Abstract
Intestinal flora (microbiota) have recently attracted attention among lipid and carbohydrate metabolism researchers. Microbiota metabolize resistant starches and dietary fibers through fermentation and decomposition, and provide short chain fatty acids (SCFAs) to the host. The major SCFAs acetates, propionate and butyrate, have different production ratios and physiological activities. Several receptors for SCFAs have been identified as the G-protein coupled receptor 41/free fatty acid receptor 3 (GPR41/FFAR3), GPR43/FFAR2, GPR109A, and olfactory receptor 78, which are present in intestinal epithelial cells, immune cells, and adipocytes, despite their expression levels differing between tissues and cell types. Many studies have indicated that SCFAs exhibit a wide range of functions from immune regulation to metabolism in a variety of tissues and organs, and therefore have both a direct and indirect influence on our bodies. This review will focus on SCFAs, especially butyrate, and their effects on various inflammatory mechanisms including atherosclerosis. In the future, SCFAs may provide new insights into understanding the pathophysiology of chronic inflammation, metabolic disorders, and atherosclerosis, and we can expect the development of novel therapeutic strategies for these diseases.
Keywords: Short chain fatty acids, Butyrate, Microbiota, Inflammation, Atherosclerosis
Introduction
There are up to 100 trillion (1 × 1014) microbes in the human intestinal tract1), including bacteria, fungi, and viruses. Collectively, these are called the intestinal microbiota. The microbiota and eukaryotic species in our intestines activate countless numbers and amounts of enzymes, and in doing so they play a fundamental role in the control of physiological functions2). The interaction between microbiota and the host influences immunological homeostasis, and changes in this interaction are associated with various inflammatory diseases3).
Fatty acids with a carbon number between 2 and 6 are considered short chain fatty acids (SCFAs) and have the following names: C2: acetic, C3: propionic, C4: butyric, C5: valeric, and C6: caproic acid. The main constitutive materials in animals are acetate, propionate, and butyrate4). In humans, SCFAs are produced from dietary fibers and resistant starches that cannot be decomposed by digestive enzymes through fermentation by the microbiota in the cecum and colon4, 5).
Atherosclerosis is associated with lipid accumulation and inflammation in the arterial wall. Aggravation of inflammation in the arterial wall induces instability of atheromatous plaques and formation of occlusive thrombosis, leading to atherosclerotic cardiovascular disease (ASCVD) events, including acute coronary syndrome and stroke. Recent studies suggest that gut microbiota and the metagenome are associated with the inflammatory condition known as atherogenesis6, 7). This brief review will focus on SCFAs, especially butyrate, and their effects on various inflammatory mechanisms including atherosclerosis. As for the connections between gut microbiota, immunity, and atherosclerosis, recent excellent reviews should also be consulted8–11).
Intestinal Bacterial Flora and Short Chain Fatty Acids
In accordance with systematic taxonomy, microbiota are organized by phylum, class, order, family, genus, and species. It has been difficult to culture microbiota, since most are obligate anaerobes, and thus many of their specific roles have remained unknown. However, recent developments in genetic analysis, including 16S ribosomal RNA sequencing and metagenomics analysis, have begun to reveal their function1). The predominant microbiota bacteria that produce SCFAs are classified as Ruminococcaceae (cluster IV) and Eubacterium (cluster XIVa) in the order Clostridia, class Clostridia, and phylum Firmicutes. The predominant producers of SCFAs are shown in Table 18, 12–15).
Table 1. SCFAs (Acetate, Propionate, Butyrate) production by microbiota in the Gut.
| SCFAs | Pathways/Reactions | Producers | References |
|---|---|---|---|
| Acetate | from pyruvate via acetyl-CoA | Most of the enteric bacteria | Louis P, et al., Nat Rev Microbiol. 2014, 12: 661–72.12) |
| (Representative of species bacteria) Akkermansia muciniphilia, | Rey FE, et al,. J Biol Chem. 2010, 285: 22082–90.13) | ||
| Bateroides spp., Bifidobacterium spp., Prevotella spp., | |||
| Ruminococcus spp. | |||
| Wood-Ljungdahl pathway | Blautia hydrogentrophica, Chrostridium spp., Streptococcus spp. | ||
| Propionate | succinate pathway | Bacteroides spp., Phascolarctobacterium succinatutens, | Louis P, et al., Nat Rev Microbiol. 2014, 12: 661–72.12) |
| Dalister spp., Veilonella spp. | Scott KP, et al., J Bacteriol. 2006, 88: 4340–9.14) | ||
| acrylate pathway | Megasphaera elsdenii, Coprpcoccus catus | ||
| propanediol pathway | Salmonella spp., Roseburia inulinivorans, Ruminocossus obeum | ||
| Butyrate | phosphotransbutyrylase/butyrate kinase route | Coprococcus comes, Coprococcus eutactus, | Duncan SH, et al., Appl Environ Microbiol. 2002, 68: 5186–90.15) |
| Louis P, et al., Nat Rev Microbiol. 2014, 12: 661–72.12) | |||
| butyryl-CoA: acetate CoA-transferase route | Anaerostripes spp. (A, L), Coprococcus catus (A), | ||
| Eubacterium rectale (A), Eubacterium hallii (A, L), Faecalibacterium prausnitzii (A), Roseburia spp. (A) | |||
Citationed from Koh A, et al, Cell 165, 1332–458)
spp., species; (A), acetate is the substrate for producing butyrate; (L), lactate is the substrate for producing butyrate
SCFAs account for 2–10% of the total energy consumption in humans, are the main energy source for large intestinal epithelial cells, and affect the production of mucins (mucus). In addition, SCFAs physiologically influence blood flow to the colon mucous membrane, the absorption of fluids and electrolytes, the autonomic nervous system, and the secretion of gut hormones8). A considerable part of the beneficial effect of prebiotics (usually non-digestive fiber compounds) is thought to be due to SCFAs produced by intestinal microbes16). Research has shown that the concentration of SCFAs is 70–140 mmol/L in the proximal colon and 20–70 mmol/L in the distal colon17). In general, acetate is thought to be more prevalent, followed by proprionate and butyrate; however, assessing the ratios of SCFAs is extremely difficult since their production depends on the various types of fermentation substrates. Animal experiments have shown that the total amount of SCFAs may be approximately 400–600 mmol/day when 60 g/day of undigested carbohydrates reach the colon18). In humans, however, since most studies on SCFAs have been conducted using fecal samples through absorption, the abovementioned estimates of intestinal concentrations may not reflect actual conditions, and many issues are yet to be elucidated. Table 2 shows the concentrations of SCFAs in the feces of adult humans that have been previously reported19–33).
Table 2. Fecal concentration of individual SCFAs (Acetate, Propionate, Butyrate) by human adults.
| Subjects (n); age | Reported measure | Acetate | Propionate | Butyrate | Total SCFAs | Unit | References | |
|---|---|---|---|---|---|---|---|---|
| Healthy subjects | 10; 21–34 years | Mean (SD) | 218 (99) | 72 (37) | 58.7 (54.5) | 378 (188) | µmol/g dry weight | Whelan K, et al., J Nutr. 2005, 135: 1896–902.20) |
| 20; 20–40 years | Mean (SEM) | 320.3 (24.9) | 97.3 (10.5) | 93.8 (9.13) | 511.4 (41.9) | µmol/g dry weight | Boler BM, et al., Br J Nutr. 2011, 106: 1864–71.21) | |
| 13; 23–58 years | Median (IQR) | 52.2 | 23.2 (13.6–37.3) | 36.8 (5–128) | 119.3 (64.5–197.0) | µmol/g wet weight | Lewis SJ, et al., Gut. 1997, 41: 245–51.22) | |
| 60; 18–24 years | Mean (SEM) | 198.4 (14.2) | 55.2 (4.7) | 50.5 (4.9) | 304.1 | µmol/g dry weight | Lecerf JM, et al., Br J Nutr. 2012, 108: 1847–58.23) | |
| 27; 18–55 years | Mean (SEM) | 35.8 (2.4) | 11.4 (1.2) | 10.0 (1.1) | 61.1 (4.4) | µmol/g | Reimer RA, et al., J Hum Nutr Diet. 2012, 25: 373–7.24) | |
| 12; 18–65 years | Mean (SD) | 48 | 13.98 | 13.31 | 80.91 | µmol/g | Fernando WM, et al., Benef Microbes. 2010, 1: 197–207.25) | |
| 46; 31–66 years | Mean (95%CI) | 44.7 females (39.7, 50.3) | 12.3 females (10.7, 14.0) | 11.7 females (9.8, 14.0) | 69.5 females (61.3, 78.7) | µmol/g | McOrist AL, et al., J Nutr. 2011, 141: 883–9.26) | |
| 58.6 males (49.8, 69.0) | 16.1 males (13.4, 19.5) | 15.4 males (12.1, 19.6) | 90.5 males (76.3, 108) | |||||
| 36 | Median (IQR) | 43.7 (34.0–52.2) | 13.1 (9.2–18.5) | 8.8 (5.2–11.5) | 91.8 (73.1–107.5) | µmol/g | Nemoto H, et al., Dig Dis Sci. 2012, 57: 2955–64.27) | |
| 20; 22–55 years | Mean (SEM) | 42.13 (3.8) | 11.5 (1.2) | 11.28 (1.4) | 67.3 (6.2) | µmol/g | Tiihonen K, et al., Br J Nutr. 2010, 103: 1070–8.28) | |
| 8; 31–59 years | Mean (SD) | NR | NR | NR | 92.7 (33.9) | µmol/g | McOrist AL, et al., Br J Nutr, 2008, 100: 138–46.29) | |
| 20; 23–28 years | Mean (SEM) | NR | NR | NR | 78.5 (6.4) | µmol/g | Hylla S, et al., Am J Clin Nutr, 1998, 67: 136–42.30) | |
| 30 | Mean (SD) | 50.5 (12.6) | 13.6 (5.2) | 14.1 (7.6) | 84.6 (22.9) | mmol/L | Schwiertz A, et al., Obesity. 2010, 18:190–5.31) | |
| Obese subjects | 20; 22–55 years | Mean (SEM) | 47.2 (3.8) | 13.6 (1.3) | 14.7 (1.5) | 78.8 (6.2) | µmol/g | Tiihonen K, et al., Br J Nutr. 2010, 103:1070–8.28) |
| 91 | Mean (SD) | 58.5 (19.1) | 17.6 (7.6) | 18.3 (9.7) | 102 (33.5) | mmol/L | Brinkworth GD, et al., Br J Nutr. 2009; 101: 1493–502.32) | |
| 32; 20–65 years | Mean (SEM) | NR | NR | NR | 34 (6) | mmol/24h | Benassi-Evans B, et al., Mutat Res, 2010; 703: 130–6.33) | |
| 35 over weight | Mean (SD) | 56.0 (18.2) | 18.3 (7.9) | 18.5 (10.1) | 98.7 (33.9) | mmol/L | Schwiertz A, et al., Obesity. 2010, 18: 190–5.31) | |
| 33 obese | 59.8 (18.3) | 19.3 (8.7) | 18.1 (10.0) | 103.9 (34.3) | ||||
Citationed from Verbeke KA, et al., Nutr Res Rev. 28:42–6619)
Mean, the mean value; SD, standard deviation; SEM, standard error of the mean; IQR, interquartile range; 95%CI, 95% confidence limit; NR, not reported.
Nearly all SCFAs absorbed by the colon are thought to pass through the portal vein from the colon capillaries and reach the liver, though the concentrations of SCFAs in the human portal vein are broad-ranging. Table 3 shows the results of the several studies that have reported on the concentrations of SCFAs in the portal vein4, 34–36).
Table 3. Portal concentrations of individual SCFAs (Acetate, Propionate, Butyrate) by human.
| Subjects | (n); age | Treatment | Reported measure | Acetate | Propionate | Butyrate | Unit | References |
|---|---|---|---|---|---|---|---|---|
| Died suddenly | 6; 16–89 years | Not fasting | Mean | 258 | 88 | 29 | µmol/L | Cummings JH, et al., Gut 1987 28, 1221–7.4) |
| Surgical patients | 5; - | Fasting | Mean | 114 | 32 | 9 | µmol/L | Dankert J, et al., Clin Chim Acta. 1981 110: 301–7.34) |
| Surgical patients | 28; 23–74 years | Fasting | Mean | 128 | 34 | 18 | µmol/L | Peters SG, et al., Gut. 1992 33: 1249–52.35) |
| Surgical patients | 10; - | Fasting 10 g Lactulose was injected into the caecum at surgery (Peak value) | Mean | 241 | 39 | 27 | µmol/L | Peters SG, et al., Gut. 1992 33: 1249–52.35) |
| Surgical patients | 6; - | Fasting 6.7 g Lactulose was injected into the caecum at surgery (Peak value) | Mean | 166 | 31 | 22 | µmol/L | Peters SG, et al., Gut. 1992 33: 1249–52.35) |
| Surgical patients | 7; 54–73 years | Fasting | Mean | 236 | 18 | 26 | µmol/L | van der Beek CM, et al., J Nutr. 2015 145: 2019–24.36) |
| Surgical patients | 7; 54–73 years | Fasting butyrate: 100 mmol/L; 60 mL enema at surgery (Peak value) | Mean | NR | NR | 92 | µmol/L | van der Beek CM, et al., J Nutr. 2015 145: 2019–24.36) |
Mean, the mean value; NR, not reported
The concentrations of SCFAs in healthy adult human peripheral blood are estimated as 100 to 150 µmoL/L for acetate, 4 to 5 µmoL/L for propionate, and 1 to 3 µmoL/L for butyrate, indicating that these concentrations in peripheral blood are vastly lower than in the intestinal tract4). In experiments using rats, a correlation has been found between the cecum content and portal or aortic serum concentrations of total SCFAs after feeding with highly fermentable fiber diets (pectin, guar gum, and fructo-oligosaccharides)37). Oral administration of tributyrin (a prodrug of butyrate) increased plasma butyrate concentrations in the portal vein to 2.4 mmol/L at 1 h and 0.7 mmol/L at 2.5 h38).
Short Chain Fatty Acids and Their Receptors
Several receptors for SCFAs have been found to be G-protein coupled receptors (GPR). Among these, GPR41 and 43 have been renamed as free fatty acid receptor (FFAR) 3 and FFAR2, respectively. GPR41/FFAR3 is distributed throughout the entire body with a high degree of expression in the intestinal tract, immune cells, and fatty tissues39, 40), and is thought to be associated with adiposity and energy homeostasis41).
GPR43/FFAR2 is expressed in intestinal tract epithelial cells and immune system cells, which suggests that it is related to cell chemotaxis and activation8, 39). Interestingly, GPR43/FFAR2 is also expressed within adipocytes in white adipose tissue, and experiments using GPR43/FFAR2 -/- mice have shown that GPR43/FFAR2 signaling with SCFAs may be effective in lipolysis control in adipocytes42).
GPR109A/hydroxycarboxylic acid receptor (HCA) 2, which is known to be a niacin receptor, has been identified as a receptor to butyrate as well as beta-hydroxybutyric acid, a ketone body43, 44). Its expression sites are intestinal tract epithelial cells, immune cells, and adipocytes8). GPR109A/HCA2 participates in homeostasis of regular T cells (Treg) in the colon and fat metabolism in adipose tissues45, 46). In addition, GPR109A/HCA2 signaling accelerates inflammation in hypertrophic adipose tissues46). Recently, olfactory receptor (Olfr) 78, which is a member of the GPR family, has been reported to be a novel SCFA receptor47, 48). Olfr78 is thought to be associated with regulation of hormone secretion and blood pressure47, 48). Table 4 shows the characteristics and physiological functions of SCFA receptors8, 47, 48).
Table 4. The characteristics and physiological functions of SCFA receptors.
| G protein | Ligand | EC50 | Expression | Physiological function | |
|---|---|---|---|---|---|
| GPR41/FFAR3 | Gi/o | C1–C5 | Colonic, colonic LP cells (mast cells), spleen, lymphnodes, bonemarrow, adipocytes, peripheral mononuclear cells, peripheral nervous system, etc. | Increased energy expenditure, leptin expression, decreased food intake, hematopoiesis of DCs from bonemarrow, increased Treg cells, etc. | |
| Propionate(C3), Butyrate(C4) | 12–274 µmol/L for C3 | ||||
| C3>C4>C2 | |||||
| GPR43/FFAR2 | Gi/o, Gq11 | C1–5 | Colonic, colonic LP cells (mast cells, neutrophils, eosinophils, and Tregs), polymorphonuclear cells, adipocytes, skeltal muscle, etc. | Anti-lipolysis, increased insulin sensitivity, preadipocyte differentiation, expansion and differentiation of Tregs, protection against IBD, etc. | |
| Acetate (C2), Propionate (C3) | 259–537 µmol/L | ||||
| C2≒C3>C4 | |||||
| GPR109A/HCA2 | Gi/o, Gβγ | Niacin | Apical membrane of colonic epithelium, macrophages, monocytes, DCs, neutrophils, adipocytes (white and brown), etc. | Anti-lipolysis, triglyceride lowering, protection against colitis, increased of Treg generation, increased IL-10 producing T cells, etc. | |
| β-D-OHB> Butyrate (C4) | 0.7 mmol/L (mouse), 1.6 mmol/L (human) for C4 | ||||
| Olfr78 | NR | Propionate (C3) > acetate (C2) | 920 µmol/L for C3 | Nurons, enteroendocrine cells, colon (epithelial enteroendocrine cells), renal afferent arteriole, juxtaglomerular cells, smooth muscle cells (blood vessels) | Regulation of hormone secretion (GLP-1, PYY), blood pressure regulation (renin-angiotensinaldosterone pathway) |
| 2.35 mmol/L for C2 | |||||
Citationed from koh A, et al., Cell. 165: 1332–458)
Citationed from Fleischer J, et al., Cell Tissue Res. 361: 697–71047), Pluznick J., Gut Microbes. 5: 202–748)
NR, not reported; EC50, half maximal (50%) effective concentration; GPR, G-protein coupled receptor; FFAR, free fatty acid receptor; HCA, hydroxycarboxylic acid receptor; Olfr, olfactory receptor; LP, lamina propria;
Treg, regulatory T cell; DC, dendritic cell; GLP-1, glucagon-like peptide; PYY, peptide YY; IBD, inflammatory bowel disease
Short Chain Fatty Acids and T Cells
SCFAs regulate T cell polarization and induction49). Propionate (at concentrations of 2.0 to 5.0 mmol/L) inhibits the proliferation of lymphocytes stimulated by mitogens50). Propionate (250 to 500 µmol/L) suppresses the Th1-type immune response in stimulated human peripheral blood mononuclear cells51). Butyrate (1.0 mmol/L) inhibits the proliferation of T lymphocytes, and more than 2.0 mmol/L of butyrate induces apoptosis in activated T lymphocytes, but not primary macrophages52). Trompette et al. have reported that there are differences in the intestinal Firmicutes/Bacteroides ratio (F/B ratio) and microbiota composition between high- and low-fiber diets in mice, and that a high-fiber diet increases blood concentrations of SCFAs (approximately 1.0 to 2.0 mmol/L) and attenuates allergic inflammation of the lungs53). The authors suggested that propionate is involved in bone marrow hematopoiesis and in the enhanced generation of macrophage and dendritic cell (DC) precursors and subsequent seeding of the lungs by DCs with high phagocytic capacity, but with an impaired ability to activate Th2 effector cells in the lung53). In addition, they suggested that these effects are induced via GPR41/FFAR3 but not GPR43/FFAR253).
Compounds acting as histone deacetylase (HDAC) inhibitors may be an effective treatment for inflammatory bowel disease and other pro-inflammatory cytokine-related diseases54). As SCFAs are widely known to have HDAC inhibitory activity, they may be involved in the expression of cytokines in T cells and the induction of Treg cells via inhibition of HDAC55). SCFAs (acetate 5–20 mmol/L, propionate 0.5–1.0 mmol/L) promote naïve CD4+ T cell polarization into Th1 and Th17 effector cells producing interleukin (IL)-17, interferon-γ, and/or IL-1056). This effect is independent of GPR41 and GPR43, but directly dependent on the HDAC inhibitor activity and subsequent enhancement of mTOR-S6 kinase activity56). More than 1 mmol/L of butyrate induces Fas-mediated apoptosis of T cells by inhibiting HDAC 1 activity to induce Fas promoter hyperacetylation and Fas upregulation in T cells57).
When butyrate is supplied into the colons of T cell-dependent colitis mouse models, the number of Treg cells increases in the colonic lamina propria, and bowel inflammation is attenuated58). The ingestion of propionate increases Treg cells in intestinal mucosa in germ-free mice via GPR43/FFAR259). By inhibition of HDAC, butyrate and to a lesser extent propionate, but not acetate, promotes transcription of the FoxP3 gene, which is the transcription factor for Treg differentiation, and increases the expression of the FoxP3 gene55). On the other hand, 1 mmol/L of butyrate causes the induction of Th17 cells and also exacerbates inflammation by the production of IL-23 in stimulated dendritic cells (DCs)60).
Thus, SCFAs, especially butyrate and propionate, play a complicated role in Treg differentiation and intestinal tract immune regulation9, 53, 61, 62).
Short Chain Fatty Acids and Neutrophils, Monocytes, and Macrophages
The chemotaxis of neutrophils is activated by inflammatory mediators [tumor necrosis factor (TNF)-α, IL-17, etc.] and chemokines [chemokine (C-X-C motif) (CXCL) 1, 8, etc.]. SCFAs (optimal concentrations for migration are 0.1–3.0 mmol/L) affect the chemotaxis and the viability of neutrophils63). SCFAs (4.0–12 mmol/L propionate, 0.4–3.2 mmol/L butyrate) inhibit TNF-α production by neutrophils in the presence of lipopolysaccharide (LPS)64). The suppression of nuclear factor-kappa B (NF-κB) activity and the inhibition of HDAC are thought to be the underlying mechanisms64, 65). On the other hand, neutrophils increase the production of IL-8, IL-6, and IL-1β at high concentrations (20 mmol/L) of SCFAs while lower concentrations (0.02–2.0 mmol/L) do not induce cytokine secretion. However, lower concentrations of SCFAs enhance TLR2-induced production of IL-8 and TNF-α production66). SCFAs suppress large intestine inflammation in dextran sulfate sodium-induced colitis mice by the induction of apoptosis of neutrophils via GPR43/FFAR267) and via HDAC inhibition68). In addition, SCFAs produce and release reactive oxygen species (ROS) as well as nitric oxide (NO) involving neutrophil bacteria phagocytosis69). Thus, SCFAs have both suppressing and promoting functions in neutrophils.
SCFAs also affect immunoregulation in monocytes and macrophages. In experiments using human monocytes, SCFAs (0.2–20 mmol/L) reduce the production of TNF-α and monocyte chemotactic protein-1 (MCP-1) under LPS stimulation and increase the production of prostaglandin E2 (PGE2)70). The suppression of NF-κB activity and inhibition of HDAC are thought to be the underlying mechanisms, as in the case of neutrophils. Butyrate increases the production of PGE2 by upregulating the expression of phospholipase A2 (PLA2) and cyclooxygenase-2 (COX2) in Kupffer cells (at butyrate concentrations of 0.5–10 mmol/L)71), in human peripheral blood mononuclear cells (1.0–2.0 mmol/L)72), and in a mouse macrophage cell line (0.2–1.0 mmol/L)73) (Fig. 1 (A)). In a human macrophage cell line, butyrate (1.0 mmol/L) increases the production and release of ROS when under LPS stimulation and increases caspase-1 expression and IL-1β production74).
Fig 1.
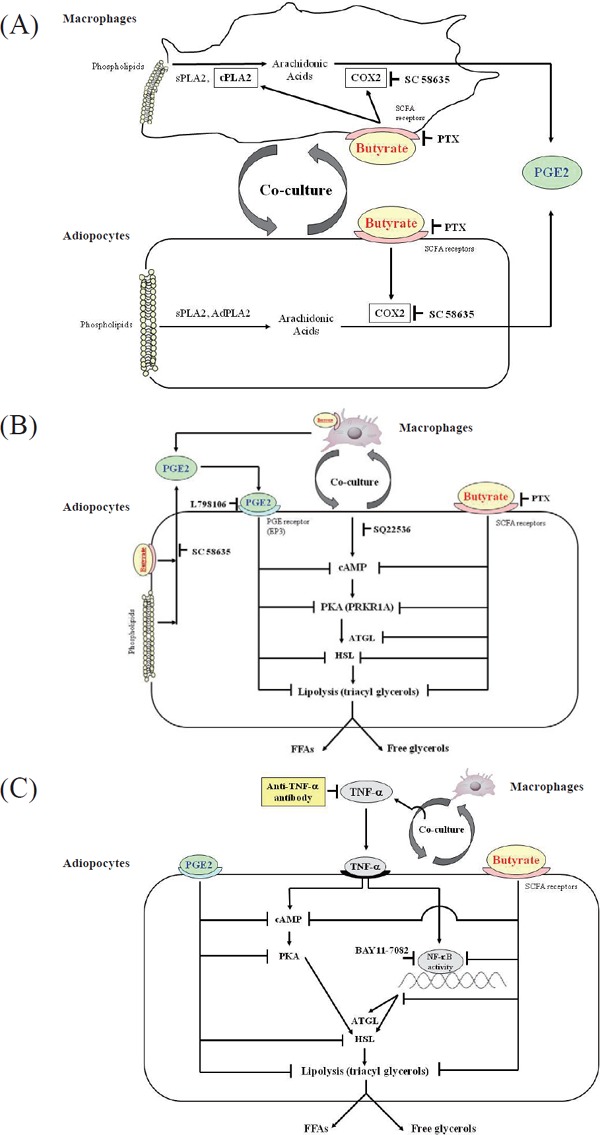
Hypothetical pathways based on the results of the suppressive effect of butyrate depends on the prostaglandin E2 (PGE2)-mediated pathway
Cited from the reference 73
(A) The effect of butyrate on PGE2 production in the interaction between co-cultured macrophages and adipocytes. Co-culture elevates calcium-dependent cytosolic phospholipase A2 (cPLA2) activity in macrophages, secretory PLA2 (sPLA2) activity in adipocytes and macrophages, and the expression of adipose-specific PLA2 (AdPLA2) protein and mRNA in adipocytes. Butyrate elevates cPLA2 activity to a greater degree in macrophages. Co-culture elevates cyclooxygenase-2 (COX2) expression in both cells, and butyrate further enhances COX2 expression in both cells. Butyrate increases PGE2 production more than coculture alone.
(B) The effects of butyrate and PGE2 on lipolysis in co-cultured adipocytes. Co-culture increases cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) levels in adipocytes and increases the release of free fatty acids (FFAs) and free glycerol into the medium (lipolysis). Butyrate suppresses cAMP and PKA levels, and exogenous PGE2 via prostaglandin E receptor 3 (EP3) has a lesser effect than butyrate. These suppressive effects may reduce the activities of lipases, including adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), thus resulting in inhibition of lipolysis.
(C) The effects of butyrate and exogenous PGE2 on cAMP- and nuclear factor-kappa B (NF-κB)–mediated lipolysis in tumor necrosis factor-α (TNF-α)–stimulated 3T3-L1 adipocytes. Co-culture increases TNF-α production. TNF-α increases cAMP, leading to increased lipolysis. Anti–TNF-α antibody, butyrate, or exogenous PGE2 decrease cAMP levels and reduce lipolysis in TNF-α–stimulated 3T3-L1 cells. The GPR109A-mediated pathway may be the predominant pathway regulating the effect of butyrate on lipolysis in TNF-α–stimulated 3T3-L1 cells.
PTX (pertussis toxin), an inhibitor blocking G-protein coupled receptor (GPR) 41- and/or GPR109A-mediated signaling; SC 58635, COX2 selective inhibitor; L798106, EP3 selective antagonist; SQ22536, adenylyl cyclase selective inhibitor; BAY11-7082, NF-αB–selective inhibitor
Short Chain Fatty Acids and Atherosclerosis
Hazen et al. have revealed that gut microbial-derived metabolites trimethylamine (TMA) and trimethylamine N-oxide (TMAO) are proatherogenic in mice and humans6, 11, 75). They have also shown that plasma TMAO concentrations can predict an enhanced risk of major adverse cardiac events in two independent cohorts76).
Recently, Aguilar et al. demonstrated that a butyrate-supplemented chow diet suppresses atherosclerotic lesions in apoE knockout mice77, 78). They also observed that butyrate reduces chemotaxis protein-1 (CCL2/MCP-1), vascular cell adhesion molecule-1, and matrix metalloproteinase-2 production in the lesion site, resulting in a lower migration of macrophages and increased collagen deposition and plaque stability77), and that peritoneal macrophages from butyrate-treated mice present lower ROS and NO release78).
On the other hand, Kasahara et al. demonstrated that the lack of microbiota in apoE-deficient mice causes a significant reduction in atherosclerotic lesion formation in spite of a significant increase in plasma and hepatic cholesterol concentrations, and suggested that this might be associated with the attenuation of LPS-mediated inflammatory responses79). They also found that gut microbiota can regulate cholesterol homeostasis via bile acid metabolism under hypercholesterolemia79). Ryan demonstrated shifts in the composition of the gut microbiome in apoE-deficient mice fed high fat/cholesterol in conjunction with plant sterol esters or oat β-glucan, and increased concentrations of cecum acetate or butyrate, and found that these feedings attenuated the microbial production of TMA and reduced serum cholesterol concentrations80).
In accordance with experimental data, recent clinical work has shown that coronary artery disease is linked with an alternation of gut microbiota, and that gut microbiota may be a diagnostic marker of morbidity from coronary artery disease81, 82). Emoto et al. reported that the incidence of coronary artery disease is related to a decreased prevalence of the phylum Bacteroidetes and increased F/B ratio in the intestinal tract81).
Therefore, it is possible that the use of SCFAs, prebiotics, or probiotics (live microbiota) to improve the gut microbiota environment can allow SCFAs to prevent metabolic disorders and prevent ASCVD.
Short Chain Fatty Acids and Visceral Adipose Tissues
Adipose tissues not only store energy through the accumulation of triglycerides, but secrete various adipokines that affect metabolism throughout the body. In hypertrophic adipose tissues, the invasion of macrophages activates the secretion of free fatty acids (FFAs), TNF-α, IL-6, MCP-1, plasminogen activator inhibitor-1, and other pro-inflammatory cytokines and chemokines, which leads to metabolic abnormalities, including insulin resistance, resulting in the promotion of atherogenesis83, 84). These interactions between adipocyte-derived FFAs and macrophagederived adipokines represent a vicious cycle85).
Using a co-culture system with adipocytes and macrophages, we found increased production of TNF-α, IL-6, MCP-1, and the release of free glycerol and FFAs into the medium. Butyrate (0.1–1.0 mmol/L) significantly reduces these effects86). Butyrate inhibits the phosphorylation of mitogen-activated protein kinases and NF-κB activity in co-cultured macrophages and suppresses lipolysis caused by the suppression of adipose triglyceride lipase expression and hormone-sensitive lipase phosphorylation in adipocytes86). In these co-culture conditions, butyrate increases the production of PGE2, and approximately 40% of the suppressive effect of butyrate on lipolysis depends on the PGE2-mediated pathway73) (Fig. 1 (A–C)).
Intraperitoneal administration of butyrate (1.0 g/kg) in db/db mice suppresses obesity-induced inflammation and the expression of IL-1, IL-6, and TNF-α mRNA in epididymal, subcutaneous adipose tissues by inhibiting the NOD-like receptor 3 inflammation signaling pathway87). In experiments using explants of human omental and subcutaneous adipose tissues, propionate (3 mmol/L) suppresses the production of resistin, which is an adipocyte-derived adipokine and pro-inflammatory cytokine88).
In hypertrophic visceral adipose tissues, a decrease of the CD4 (+) Treg and an increase of the CD153+PD-1+CD44hiCD4+ T cell population ratio have been observed. This change in T cell populations may induce macrophages into adipose tissues as well as decreases in the M2 macrophage population ratio, and an increase in the M1 macrophage population ratio89–91). However, it has not been clarified whether SCFAs are directly associated with changes in the M1/M2 macrophage ratio or in T cell differentiation and population in adipose tissues. This issue therefore requires further study. Finally, mast cells may be involved in the inflammation of visceral adipose tissues prior to macrophage invasion92). Nevertheless, the effect of SCFAs on mast cells remains unknown.
Short Chain Fatty Acids and Metabolic Abnormality
Several reports have shown that obesity is associated with changes in the relative abundance of the two dominant bacterial phyla, Firmicutes and Bacteroides. Increased F/B ratios are observed in the guts of obese adults81, 93). Recent cohort studies in Chinese and European women have shown that regardless of the difference in race and eating habits, type 2 diabetes patients are characterized by a moderate degree of gut microbial decrease in the abundance of some universal butyrate-producing bacteria94, 95).
Dietary fibers promote metabolic benefits on body weight and glucose control, and several studies have demonstrated the impact of an SCFA-enriched diet, establishing a direct causal relationship between fiber fermentation and improved metabolism in humans8, 96, 97). Lin et al. have shown that butyrate, propionate, and acetate protect against diet-induced obesity and insulin resistance and that butyrate and propionate, but not acetate, induce gut hormones and reduce food intake98). De Vadder et al. have demonstrated that propionate and butyrate activate intestinal gluconeogenesis via complementary mechanisms99). Increased production of acetate by an altered gut microbiota in rodents leads to activation of the parasympathetic nervous system, which promotes increased glucose-stimulated insulin secretion, increased ghrelin secretion, hyperphagia, obesity, and related sequelae100). Oral supplementation of SCFAs may thus improve impaired glucose metabolism in humans101).
Dietary acetic acid reduces serum cholesterol and triglycerides (TG) in rats fed a cholesterol-rich diet102). Propionate inhibits fatty acid synthesis and to a lesser extent cholesterol synthesis, while butyrate is a potent activator of both synthetic pathways in rat isolated liver cells103). A high propionate diet reduces hepatic gene and protein expression of lipogenic enzymes leading to reduced hepatic TG concentrations in high-fat fed mice104). Although it has been reported that a high-cholesterol diet does not alter gut microbiota composition in mice105), further examination is needed to reveal the association between SCFAs and lipid metabolism. Interestingly, Tarini et al. demonstrated in healthy adult humans that supplementation with the fermentable dietary fiber inulin significantly increased postprandial serum acetate, propionate, and butyrate concentrations at 4–6 h, and significantly decreased postprandial serum FFAs concentrations at 4 h. They also showed that inulin significantly increased plasma glucagon-like peptide-1 concentrations at 30 min, and reduced ghrelin at 4.5 h and 6 h106). Thus, compositional changes of microbiota might influence adiposity and glucose and lipid metabolism by regulating food intake.
Conclusion and Perspectives
SCFAs may suppress inflammation by reducing migration and proliferation of immune cells, reducing many types of cytokines, and inducing apoptosis. Thus, SCFAs are thought to have anti-inflammatory effects. However, marked changes of SCFAs concentrations in blood or various tissues are thought to cause disorders related to immunological and metabolic imbalances. Thus, gut bacteria exert both beneficial and harmful effects107). Therefore, it may be important to estimate the appropriate concentrations of SCFAs to maintain a normal metabolism and immune system for the prevention and treatment of diseases using diet and SCFAs. Recently, Bergeron et al. demonstrated that a lower carbohydrate diet (39–40% energy) high in resistant starches was associated with higher plasma TMAO levels in spite of reduced postprandial insulin and glucose responses, while there was no difference in TMAO affected by resistant starches when carbohydrate intake was high (51–53% energy)108). It may be necessary to develop food patterns or medications to reduce plasma TMAO concentrations and maintain appropriate concentrations of SCFAs.
Although there have been only a small number of studies thus far, in the future SCFAs may provide new insights into the pathophysiology of inflammatory diseases, and we can expect the development of novel therapeutic strategies against chronic inflammation, metabolic disorders, and atherosclerosis.
Conflict of Interest
All authors declare that they have no conflict of interest.
Sources of Funding
Some studies in this review were supported by a Grant-in-Aid for Scientific Research (C), JSPS KAK-ENHI grant number 26350166.
Author Contributions
All authors contributed equally to the preparation of the manuscript and approved the final manuscript.
References
- 1). Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta-HIT Consortium, Bork P, Ehrlich SD, Wang J: A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 2010; 464: 59-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Lepage P, Leclerc MC, Joossens M, Mondot S, Blottière HM, Raes J, Ehrlich D, Doré J: A metagenomic insight into our gut's microbiome. Gut, 2013; 62: 146-158 [DOI] [PubMed] [Google Scholar]
- 3). Sonnenburg JL, Bäckhed F: Diet-microbiota interactions as moderators of human metabolism. Nature, 2016; 535: 56-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT: Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut, 1987; 28: 1221-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Macfarlane GT, Macfarlane S: Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int, 2012; 95: 50-60 [DOI] [PubMed] [Google Scholar]
- 6). Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011; 472: 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J: Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun, 2012; 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F: From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell, 2016; 165: 1332-1345 [DOI] [PubMed] [Google Scholar]
- 9). Yamashita T: Intestinal immunity and gut microbeta in Atherosclerosis. J Atheroscler Thromb, 2017; 24: 110-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Kasabuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I: Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients, 2015; 7: 2839-2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tang WH, Hazen SL: The contributory role of gut microbeta in cardiovascular disease. J Clin Invest, 2014; 124: 4204-4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Louis P, Hold GL, Flint HJ: The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol, 2014; 12: 661-672 [DOI] [PubMed] [Google Scholar]
- 13). Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI: Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem, 2010; 285: 22082-22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Scott KP, Martin JC, Campbell G, Mayer CD, Flint HJ: Whole-genome transcription profiling reveals genes upregulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J Bacteriol, 2006; 188: 4340-4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ: Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol, 2002; 68: 5186-5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S: Host-gut microbiota metabolic interactions. Science, 2012; 336: 1262-1267 [DOI] [PubMed] [Google Scholar]
- 17). Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ: Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol, 2006; 40: 235-243 [DOI] [PubMed] [Google Scholar]
- 18). Bergman EN: Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev, 1990; 70: 567-590 [DOI] [PubMed] [Google Scholar]
- 19). Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, Nauta A, Raes J, van Tol EA, Tuohy KM: Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev, 2015; 28: 42-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Whelan K, Judd PA, Preedy VR, Simmering R, Jann A, Taylor MA: Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr, 2005; 135: 1896-1902 [DOI] [PubMed] [Google Scholar]
- 21). Boler BM, Serao MC, Bauer LL, Staeger MA, Boileau TW, Swanson KS, Fahey GC, Jr: Digestive physiological outcomes related to polydextrose and soluble maize fibre consumption by healthy adult men. Br J Nutr, 2011; 106: 1864-1871 [DOI] [PubMed] [Google Scholar]
- 22). Lewis SJ, Heaton KW: Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut, 1997; 41: 245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Lecerf JM, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, Jacobs H, Lambrey G, Abdelnour AM, Pouillart PR: Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr, 2012; 108: 1847-1858 [DOI] [PubMed] [Google Scholar]
- 24). Reimer RA, Pelletier X, Carabin IG, Lyon MR, Gahler RJ, Wood S: Faecal short chain fatty acids in healthy subjects participating in a randomised controlled trial examining a soluble highly viscous polysaccharide versus control. J Hum Nutr Diet, 2012; 25: 373-377 [DOI] [PubMed] [Google Scholar]
- 25). Fernando WM, Hill JE, Zello GA, Tyler RT, Dahl WJ, Van Kessel AG: Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef Microbes, 2010; 1: 197-207 [DOI] [PubMed] [Google Scholar]
- 26). McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DL, Conlon MA: Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr, 2011; 141: 883-889 [DOI] [PubMed] [Google Scholar]
- 27). Nemoto H, Kataoka K, Ishikawa H, Ikata K, Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T, Yasutomo K: Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci, 2012; 57: 2955-2964 [DOI] [PubMed] [Google Scholar]
- 28). Tiihonen K, Ouwehand AC, Rautonen N: Effect of overweight on gastrointestinal microbiology and immunology: correlation with blood biomarkers. Br J Nutr, 2010; 103: 1070-1078 [DOI] [PubMed] [Google Scholar]
- 29). McOrist AL, Abell GC, Cooke C, Nyland K: Bacterial population dynamics and faecal short-chain fatty acid (SCFA) concentrations in healthy humans. Br J Nutr, 2008; 100: 138-146 [DOI] [PubMed] [Google Scholar]
- 30). Hylla S, Gostner A, Dusel G, Anger H, Bartram HP, Christl SU, Kasper H, Scheppach W: Effects of resistant starch on the colon in healthy volunteers: possible implications for cancerprevention. Am J Clin Nutr, 1998; 67: 136-142 [DOI] [PubMed] [Google Scholar]
- 31). Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD: Microbiota and SCFA in lean and overweight healthy subjects. Obesity, 2010; 18: 190-195 [DOI] [PubMed] [Google Scholar]
- 32). Brinkworth GD, Noakes M, Clifton PM, Bird AR: Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr, 2009; 101: 1493-1502 [DOI] [PubMed] [Google Scholar]
- 33). Benassi-Evans B, Clifton P, Noakes M, Fenech M: Highprotein/high red meat and high-carbohydrate weight-loss diets do not differ in their effect on faecal water genotoxicity tested by use of the WIL2-NS cell line and with other biomarkers of bowel health. Mutat Res, 2010; 703: 130-136 [DOI] [PubMed] [Google Scholar]
- 34). Dankert J, Zijlstra JB, Wolthers BG: Volatile fatty acids in human peripheral and portal blood: quantitative determination vacuum distillation and gas chromatography. Clin Chim Acta, 1981; 110: 301-307 [DOI] [PubMed] [Google Scholar]
- 35). Peters SG, Pomare EW, Fisher CA: Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut, 1992; 33: 1249-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, Dejong CH: Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J Nutr, 2015; 145: 2019-2024 [DOI] [PubMed] [Google Scholar]
- 37). Jakobsdottir G, Jädert C, Holm L, Nyman ME: Propionic and butyric acids, formed in the caecum of rats fed highly fermentable dietary fibre, are reflected in portal and aortic serum. Br J Nutr, 2013; 110: 1565-1572 [DOI] [PubMed] [Google Scholar]
- 38). Miyoshi M, Sakaki H, Usami M, Iizuka N, Shuno K, Aoyama M, Usami Y: Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin Nutr, 2011; 30: 252-258 [DOI] [PubMed] [Google Scholar]
- 39). Stoddart LA, Smith NJ, Milligan G: International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol Rev, 2008; 60: 405-417 [DOI] [PubMed] [Google Scholar]
- 40). Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M: Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem, 2003; 278: 25481-25489 [DOI] [PubMed] [Google Scholar]
- 41). Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI: Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A, 2008; 105: 16767-16772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y: Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology, 2008; 149: 4519-4526 [DOI] [PubMed] [Google Scholar]
- 43). Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S: PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med, 2003; 9: 352-355 [DOI] [PubMed] [Google Scholar]
- 44). Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG: (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem, 2005; 280: 26649-26652 [DOI] [PubMed] [Google Scholar]
- 45). Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V: Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity, 2014; 40: 128-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Feingold KR, Moser A, Shigenaga JK, Grunfeld C: Inflammation stimulates niacin receptor (GPR109A/HCA2) expression in adipose tissue and macrophages. J Lipid Res, 2014; 55: 2501-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Fleischer J, Bumbalo R, Bautze V, Strotmann J, Breer H, Expression of: odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res, 2015; 361: 697-710 [DOI] [PubMed] [Google Scholar]
- 48). Pluznick J: A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes, 2014; 5: 202-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Belkaid Y, Hand TW: Role of the microbiota in immunity and inflammation. Cell, 2014; 157: 121-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Curi R, Bond JA, Calder PC, Newsholme EA: Propionate regulates lymphocyte proliferation and metabolism. Gen Pharmacol, 1993; 24: 591-597 [DOI] [PubMed] [Google Scholar]
- 51). Maier E, Kurz K, Jenny M, Schennach H, Ueberall F, Fuchs D: Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food Chem Toxicol, 2010; 48: 1950-1956 [DOI] [PubMed] [Google Scholar]
- 52). Bailón E, Cueto-Sola M, Utrilla P, Rodríguez-Cabezas ME, Garrido-Mesa N, Zarzuelo A, Xaus J, Gálvez J, Comalada M: Butyrate in vitro immune-modulatory effects might be mediated through a proliferation-related induction of apoptosis. Immunobiology, 2010; 215: 863-873 [DOI] [PubMed] [Google Scholar]
- 53). Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ: Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med, 2014; 20: 159-166 [DOI] [PubMed] [Google Scholar]
- 54). Felice C, Lewis A, Armuzzi A, Lindsay JO, Silver A: Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment Pharmacol Ther, 2015; 41: 26-38 [DOI] [PubMed] [Google Scholar]
- 55). Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY: Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature, 2013; 504: 451-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH: Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol, 2015; 8: 80-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K: Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fasmediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol, 2012; 302: G1405-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H: Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 2013; 504: 446-450 [DOI] [PubMed] [Google Scholar]
- 59). Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS: The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 2013; 341: 569-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, Veniaminova NA, Merchant JL, Chen CC, Huffnagle GB, Kao JY: Butyrate increases IL-23 production by stimulated dendritic cells. Am J Physiol Gastrointest Liver Physiol, 2012; 303: G1384-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K: Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation, 2009; 120: 1996-2005 [DOI] [PubMed] [Google Scholar]
- 62). Li MO, Rudensky AY: T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol, 2016; 16: 220-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R: SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One, 2011; 6: e21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R: Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem, 2011; 22: 849-855 [DOI] [PubMed] [Google Scholar]
- 65). Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA: Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology, 2016; 5: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT: Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol, 2012; 67: 391-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature, 2009; 461: 1282-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Aoyama M, Kotani J, Usami M: Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition, 2010; 26: 653-661 [DOI] [PubMed] [Google Scholar]
- 69). Rodrigues HG, Takeo Sato F, Curi R, Vinolo MA: Fatty acids as modulators of neutrophil recruitment, function and survival. Eur J Pharmacol, 2016; 785: 50-58 [DOI] [PubMed] [Google Scholar]
- 70). Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH: Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol, 2009; 15: 5549-5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Perez R, Stevenson F, Johnson J, Morgan M, Erickson K, Hubbard NE, Morand L, Rudich S, Katznelson S, German JB: Sodium butyrate upregulates Kupffer cell PGE2 production and modulates immune function. J Surg Res, 1998; 78: 1-6 [DOI] [PubMed] [Google Scholar]
- 72). Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J: Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res, 2008; 28: 321-328 [DOI] [PubMed] [Google Scholar]
- 73). Ohira H, Tsutsui W, Mamoto R, Yamaguchi S, Nishida M, Ito M, Fujioka Y: Butyrate attenuates lipolysis in adipocytes co-cultured with macrophages through non-prostaglandin E2-mediated and prostaglandin E2-mediated pathways. Lipids Health Dis, 2016; 15: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Ohira H, Fujioka Y, Katagiri C, Yano M, Mamoto R, Aoyama M, Usami M, Ikeda M: Butyrate enhancement of inteleukin-1β production via activation of oxidative stress pathways in lipopolysaccharide-stimulated THP-1 cells. J Clin Biochem Nutr, 2012; 50: 59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med, 2013; 368: 1575-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Lüscher TF: Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J, 2017; 38: 814-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Aguilar EC, Leonel AJ, Teixeira LG, Silva AR, Silva JF, Pelaez JM, Capettini LS, Lemos VS, Santos RA, Alvarez-Leite JI: Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr Metab Cardiovasc Dis, 2014; 24: 606-613 [DOI] [PubMed] [Google Scholar]
- 78). Aguilar EC, Santos LC, Leonel AJ, de Oliveira JS, Santos EA, Navia-Pelaez JM, da Silva JF, Mendes BP, Capettini LS, Teixeira LG, Lemos VS, Alvarez-Leite JI: Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase downregulation in endothelial cells. J Nutr Biochem, 2016; 34: 99-105 [DOI] [PubMed] [Google Scholar]
- 79). Kasahara K, Tanoue T, Yamashita T, Yodoi K, Matsumoto T, Emoto T, Mizoguchi T, Hayashi T, Kitano N, Sasaki N, Atarashi K, Honda K, Hirata KI: Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflamemation in Atherosclerosis. J Lipid Res, 2017; 58: 519-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Ryan PM, London LE, Bjorndahl TC, Mandal R, Murphy K, Fitzgerald GF, Shanahan F, Ross RP, Wishart DS, Caplice NM, Stanton C: Microbiome and metabolome modifying effects of several cardiovascular disease interventions in apo-E-/- mice. Microbiome, 2017; 5: 30. 10.1186/s40168-017-0246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K: Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb, 2016; 23: 908-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). Emoto T, Yamashita T, Kobayashi T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata KI: Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels, 2017; 32: 39-46 [DOI] [PubMed] [Google Scholar]
- 83). Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest, 2007; 117: 175-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84). Wellen KE, Hotamisligil GS: Obesity-induced inflammatory changes in adipose tissue. J Clin Invest, 2003; 112: 1785-1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Suganami T, Nishida J, Ogawa Y: A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol, 2005; 25: 2062-2068 [DOI] [PubMed] [Google Scholar]
- 86). Ohira H, Fujioka Y, Katagiri C, Mamoto R, Aoyama-Ishikawa M, Amako K, Izumi Y, Nishiumi S, Yoshida M, Usami M, Ikeda M: Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb, 2013; 20: 425-442 [DOI] [PubMed] [Google Scholar]
- 87). Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B: Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci Rep, 2015; 5: 12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88). Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, Rezaee F, Venema K, Vonk RJ: Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Invest, 2010; 40: 401-407 [DOI] [PubMed] [Google Scholar]
- 89). Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T: Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia, 2012; 55: 2583-2592 [DOI] [PubMed] [Google Scholar]
- 90). Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M: Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest, 2016; 126: 4626-4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91). Cipolletta D: Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology, 2014; 142: 517-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP: Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med, 2009; 15: 940-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93). Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: human gut microbes associated with obesity. Nature, 2006; 444: 1022-1023 [DOI] [PubMed] [Google Scholar]
- 94). Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J: A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 2012; 490: 55-60 [DOI] [PubMed] [Google Scholar]
- 95). Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F: Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature, 2013; 498: 99-103 [DOI] [PubMed] [Google Scholar]
- 96). Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G: Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut, 2015; 64: 1744-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97). Freeland KR, Wolever TM: Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr, 2010; 103: 460-466 [DOI] [PubMed] [Google Scholar]
- 98). Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ: Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One, 2012; 7: e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99). De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G: Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell, 2014; 156: 84-96 [DOI] [PubMed] [Google Scholar]
- 100). Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI: Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature, 2016; 534: 213-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101). Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, Morrison DJ, Preston T, Wallis GA, Tedford C, Castañera González R, Huang GC, Choudhary P, Frost G, Persaud SJ: The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab, 2017; 19: 257-265 [DOI] [PubMed] [Google Scholar]
- 102). Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T: Dietary acetic acid reduce serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr, 2006; 95: 916-92416611381 [Google Scholar]
- 103). Demigné C, Morand C, Levrat MA, Besson C, Moundras C, Rémésy C: Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr, 1995; 74: 209-219 [DOI] [PubMed] [Google Scholar]
- 104). Weitkunat K, Schumann S, Nickel D, Kappo KA, Petzke KJ, Kipp AP, Blaut M, Klaus S: Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat dietinduced obesity. Mol Nutr Food Res, 2016; 60: 2611-2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105). Dimova LG, Zlatkov N, Verkade HJ, Uhlin BE, Tietge UJ: High-cholesterol diet does not alter gut microbiota composition in mice. Nutr Metab (Lond), 2017; 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106). Tarini J, Wolever TM: The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reducesfree-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab, 2010; 35: 9-16 [DOI] [PubMed] [Google Scholar]
- 107). Woting A, Blaut M: The Intestinal Microbiota in Metabolic Disease. Nutrients, 2016; 8: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108). Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, Wang Z, Knight R, Jansson JK, Hazen SL, Krauss RM: Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr, 2017; 116: 2020-2029 [DOI] [PMC free article] [PubMed] [Google Scholar]


