; p < 0.00001; with heterogeneity I2 = 64%; p < 0.0001, and the test for subgroup differences was [Chi2 = 0.80, df= 3 (p = 0.85), I2 = 0%]. (Fig. 4b)
Fig. 4b.
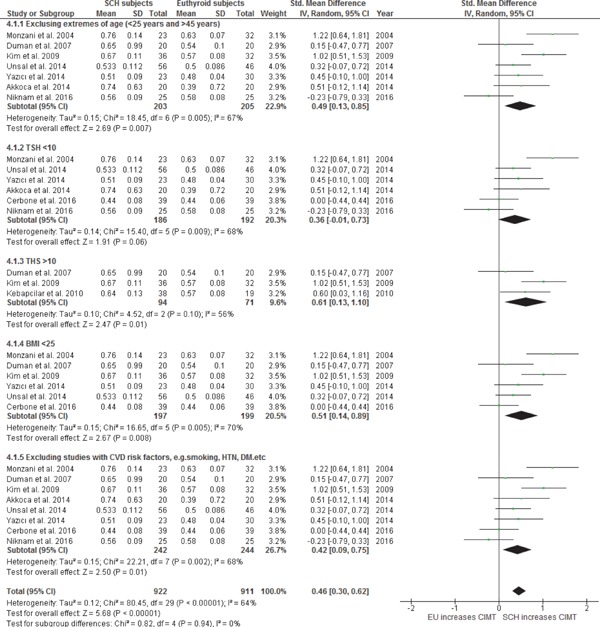
Subgroup analysis: WMD with 95% CI of CIMT in SCH and EU at baseline.
Effect of Thyroxin Treatment on CIMT Values in Subjects with SCH
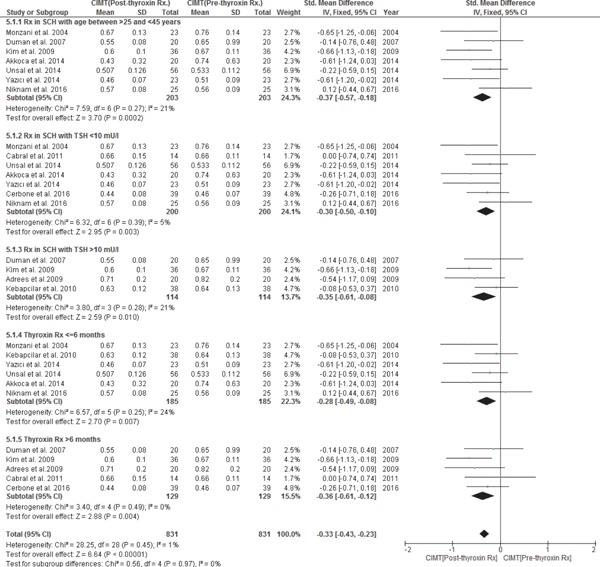
All 12 studies discussed the effects of thyroxin treatment on CIMT reduction from pre- to post-treatment in subjects with SCH (n = 314) with the duration of treatment ranging from 2 to 24 months; however, 11 studies were included in the meta-analysis as one study by Koroglu et al. 201224) did not report the mean CIMT values in subjects with SCH at pre- and post-treatment with thyroxin. After treatment with thyroxin in subjects with SCH, there was a statistically significant decrease in CIMT from pre- to post-treatment. In a fixed-effect model, the pooled WMD of CIMT decrease in subjects with SCH from pre- to post-treatment with thyroxin was −0.32 mm with 95% CI (−0.47, −0.16), p = < 0.0001 with heterogeneity, I2 = 2%; p = 0.42. CIMT of SCH treated subjects was no longer different from matched EU subjects with WMD of 0.13 mm and 95% CI (−0.04, 0.30); p = 0.14 and heterogeneity, I2 = 27%; p = 0.20) (Data not shown). (Figs. 5a & 5b)
Fig. 5b.
Subgroup analysis: WMD with 95% CI of CIMT in subjects with SCH at pre- to post-treatment with thyroxin.
A subgroup analysis was also conducted by considering prior thyroxin treatment TSH cut-off values as ≤ 10 mIU/l and > 10 mIU/l in subjects with SCH and calculating the mean decrease in CIMT with thyroxin treatment from pre- to post-treatment periods. WMD of a decrease in CIMT from pre- to post-treatment in subjects with SCH with a prior treatment TSH ≤ 10 mIU/l was [WMD −0.30 mm; 95% CI (−0.50, −0.10); p = 0.003); with heterogeneity, I2 = 5%; p = 0.39] and in subjects with SCH with a prior treatment TSH ≥ 10 mIU/l was [WMD −0.35 mm; 95% CI (−0.61, −0.08); p = 0.010; with heterogeneity, I2 = 21%; p = 0.28]. Although WMD of a decrease in CIMT with thyroxin treatment was higher in subjects with SCH with a prior treatment TSH > 10 mIU/l as compared to those with TSH ≤ 10 mIU/l (WMD −0.35 vs. −0.30), this subgroup analysis indicates that thyroxin treatment is effective in both groups of subjects with SCH based on a prior treatment TSH level (i.e., TSH as ≤ 10 mIU/l or TSH ≥ 10 mIU/l) in significantly reducing CIMT. In a subgroup sensitivity analysis by the duration of follow-up with thyroxin treatment keeping 6 months of treatment as cut-off, subjects with > 6 months' thyroxin treatment had a higher decrease in CIMT from pre- to post-treatment with WMD [−0.36.; 95% CI (−0.61, −0.12); p = 0.004] with heterogeneity I2 = 0% (p = 0.49), as compared to subjects with ≤ 6 months' thyroxin treatment with WMD [−0.28; 95% CI (−0.49, −0.08); p = 0.007] with heterogeneity I2 = 24% (p = 0.25). We also conducted a subgroup analysis based on the JADAD score. We excluded all low-quality studies with the JADAD score ≤ 2. In all studies with the JADAD score ≥ 3, WMD of a decrease in CIMT from preto post-treatment in subjects with SCH was [WMD −0.40 mm; 95% CI (−0.61, −0.19); p = 0.0002); with heterogeneity, I2 = 8%; p = 0.37]. In a subgroup analysis, the test for the overall effect of CIMT reduction with thyroxin treatment was significant with Z = 5.54; p < 0.00001; with heterogeneity I2 = 0%; p = 0.50, and the test for subgroup differences was [Chi2 = 0.32, df = 3 (p = 0.96), I2 = 0%]. (Fig. 5a).
Fig. 5a.
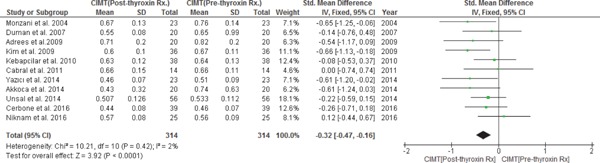
WMD with 95% CI of CIMT pre- to post-thyroxin Rx. in subjects with SCH.
Changes of Metabolic Parameters
Table 3 shows the differences in metabolic parameters between subjects with SCH and EU controls at baseline as well as after thyroxin treatment in subjects with SCH. As compared to EU controls, subjects with SCH had a significant increase in the levels of TC [WMD, 0.71 mg/dl, 95% CI (0.23, 1.19); p = 0.004), TG [WMD, 0.51 mg/dl, 95% CI (0.13, 0.90); p = 0.009], LDL [WMD, 0.63 mg/dl, 95% CI (0.30, 0.95); p = 0.0001], SBP [WMD, 6.16 mmHg l, 95% CI (1.88, 10.45); p = 0.005], and DBP [WMD, 0.43 mmHg, 95% CI (0.11, 0.76); p = 0.009]. There was no significant difference in the HDL level in subjects with SCH and EU at baseline [WMD, 0.02 mg/dl, 95% CI (−0.24, 0.27); p = 0.90]. We also discussed the changes in metabolic parameters in subjects with SCH at pre- to post-treatment with thyroxin along a treatment follow-up period. As compared to pre-treatment with thyroxin, the levels of TC (WMD, −0.53 mg/dl, 95% CI (−0.97, −0.09); p = 0.02), TG [WMD, −0.55 mg/dl, 95% CI (−0.96, −0.13); p = 0.01], LDL [WMD, −0.57 mg/dl, 95% CI (−0.98, −0.15); p = 0.007], SBP [WMD, −0.33 mmHg l, 95% CI (−0.62, −0.05); p = 0.02], and DBP [WMD, −0.38 mmHg, 95% CI (−0.68, −0.08); p = 0.01] were significantly decreased at post-treatment with thyroxin. Thyroxin treatment did not have any significant effect in increasing the HDL levels as there was no significant change in HDL (WMD, 0.03 mg/dl, 95% CI −0.16, 0.22; p = 0.75) in subjects with SCH from pre- to post-treatment with thyroxin.
Table 3. Metabolic Parameters at baseline in Subclinical Hypothyroid (SCH) subjects and Euthyroid (EU) controls and at Pre-and-Post Rx with thyroxin in SCH subjects.
| Metabolic Parameters at baseline b/w SCH and EU groups |
Changes of Metabolic Parameters at pre-and-post thyroxin Rx in SCH |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies # | Sample size (SCH/EU) | WMD (95%CI) | I2 Statistic %; P | P value | # of studies | Sample size SCH (Pre-Rx/Post-Rx) | WMD (95%CI) | I2 Statistic %; P | P value | |
| TC | 11 | 324/321 | 0.71 [0.23, 1.19] |
87%; 0.00001 | 0.004 | 10 | 299/295 | −0.53 [−0.97, −0.09] |
85%; 0.00001 | 0.02 |
| TG | 11 | 324/321 | 0.51 [0.13, 0.90] |
81%; 0.00001 | 0.009 | 10 | 299/295 | −0.55 [−0.96, −0.13] |
83%; 0.00001 | 0.01 |
| LDL | 12 | 380/367 | 0.63 [0.30, 0.95] |
78%; 0.00001 | 0.0001 | 10 | 299/295 | −0.57 [−0.98, −0.15] |
83%; 0.00001 | 0.007 |
| HDL | 12 | 380/367 | 0.02 [−0.24, 0.27] |
65%; 0.0009 | 0.90 | 8 | 224/220 | 0.03 [−0.16, 0.22] |
0%; 0.57 | 0.75 |
| SBP | 8 | 265/258 | 6.16 [1.88, 10.45] |
62%; 0.009 | 0.005 | 6 | 209/205 | −0.33 [−0.62, −0.05] |
52%; 0.07 | 0.02 |
| DBP | 8 | 265/258 | 0.43 [0.11, 0.76] |
69%; 0.002 | 0.009 | 6 | 209/205 | −0.38 [−0.68, −0.08] |
56%; 0.04 | 0.01 |
TC = Total cholesterol, TG = Triglycerides, LDL = Low density lipoprotein, HDL = High density lipoprotein, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure.
Assessment of Publication Bias
We included at least 3 studies from a total of 12 studies that were eligible for this meta-analysis, which discussed the comparison of CIMT values. To assess any publication bias, we generated funnel plots of effect estimates against their standard errors (on a reversed scale) using free version of Review Manager software 5 (RevMan) (Figs. 6 and 7). We assessed the potential risk of publication bias through visual analysis of funnel plots. The studies reporting CIMT values in subjects with SCH and EU controls at baseline (n = 9) generated a roughly symmetrical funnel plot indicating a positive standardized mean difference, while 2 studies were published with significantly higher values for a standardized mean difference and 1 study with a lower standardized mean difference. There is little possibility of publication bias toward reporting lower CIMT among EU controls. Studies reporting CIMT from pre- to post-treatment with thyroxin in subjects with SCH produced a symmetrical funnel plot with a relatively low SE hinting at a low risk of publication bias (Higgins 2011). One should be mindful that interpreting a funnel plot is subjective and an asymmetric funnel plot could be generated due to variability in selected sources, and it is not necessary that an actual publication bias can lead to an asymmetrical funnel plot. We attempted to avoid bias in study selection by defining our search criteria a priori. These are discussed in detail under sensitivity analysis as well as in limitations and strengths of the study. We also addressed location bias by searching multiple databases, which included studies that were conducted in different countries or research settings in heterogeneous populations.
Fig. 6.
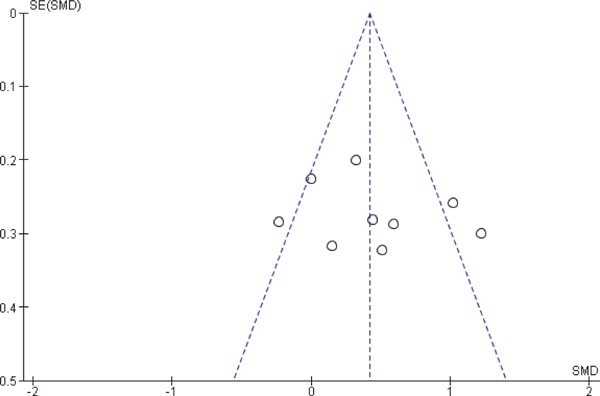
Funnel plot of CIMT values in subjects with SCH and EU at baseline.
Fig. 7.
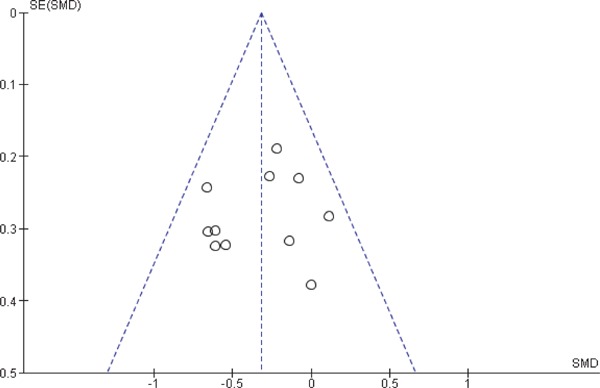
Funnel plot of CIMT pre- to post-thyroxin Rx. in subjects with SCH.
Abbreviations: SCH: Subclinical hypothyroidism/Subclinical hypothyroid, EU: Euthyroid/Controls
Medication doses provided to subjects with SCH were not similar across the studies. To account for this variability, we conducted a meta-regression analysis and accounted for different dose-related information. Results showed that there was an inverse relationship between increasing medication dosage and low CIMT, but the association was not significant. For each unit increase in dose, CIMT decreased by 0.004 units (β: −0.004; 95% CI: −0.03, 0.02; p > 0.70). This could be due to the limited number of studies (n = 9) included in the model.
Discussion
We conducted a meta-analysis to demonstrate the differences of CIMT values between subjects with SCH and EU controls at baseline. We also provided evidence of the effect of thyroxin treatment on CIMT reduction in subjects with SCH from pre- to post-treatment. The main results of this meta-analysis were as follows: (1) CIMT was significantly higher in subjects with SCH as compared to matched EU controls at baseline. (2) Thyroxin treatment in subjects with SCH was related to a significant reduction in CIMT values from pre- to post-treatment over a follow-up period and CIMT values in post-treated subjects with SCH were no longer different from CIMT values of matched EU controls. (3) More than 6 months of thyroxin treatment showed a higher reduction in CIMT values as compared to less than 6 months of thyroxin treatment. (4) As compared to EU controls, SCH was also associated with a significant increase in TC, TG, LDL, SBP, and DBP. (4) Thyroxin treatment in subjects with SCH was related to a significant reduction in TC, TG, LDL, SBP, and DBP from pre- to post-treatment over a follow-up period. (5) Neither SCH was associated with a low level of HDL nor the treatment of SCH caused a significant increase in the HDL level.
There is an increasing body of literature associating SCH with possible subclinical as well as clinical CVD outcomes15, 17, 18, 20–28, 34). The subclinical CVD outcome can be presented as increased inflammatory markers, risk of hypertension, lipid disorders, increased CIMT, endothelial dysfunction, and arterial stiffness17, 18, 22–26, 28, 35–40). Furthermore, some studies emphasized a positive association between SCH and clinical CVD outcomes, such as heart failure progression with less evidence in the oldest old population41, 42). Rodondi et al. showed in a meta-analysis that SCH was associated with an increased risk of CHD43). In another meta-analysis of 11 prospective cohort studies, Rodondi et al. showed that SCH was associated with an increased risk of coronary heart disease (CHD) events and mortality. Such risk was greater among those with higher TSH levels, predominantly among those with a TSH level ≥ 10 mIU/L44). According to some studies, SCH is not only related to worse CVD outcomes but also associated with pregnancy outcomes, infertility, other neuropsychiatric issues, and even cancer mortality45, 46). However, few studies failed to demonstrate such association between SCH and CVD outcomes47). Capola et al. showed a strong association between SCH and atrial fibrillation but did not support the association between SCH with other CVD-related morbidity and mortality48, 49). The main reason for the lack of evidence could be the use of a small sample size. The main mechanism of development of CVD in SCH could be related to low-grade chronic inflammation, abnormal lipid profile, insulin resistance, oxidative stress, arterial stiffness, and endothelial dysfunction38, 50–52). Moreover, these risk factors are accelerated in case of progression of SCH to overt thyroid disorders because there is a minimum of 2 to 5% per year to a maximum of 5 to 8% per year risk of progression of SCH to overt hypothyroidism depending on the degree of serum TSH elevation53–55).
There is also an increasing controversy on whether or not subjects with SCH should be treated. Most research workers agree with treating SCH with persistent serum TSH ≥ 10.0 mIU/L and following an individualized therapy for those with TSH < 10.0 mIU/L54). This is because the risk of CVD increases as the TSH level increases beyond 10 mIU/L. The 2013 ETA Guidelines recommended thyroxin treatment of young patients with SCH (< 70 years) if TSH ≥ 10 mIU/L, and followed an individualized approach in young patients with SCH (< 70 years) with TSH ≤ 10 mIU/L depending upon the presence and absence of symptoms of SCH. These guidelines recommended following the age-specific local reference ranges for serum TSH levels to decide in taking a step to treat subjects with SCH or simply follow-up with monitoring of TSH in both situations55). Our meta-analysis showed a significant increased CIMT in subjects with SCH versus EU controls only at TSH values of > 10.0 mIU/l, but showed a near significant increase in CIMT at TSH values of ≤ 10.0 mIU/l. However, this review emphasizes thyroxin treatment of subjects with SCH at any level of TSH, because such treatment has a significant effect on CIMT reduction both at a prior treatment TSH ≤ 10.0 mU/l as well as TSH > 10.0 mU/l (Fig. 5b). We also observed that subjects with SCH with a prior treatment TSH > 10.0 mU/l exhibited a significantly higher WMD decrease in CIMT as compared to SCH with a prior treatment TSH ≤ 10.0 mU/l (WMD −0.35 vs. −0.30), but both showed a significant CIMT reduction from pre- to post-treatment. Some studies report against treating SCH because these studies did not find any evidence of SCH association with CVD outcomes at any level of TSH meeting SCH diagnosis. The study by Cappola et al. did not find any association between SCH and incidence and prevalence of atherosclerotic disease, as well as showed no significant positive effect on CVD outcome with thyroxin treatment56).
Our meta-analysis provided a strong evidence of increased subclinical CVD risks as increased CIMT, higher-level atherogenic lipids, and increased SBP and DBP in subjects with SCH. An increase in CIMT was significantly correlated with an increase in the level of atherogenic lipids. The altered lipid levels could be the major mechanism causing early atherosclerotic vascular changes in subjects with SCH. In all subgroup analyses, the association between increased CIMT in subjects with SCH remained significant except in subjects with SCH with a mean TSH ≤ 10.0 mIU/l, where SCH was no longer associated with an increased CIMT. Furthermore, we found a greater improvement in CIMT values in subjects with SCH with a thyroxin treatment duration of more than 6 months versus less than 6 months (WMD of CIMT reduction −0.36 vs. −0.28). Many studies vote for a lifelong treatment with thyroxin and a regular monitoring of thyroid function tests for clinical/overt hypothyroidism; however, no robust evidence exists regarding the duration of long-term treatment of otherwise healthy subjects with SCH. Moreover, some studies suggest little or no symptomatic benefit from the treatment of SCH57–59). According to the 2013 SCH treatment guidelines by Pearce et al., there is emphasis on starting the treatment of SCH if a patient has signs and symptoms or other conditions, such as diffuse or nodular goiter, diabetes, dyslipidemia, or TSH > 10 mU/l, and if there is an improvement in symptoms, then to consider a lifelong treatment55). Our meta-analysis also showed a significant risk of an increase in CIMT and dyslipidemia especially at TSH > 10.0 mU/l and recommended the start of thyroxin treatment in SCH in otherwise healthy young subjects. A meta-analysis by Goa et al. showed that SCH was associated with only SBP but not DBP34), but a meta-analysis by Cai et al.60) and the present meta-analysis showed that SCH was significantly associated with higher SBP as well as higher DBP when compared with matched EU controls. Furthermore, this meta-analysis also observed a significant decrease in both SBP (p = 0.02) and DBP (p = 0.01) with thyroxin treatment. This evidence suggested that subjects with SCH are at a significantly higher risk of subclinical CVD risk factors and can obtain benefits from in time long-term thyroxin treatment.
The strengths of the present meta-analysis are as follows: (1) We defined a strict inclusion and exclusion criteria. (2) We conducted a different subgroup analysis to reduce heterogeneity and publication bias. (3) We also conducted an analysis for CIMT differences in subjects with SCH and EU controls after removing all studies that used subjects with any other conditions, which can potentially affect CIMT; for example, subjects with a history of smoking, hypertension, and diabetes, obese with non-alcoholic fatty liver disease, with BMI > 25 kg/m2, subjects with Polycystic Ovary Syndrome (PCOS); the results were still significant. (4) We conducted the dose meta-regression, which showed that an increase in each unit thyroxin dose has an addictive effect on CIMT reduction, although the model was not significant due to the limited number of studies included in the model.
The present meta-analysis has the following limitations: (1) Most of the clinical trials discussing the treatment of subjects with SCH were not doubleblind, randomized controlled trials; however, such approach would be linked with a less bias. (2) There was significant heterogeneity among the studies discussing baseline differences among CIMT values in SCH and EU controls, although a subgroup analysis was conducted to calculate the less biased association between SCH and CVD risk factors. The main reasons for heterogeneity would be related to the differences across the studies included in the review, such as great variations in sample size, type of study population used, study design, different TSH cut-off value to diagnose SCH, differences in inclusion and exclusion criteria, the method of TSH and CIMT measurements, and different confounding factors used for adjustment. (3) The duration of treatment was quite broad ranging from 2 months to 24 months, which can potentially create a bias, although we conducted a subgroup analysis keeping the cut-off treatment duration as 6 months and noticed that the benefit of CVD risks is greater with the treatment duration longer than 6 months. Randomized controlled clinical trials with longer treatment durations are needed to verify these changes. (4) We could not conduct a subgroup analysis of specific gender's (females vs. males) risk of SCH with CIMT changes because some studies did not clearly provide such information. (5) The studies used in this meta-analysis used a broad age range, which could be another source of heterogeneity. (6) Heterogeneity could be due to a selection or language bias as we used articles from all languages; citation bias as “negative” studies are quoted less frequently and, therefore, are more likely to be missed in the search for relevant trials. All these types of biases are more likely to affect studies with smaller participants to a greater degree than large trials.
Conclusion
In summary, this meta-analysis suggests that there is a strong association of SCH and increased CIMT, along with dyslipidemia and incre ased SBP and DBP. Such association has been proven by many other studies. The increased CIMT could be related to the associated increase in TSH level, dyslipidemia, obesity, and hypertension. This meta-analysis also suggests that thyroxin treatment has significant beneficial effects on CIMT reduction, weight, hypertension, and a positive improvement in lipids, especially thyroxin treatment longer than 6 months. Double-blind, randomized controlled clinical trials with a longer duration of follow-up are needed to clearly delineate the risk of SCH with CVD risk factors.
Abbreviations Frequently Used in This Manuscript
- SCH
Subclinical hypothyroidism/subclinical hypothyroid
- EU
Euthyroid (controls)
- CIMT
Carotid Intima–Media Thickness
- T4
Thyroxin treatment
- TC
Total cholesterol
- TG
Triglycerides
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
Conflict of Interests
M. Aziz, Y. Kandimalla, A. Machavarapu, A. Sexena, S. Das, A. Younus, M. Nguyen, R. Malik, C. MA. Latif, Humayun, IM. Khan, A. Adus, A. Rasool, E. Veledar, K. Nasir state that no conflict of interest exists. No off-label or investigational use of a drug was performed as part of this research.
References
- 1). Surks MI, Ocampo E: Subclinical thyroid disease. Am J Med, 1996; 100: 217-223 [DOI] [PubMed] [Google Scholar]
- 2). Canaris GJ, Manowitz NR, Mayor G, Ridgway EC: The Colorado thyroid disease prevalence study. Arch Intern Med, 2000; 160: 526-534 [DOI] [PubMed] [Google Scholar]
- 3). Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V: Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med, 2005; 165: 2467-2472 [DOI] [PubMed] [Google Scholar]
- 4). Centre WM: WHO | Diabetes. WHO, 2016 [Google Scholar]
- 5). Bastenie PA, Vanhaelst L, Bonnyns M, Neve P, Staquet M: Preclinical hypothyroidism: a risk factor for coronary heart-disease. Lancet, 1971; 1: 203-204 [DOI] [PubMed] [Google Scholar]
- 6). Bastenie PA, Vanhaelst L, Neve P: Coronary-artery disease in hypothyroidism. Lancet, 1967; 2: 1221-1222 [DOI] [PubMed] [Google Scholar]
- 7). Cappola AR, Ladenson PW: Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab, 2003; 88: 2438-2444 [DOI] [PubMed] [Google Scholar]
- 8). Danese MD, Ladenson PW, Meinert CL, Powe NR: Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab, 2000; 85: 2993-3001 [DOI] [PubMed] [Google Scholar]
- 9). Canturk Z, Cetinarslan B, Tarkun I, Canturk NZ, Ozden M, Duman C: Hemostatic system as a risk factor for cardiovascular disease in women with subclinical hypothyroidism. Thyroid, 2003; 13: 971-977 [DOI] [PubMed] [Google Scholar]
- 10). Ladenson PW: Cardiovascular consequences of subclinical thyroid dysfunction: more smoke but no fire. Ann Intern Med, 2008; 148: 880-881 [DOI] [PubMed] [Google Scholar]
- 11). Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC: Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med, 2000; 132: 270-278 [DOI] [PubMed] [Google Scholar]
- 12). Allan PL, Mowbray PI, Lee AJ, Fowkes FG: Relationship between carotid intima-media thickness, symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke, 1997; 28: 348-353 [DOI] [PubMed] [Google Scholar]
- 13). O'Leary DH, Polak JF: Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol, 2002; 90: 18L-21L [DOI] [PubMed] [Google Scholar]
- 14). Cikim AS, Oflaz H, Ozbey N, Cikim K, Umman S, Meric M, Sencer E, Molvalilar S: Evaluation of endothelial function in subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid, 2004; 14: 605-609 [DOI] [PubMed] [Google Scholar]
- 15). Kebapcilar L, Comlekci A, Tuncel P, Solak A, Secil M, Gencel O, Sahin M, Sari I, Yesil S: Effect of levothyroxine replacement therapy on paraoxonase-1 and carotid intima-media thickness in subclinical hypothyroidism. Med Sci Monit, 2010; 16: CR41-CR47 [PubMed] [Google Scholar]
- 16). Knapp M, Lisowska A, Sobkowicz B, Tycinska A, Sawicki R, Musial WJ: Myocardial perfusion and intimamedia thickness in patients with subclinical hypothyroidism. Adv Med Sci, 2013; 58: 44-49 [DOI] [PubMed] [Google Scholar]
- 17). Kim S-K, Kim S-H, Park K-S, Park S-W, Cho Y-W: Regression of the Increased Common Carotid Arteryintima Media Thickness in Subclinical Hypothyroidism after Thyroid Hormone Replacement. Endocrine Journal, 2009; 56: 753-758 [DOI] [PubMed] [Google Scholar]
- 18). Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, Taddei S, Palombo C, Ferrannini E: Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: A double-blind, placebo-controlled study. J Clin Endocrinol Metab, 2004; 89: 2099-2106 [DOI] [PubMed] [Google Scholar]
- 19). Zha K, Zuo C, Wang A, Zhang B, Zhang Y, Wang B, Wang Y, Zhao J, Gao L, Xu C: LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis, 2015; 14: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Adrees M, Gibney J, El-Saeity N, Boran G: Effects of 18 months of l-T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol, 2009; 71: 298-303 [DOI] [PubMed] [Google Scholar]
- 21). Akkoca AN, Özdemir ZT, Özler GS, Karabulut L: The Evaluation of Carotid Intima Thickness in Clinical and Subclinical Hypothyroidism and Effects of Thyroid Hormone Treatment. Am J of Clin and Exp Med, 2014; 2: 59 [Google Scholar]
- 22). Cabral MD, Teixeira P, Soares D, Leite S, Salles E, Waisman M: Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. In: Clinics (Sao Paulo), pp1321-1327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Duman D, Demirtunc R, Sahin S, Esertas K: The effects of simvastatin and levothyroxine on intima-media thickness of the carotid artery in female normolipemic patients with subclinical hypothyroidism: a prospective, randomized-controlled study. J Cardiovasc Med (Hagerstown, Md), 2007; 8: 1007-1011 [DOI] [PubMed] [Google Scholar]
- 24). Ersoy I, Banu KK, Bagci O, Aksu O, Balkarli A, Alanoglu E, Tamer M: Effects of levothyroxine treatment on cardiovascular risk profile and carotid intima media thickness in patients with subclinical hypothyroidism. Acta Endocrinologica-Bucharest, 2012; 8: 433-442 [Google Scholar]
- 25). Unsal IO, Topaloglu O, Cakir E, Colak Bozkurt N, Karbek B, Gungunes A, Sayki Arslan M, Tutal Akkaymak E, Ucan B, Demirci T, Karakose M, Caliskan M, Cakal E, Delibasi T: Effect of L-thyroxin therapy on thyroidvolume and carotid artery lntima-media thickness in the patients with subclinical hypothyroidism. J of Med Disorders, 2014; 2: 1 [Google Scholar]
- 26). Cerbone M, Capalbo D, Wasniewska M, Alfano S, Mattace Raso G, Oliviero U, Cittadini A, De Luca F, Salerno M: Effects of L-thyroxine treatment on early markers of atherosclerotic disease in children with subclinical hypothyroidism. Eur J Endocrinol, 2016; 175: 11-19 [DOI] [PubMed] [Google Scholar]
- 27). Niknam N, Khalili N, Khosravi E, Nourbakhsh M: Endothelial dysfunction in patients with subclinical hypothyroidism and the effects of treatment with levothyroxine. Adv Biomed Res, India, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Yazici D, Ozben B, Toprak A, Yavuz D, Aydin H, Tarcin O, Deyneli O, Akalin S: Effects of restoration of the euthyroid state on epicardial adipose tissue and carotid intima media thickness in subclinical hypothyroid patients. Endocrine, 2015; 48: 909-915 [DOI] [PubMed] [Google Scholar]
- 29). Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev, 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Cabral MD, Teixeira P, Soares D, Leite S, Salles E, Waisman M: Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics, 2011; 66: 1321-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). RevMan 5 download | Cochrane Community. 2016; [Google Scholar]
- 32). Begg CB, Mazumdar M: Operating characteristics of a rank correlation test for publication bias. Biometrics, 1994; 50: 1088-1101 [PubMed] [Google Scholar]
- 33). Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed), 1997; 315: 629-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH: Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis, 2013; 227: 18-25 [DOI] [PubMed] [Google Scholar]
- 35). Huang LC, Lin RT, Chen CF, Chen CH, Juo SH, Lin HF: Predictors of Carotid Intima-Media Thickness and Plaque Progression in a Chinese Population. J Atheroscler Thromb, 2016; 23: 940-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Nezu T, Hosomi N, Aoki S, Matsumoto M: Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb, 2016; 23: 18-31 [DOI] [PubMed] [Google Scholar]
- 37). Nagasawa SY, Ohkubo T, Masaki K, Barinas-Mitchell E, Miura K, Seto T, El-Saed A, Kadowaki T, Willcox BJ, Edmundowicz D, Kadota A, Evans RW, Kadowaki S, Fujiyoshi A, Hisamatsu T, Bertolet MH, Okamura T, Nakamura Y, Kuller LH, Ueshima H, Sekikawa A: Associations between Inflammatory Markers and Subclinical Atherosclerosis in Middle-aged White, Japanese-American and Japanese Men: The ERA-JUMP Study. J Atheroscler Thromb, 2015; 22: 590-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Nagasaki T, Inaba M, Kumeda Y, Hiura Y, Yamada S, Shirakawa K, Ishimura E, Nishizawa Y: Central pulse wave velocity is responsible for increased brachial-ankle pulse wave velocity in subclinical hypothyroidism. Clin Endocrinol (Oxf), 2007; 66: 304-308 [DOI] [PubMed] [Google Scholar]
- 39). Shimabukuro M, Hasegawa Y, Higa M, Amano R, Yamada H, Mizushima S, Masuzaki H, Sata M: Subclinical Carotid Atherosclerosis Burden in the Japanese: Comparison between Okinawa and Nagano Residents. J Atheroscler Thromb, 2015; 22: 854-868 [DOI] [PubMed] [Google Scholar]
- 40). Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, Humayun C, Anugula D, Abbas G, Salami J, Elizondo JV, Veledar E, Nasir K: Association of Subjective and Objective Sleep Duration as well as Sleep Quality with Non-Invasive Markers of Sub-Clinical Cardiovascular Disease (CVD): A Systematic Review. J Atheroscler Thromb, 2017; 24: 208-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F: Subclinical hypothyroidism and heart failure risk in older people. Endocr Metab Immune Disord Drug Targets, 2013; 13: 13-21 [DOI] [PubMed] [Google Scholar]
- 42). Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM: Subclinical Hypothyroidism and Survival: The Effects of Heart Failure and Race. In: J. Clin. Endocrinol. Metab, pp2326-2336, Chevy Chase, MD, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Rodondi N, Aujesky D, Vittinghoff E, Cornuz J, Bauer DC: Subclinical hypothyroidism and the risk of coronary heart disease: a meta-analysis. Am J Med, 2006; 119: 541-551 [DOI] [PubMed] [Google Scholar]
- 44). Rodondi N, den Elzen WPJ, Bauer DC, Cappola AR, Razvi S, Walsh JP, Åsvold BO, Iervasi G, Imaizumi M, Collet T-H, Bremner A, Maisonneuve P, Sgarbi JA, Khaw K-T, Vanderpump MPJ, Newman AB, Cornuz J, Franklyn JA, Westendorp RGJ, Vittinghoff E, Gussekloo J: Subclinical Hypothyroidism and the Risk of Coronary Heart Disease and Mortality. JAMA, 2010; 304: 1365-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Raza SA, Mahmood N: Subclinical hypothyroidism: Controversies to consensus. In: Indian J Endocrinol Metab, ppS636-642, India, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Tseng FY, Lin WY, Li CI, Li TC, Lin CC, Huang KC: Subclinical Hypothyroidism Is Associated with Increased Risk for Cancer Mortality in Adult Taiwanese—A 10 Years Population-Based Cohort. In: PloS one, ed by Björklund P, San Francisco, CA USA, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Zhou Y, Zhao L, Wang T, Hong J, Zhang J, Xu B, Huang X, Xu M, Bi Y: Free Triiodothyronine Concentrations are Inversely Associated with Elevated Carotid Intima-Media Thickness in Middle-Aged and Elderly Chinese Population. J Atheroscler Thromb, 2016; 23: 216-224 [DOI] [PubMed] [Google Scholar]
- 48). Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW: Thyroid Status, Cardiovascular Risk, and Mortality in Older Adults: The Cardiovascular Health Study. JAMA, 2006; 295: 1033-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Martinez-Comendador J, Marcos-Vidal JM, Gualis J, Martin CE, Martin E, Otero J, Castano M: Subclinical Hypothyroidism Might Increase the Risk of Postoperative Atrial Fibrillation after Aortic Valve Replacement. Thorac Cardiovasc Surg Rep, 2016; 64: 427-433 [DOI] [PubMed] [Google Scholar]
- 50). Lu M, Yang C, Gao L, Zhao J: Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review). In: Exp Ther Med, pp3-10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Vyakaranam S, Vanaparthy S, Nori S, Palarapu S, Bhongir AV: Study of Insulin Resistance in Subclinical Hypothyroidism. Int J Health Sci Res, 2014; 4: 147-153 [PMC free article] [PubMed] [Google Scholar]
- 52). Peleg RK, Efrati S, Benbassat C, Fygenzo M, Golik A: The effect of levothyroxine on arterial stiffness and lipid profile in patients with subclinical hypothyroidism. THYROID, 2008; 18: 825-830 [DOI] [PubMed] [Google Scholar]
- 53). Khandelwal D, Tandon N: Overt and subclinical hypothyroidism: who to treat and how. DRUGS, 2012; 72: 17-33 [DOI] [PubMed] [Google Scholar]
- 54). Fatourechi V: Subclinical Hypothyroidism: An Update for Primary Care Physicians. In: Mayo Clin Proc, pp65-71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Pearce SHS, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, Wemeau JL: 2013 ETA Guideline: Management of Subclinical Hypothyroidism. In: Eur Thyroid J, pp215-228, Switzerland, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW: Thyroid status, cardiovascular risk, and mortality in older adults. JAMA, 2006; 295: 1033-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG: Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab, 2006; 91: 145-153 [DOI] [PubMed] [Google Scholar]
- 58). Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Muller B: TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study). J Clin Endocrinol Metab, 2001; 86: 4860-4866 [DOI] [PubMed] [Google Scholar]
- 59). Jaeschke R, Guyatt G, Gerstein H, Patterson C, Molloy W, Cook D, Harper S, Griffith L, Carbotte R: Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med, 1996; 11: 744-749 [DOI] [PubMed] [Google Scholar]
- 60). Cai Y, Ren Y, Shi J: Blood pressure levels in patients with subclinical thyroid dysfunction: a meta-analysis of cross-sectional data. Hypertens Res, 2011; 34:1098-1105 [DOI] [PubMed] [Google Scholar]


