Abstract
Aim: The prognostic value of homocysteine (HCY) in patients with coronary artery diseases (CAD) is still controversial. The objective of this study was to investigate whether elevated HCY level at admission predict long-term outcomes in patients after percutaneous coronary interventions (PCI) with coronary artery stenting.
Methods: From the institutional registry of Cardiovascular Atherosclerosis and Percutaneous TrAnsluminal INterventions (CAPTAIN), we enrolled a total of 1,307 patients with documented CAD undergone PCI with bare metal stents from July 2003 to December 2014. They were divided into two groups according to the fasting plasma HCY levels before catheterization: group I (883 patients, < 12 µmol/L) and group II (424 patients, ≥ 12 µmol/L). The primary endpoint was occurrence of major adverse cardiac events (MACE), including cardiac death, nonfatal myocardial infarction, stroke, target lesion revascularization, new lesion stenting, and requiring bypass surgery.
Results: After a mean follow-up period of 58 ± 41 months, the group II patients had a higher MACE rate (33.3% vs. 25.6%, p = 0.005). The main differences between two groups were cardiac death (8.0% vs. 3.4%, p = 0.001) and new lesion stenting (13.6% vs. 9.5%, p = 0.034). The risks of long-term MACE remained significantly higher in patients with elevated HCY level (≥ 12 µmol/L) after adjusting for clinical variables, with a hazard ratio of 1.29 (95% CI, 1.02–1.64, p = 0.036).
Conclusions: Elevated HCY level (≥ 12 µmol/L) was independently associated with increased risk of long-term cardiovascular events in patients after coronary artery bare metal stents implantations. Thus, hyperhomocysteinemia may remain a useful prognostic marker for the risk assessment in clinical care of CAD patients.
Keywords: Homocysteine, Coronary artery stent, Clinical outcome
Introduction
Coronary artery disease (CAD) is increasingly prevalent worldwide and is a major cause of cardiovascular mortality. Even if percutaneous coronary intervention (PCI) and effective medical therapies have significantly improved clinical outcomes, certain high risk patients still experienced late cardiovascular events1, 2). Progression of atherosclerosis and late stent failure were the major causes of late cardiovascular events in these CAD patients. A serum marker with prognostic value for late events would help physicians to identify these high-risk patients. Several epidemiologic studies have suggested that elevated homocysteine (HCY) levels are associated with increased risks of atherothrombotic diseases such as CAD, stroke, and peripheral artery disease3–8). Furthermore, Morita H et al.9) and Schnyder G et al.10) reported HCY levels were associated with the late lumen loss after peripheral artery or coronary artery stenting, respectively. However, the prognostic value of HCY levels was not well studied and the conclusions of past studies were inconsistent11, 12). The aim of this study is to clarify the predictive value of plasma HCY levels at index admission for long-term outcomes in patients with coronary artery stenting.
Materials and Methods
Patient Population and Laboratory Analysis
The Cardiovascular Atherosclerosis and Percutaneous TrAnsluminal INterventions (CAPTAIN) registry, a prospective, physician-initiated, single-center database, which includes 6,300 patients undergoing elective or emergent PCI with stenting at Chang Gung Memorial Hospital. We enrolled consecutive patients in the CAPTAIN registry from July 2003 to December 2014, who had acute myocardial infarction or stable CAD with > 70% stenosis in coronary arteries underwent stent implantations. To minimize the effects of stent property differences between drug eluting stents and bare metal stents (BMS) on long-term clinical outcomes, only patients with BMS implantations were included into analysis. We excluded patients with severe multivessel disease requiring bypass surgery, intolerable for dual antiplatelet therapy, or not follow the study protocol. Patients with renal insufficiency (serum creatinine level > 1.5 mg/dL) or concurrent active infections, inflammatory disorders, or cancer were also excluded to minimize the influence of comorbidities on HCY levels. Stent implantation was performed through the femoral or radial artery according to standard techniques. The type of stent was based on the indication and available stent size. After initial stent deployment, high-pressure balloon inflation (≥ 14 atm) was applied in most patients. The creatine kinase myocardial-band (CKMB) isoenzyme and troponin I were measured in all of the patients immediately and 6 h after the procedure to detect periprocedure myocardial infarction (MI). All of the patients received standard ischemia heart disease therapies after procedure, including dual antiplatelet therapy with aspirin and clopidogrel or ticagrelor, beta-blockers, statins, angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Blood samples for plasma total HCY were collected after overnight fasting before index coronary angiography and intervention. The plasma total HCY level was measured by high-performance liquid chromatography with fluorescence detection (Hitachi Labospect 008, AS, Japan). We use a cutoff value of 12 µmol/L for hyperhomocysteinemia10). The study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation. All participants provided informed consents to undergo the procedure and follow-up protocol.
Angiographic Evaluation
The minimal luminal diameter (MLD), reference vessel diameter (RVD), percent of diameter stenosis (DS%), and balloon diameter were measured from multiple projections using the automatic edge-detection method. Acute gain was defined as the difference in MLD between before and after stenting. The quantitative coronary angiographic analysis was performed by two experienced interventional cardiologists, and images were selected with a end-diastolic cine-frame of the most severe and nonforeshortened projection. A contrast-filled guiding catheter was used as a reference for calibration. The interobserver correlation coefficient (r) was 0.93 (p < 0.01), and the intraobserver correlation coefficient was 0.95 (p < 0.01).
Definitions of the Endpoints
The study endpoint was the occurrence of major adverse cardiac events (MACE), a composite of cardiac death, nonfatal MI, stroke, target lesion revascularization, new lesion stenting, and necessitation of coronary bypass surgery. Cardiac death was defined as any death due to immediate cardiac cause, such as MI, low-output pumping failure, or fatal arrhythmia. The MI was diagnosed if the patient experienced prolonged chest pain, ischemic ST-T changes shown on electrocardiography, and elevated CK-MB isoenzyme or troponin I levels. Clinical follow-up visits at the outpatient clinics were scheduled at 1, 2, 3, 6, 9, and 12 months, and every 3 months thereafter. Information regarding clinical events was obtained from hospital medical records, the referring physician, and phone interviews with the patients or their relatives through December 30, 2015.
Statistical Analysis
Data were prospectively collected and analyzed using SPSS statistical software package (version 22.0, IBM) for all statistical analyses. Continuous variables were represented as means ± standard deviation (SD) and compared between groups using a two-tailed t test. Categorical variables were presented as numbers (percentages) and compared with a Fisher's exact test. Cox proportional hazard regression models were used to determine the relationship between plasma HCY levels and MACE rates. They were presented as hazard ratios (HRs) with 95% confidence intervals (CIs) and adjusted for confounding factors including variants in clinical and angiographic characteristics. MACE-free survival curve was depicted by the Kaplan–Meier method and differences between the two groups were assessed by the log-rank test. A p value less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 1,340 patients were in accordance with eligibility criteria for study participants in the CAPTAIN registry. There were 33 patients excluded from relevant analyses for missing data. Finally, 1,307 patients (mean age 62 ± 12 years, range 24 – 93 years; 81.5% male) with 1,604 lesions and 1,800 BMS implantations were consecutively enrolled for analyses (Fig. 1). The patients were divided into two groups according to baseline plasma total HCY level with a cutoff value of 12 µmol/L: group I (883 patients, HCY < 12 µmol/L), group II (424 patients, ≥ 12 µmol/L). Comparisons of the baseline characteristics are shown in Table 1. The patients in group II were significantly older (65.2 ± 12 vs. 61.1 ± 12 years, p < 0.001), and there were more patients with diabetes (36.1% vs. 27.9%, p = 0.003), hypertension (63.7% vs. 56.3%, p = 0.033), history of stroke (7.8% vs. 4.8%, p = 0.031), and left ventricular systolic dysfunction (ejection fraction < 40%) at the initial event (17.9% vs. 9.3%, p < 0.001). The mean plasma total HCY levels in group I and II were 8.7 µmol/L and 16.4 µmol/L, respectively (Table 1). The mean serum creatinine level in group II was higher than that in group I (1.12 ± 0.24 vs. 1.01 ± 0.24 mg/dL, p < 0.001) and the mean estimated glomerular filtration rates (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) equation, in group I and II were 74.9 ± 26.8 and 65.8 ± 20.1 mL/min/1.73 m2, respectively (p < 0.001).
Fig. 1.
Flow diagram of patient enrollment and analysis
HCY: plasma homocysteine.
Table 1. Baseline clinical characteristics (n = 1,307).
| Homocysteine level | < 12.0 µmol/L | ≥ 12.0 µmol/L | P value |
|---|---|---|---|
| (n = 883) | (n = 424) | ||
| Age ± SD (years) | 61.1 ± 12 | 65.2 ± 12 | < 0.001 |
| Woman, n (%) | 169 (19.1) | 73 (17.2) | 0.447 |
| Diabetes mellitus, n (%) | 246 (27.9) | 153 (36.1) | 0.003 |
| Hypertension, n (%) | 497 (56.3) | 270 (63.7) | 0.033 |
| Currently smoking, n (%) | 397 (45.0) | 170 (40.1) | 0.107 |
| Hyperlipidemia, n (%) | 462 (51.6) | 203 (46.1) | 0.063 |
| Hx of PCI, n (%) | 99 (11.2) | 60 (14.2) | 0.148 |
| Hx of stroke, n (%) | 42 (4.8) | 33 (7.8) | 0.031 |
| Hx of MI, n (%) | 468 (53.0) | 231 (54.5) | 0.636 |
| Homocysteine, mean ± SD, µmol/l | 8.7 ± 2.0 | 16.4 ± 8.5 | < 0.001 |
| Serum creatinine, mean ± SD, mg/dL | 1.01 ± 0.23 | 1.12 ± 0.24 | < 0.001 |
| eGFR, mean ± SD, ml/min/1.73 m2 | 74.9 ± 26.8 | 65.8 ± 20.1 | < 0.001 |
| Stable angina, n (%) | 486 (55.0) | 242 (57.1) | 0.488 |
| ACS, n (%) | 397 (45.0) | 182 (42.9) | 0.521 |
| LVEF < 40%, n (%) | 76 (9.3) | 70 (17.9) | < 0.001 |
| Cardiogenic shock, n (%) | 46 (5.2) | 27 (6.4) | 0.440 |
| Multivessel disease, n (%) | 578 (65.5) | 295 (69.6) | 0.149 |
ACS: acute coronary syndrome; eGFR: estimated glomerular filtration rate, calculated by the Modification of Diet in Renal Disease (MDRD) equation. Hx: history of occurrence 6 months or longer before the study; LVEF: left ventricular ejection fraction, measured by angiography; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation.
Angiographic Characteristics
There were total 1,082 lesions in group I and 522 lesions in group II, of which 1,253 (78.1%) were complex lesions (type B2 and C), 107 (6.7%) were bifurcation lesions, and 117 (7.3%) were ostial lesions. The mean lesion length was 20 ± 10 mm. There was no significant difference in lesion location, length, or complexity between the two groups. However, patients in groups II had more calcified lesions (17.0% vs. 11.6%, p = 0.004) and fewer thrombus-containing lesions (11.1% vs. 15.1%, p = 0.037). For PCI-related characteristics, there was no significant difference in the pre- or post-procedure DS%, MLD, or RVD of the target lesions. Also, there was no significant difference in the sizes and lengths of the implanted coronary artery stents between the two groups (Table 2). A total of 1,800 stents were implanted including 40 Bx Velocity (Johnson and Johnson, Miami Lakes, Florida, USA), 427 Driver (Medtronic, Santa Rosa, CA, USA), 122 Express (Boston Scientific, Natick, Massachusetts, USA), 45 Integrity (Medtronic), 88 Liberte (Boston Scientific), 14 Multilink (Guidant, Santa Clara, CA, USA), 1 Multi-Link TETRA (Guidant), 312 Multi-Link PENTA (Guidant), 76 Multi-Link PIXEL (Guidant), 469 Multi-Link VISION (Abbott Vascular, Santa Clara, CA, USA), 53 Multi-Link ZETA (Abbott), 27 Omega (Boston Scientific), 61 R (Orbus-Neich, Hong Kong), and 65 S7 (Medtronic) stents.
Table 2. Angiographic and PCI-related characteristics (n = 1,604).
| Homocysteine level | < 12.0 µmol/L | ≥ 12.0 µmol/L | P value |
|---|---|---|---|
| (n = 1082) | (n = 522) | ||
| Complex lesion (type B2 or C*), n (%) | 832 (76.9) | 421 (80.7) | 0.094 |
| Target vessel site | 0.924 | ||
| Left main, n (%) | 24 (2.2) | 14 (2.7) | |
| LAD, n (%) | 442 (40.9) | 221 (42.3) | |
| LCx, n (%) | 207 (19.1) | 94 (18.0) | |
| RCA, n (%) | 402 (37.2) | 189 (36.2) | |
| Graft, n (%) | 7 (0.6) | 4 (0.8) | |
| Ostial lesion, n (%) | 78 (7.2) | 39 (7.5) | 0.838 |
| Bifurcation, n (%) | 76 (7.0) | 31 (5.9) | 0.456 |
| Lesion length, mean ± SD (mm) | 20.2 ± 10 | 20.5 ± 11 | 0.629 |
| Stent size, mean ± SD (mm) | 3.3 ± 0.7 | 3.3 ± 0.6 | 0.470 |
| Calcified, n (%) | 126 (11.6) | 89 (17.0) | 0.004 |
| Thrombus containing, n (%) | 163 (15.1) | 58 (11.1) | 0.037 |
| Number of stents per lesion | 1.10 | 1.12 | 0.178 |
| Pre-procedure target lesion | |||
| % diameter stenosis, mean ± SD | 82.5 ± 13 | 82.9 ± 13 | 0.547 |
| MLD (mm), mean ± SD | 0.58 ± 0.5 | 0.57 ± 0.5 | 0.627 |
| RVD (mm), mean ± SD | 3.32 ± 0.6 | 3.33 ± 0.6 | 0.884 |
| Post-procedure target lesion | |||
| % diameter stenosis, mean ± SD | 6.4 ± 6 | 6.6 ± 6 | 0.523 |
| MLD (mm), mean ± SD | 3.36 ± 0.5 | 3.10 ± 0.5 | 0.479 |
| RVD (mm), mean ± SD | 3.32 ± 0.6 | 3.36 ± 0.6 | 0.894 |
| Acute gain (mm), mean ± SD | 2.52 ± 0.6 | 2.53 ± 0.6 | 0.812 |
LAD: left anterior descending artery; LCx: left circumflex artery; LIMA: left internal mammary artery; OM: obtuse margin; MLD: minimal luminal diameter; PCI percutaneous coronary intervention; PDA: posterior descending artery; PL: posterolateral artery; RCA: right coronary artery; RVD: reference vessel diameter; SD: standard deviation.
A complex lesion was defined as a lesion of type B2 and C according to the classification of the American College of Cardiology and the American Heart Association.
In-Hospital Cardiac Events
The in-hospital cardiac events in the two groups are shown in Table 3. The overall and cardiac-related mortality rates were higher in group II than in group I (2.9% vs. 0.7%, p = 0.004; 2.4% vs. 0.7%, p = 0.014, respectively). However, there were no significant differences in the incidence of MI, acute, and subacute stent thrombosis, emergency bypass surgery, nonfatal stroke, or in-hospital MACE between the two groups.
Table 3. In-hospital cardiac events.
| Homocysteine level | < 12.0 µmol/L | ≥ 12.0 µmol/L | P value |
|---|---|---|---|
| Number of patients | 883 | 424 | |
| Death, n (%) | 6 (0.7) | 12 (2.9) | 0.004 |
| Cardiac | 6 (0.7) | 10 (2.4) | 0.014 |
| Non-Cardiac | 0 (0) | 2 (0.5) | 0.105 |
| Non-fatal MI, n (%) | 0.607 | ||
| NSTEMI | 18 (2.0) | 8 (1.9) | 1.00 |
| STEMI | 2 (0.2) | 0 | 1.00 |
| Acute stent thrombosis, n (%) | 3 (0.3) | 2 (0.5) | 0.662 |
| Subacute stent thrombosis, n (%) | 5 (0.6) | 4 (0.9) | 0.483 |
| Emergent bypass surgery, n (%) | 2 (0.2) | 1 (0.2) | 1.000 |
| Non-fatal stroke, n (%) | 2 (0.2) | 0 | 1.00 |
| MACE, n (%) | 33 (3.7) | 19 (4.5) | 0.546 |
MACE: major adverse cardiac event; MI: myocardial infarction; NSTEMI: non ST-segment elevation myocardial infarction; STEMI: ST- segment elevation myocardial infarction
Long-Term Clinical Outcomes
A total of 1,289 patients, who survived and discharged from hospital after PCI, were followed up for a mean of 58 ± 41 months. The patients in group II had a significantly higher mortality rate than those in group I (12.1% vs. 6.4%, p = 0.001) and majority were from cardiac death (8.0% vs. 3.4%, p = 0.001). In addition, the patients in group II had a higher percentage of new coronary artery lesions requiring stenting than patients in group I (13.6% vs. 9.5%, p = 0.034). Overall, the group II patients had a higher MACE rate than group I patients (33.3% vs. 25.6%, p = 0.005) (Table 4). In Kaplan–Meier analysis, there was a significant difference in the MACE-free survival rate between the two groups (log-rank test; p = 0.001) (Fig. 2). The univariate Cox proportional hazards analysis showed that the MACE rates were significantly associated with diabetes mellitus, LVEF < 40%, multivessel disease, complex type B2 or C lesion, postprocedure MLD, serum creatinine level, and plasma HCY level ≥ 12 µmol/L. After adjusting for factors of age, gender, hypertension, diabetes mellitus, hyperlipidemia, serum creatinine, HCY level > 12 µmol/L, multivessel disease, type B2 or C lesions, post-procedure MLD, and LVEF < 40% in multivariable analysis, elevated plasma HCY levels (≥ 12 µmol/L) remained a statistically significant predictor for long-term cardiovascular outcomes, with a HR of 1.29 (95% CI: 1.02–1.64, p = 0.036). Other significant predictors were LVEF < 40% (HR: 1.52, 95% CI: 1.11 – 2.09, p = 0.010), multivessel disease (HR: 1.51, 95% CI: 1.17– 1.95, p = 0.002), post-procedure MLD (HR: 0.66, 95% CI: 0.53 — 0.83, p < 0.001), and serum creatinine (HR: 1.69, 95% CI: 1.06 — 2.71, p = 0.027) (Table 5). Moreover, the plasma HCY values were divided into quintiles to examine their relationship with the long-term MACEs (Table 6). There was a graded association between HCY levels and MACE rates, which compared with quintiles 1–3, the adjusted HRs in quintile 4 (HCY range 11.3–13.4 µmol/L) and quintile 5 (HCY range > 13.4 µmol/L) were 1.06 (95% CI: 0.78–1.44) and 1.37 (95% CI: 1.03–1.82), respectively.
Table 4. Clinical events during long-term follow-up (n = 1,289, 58 ± 41 months).
| Homocysteine level | < 12.0 µmol/L | ≥ 12.0 µmol/L | P value |
|---|---|---|---|
| Number of patients | 876 | 413 | |
| Overall mortality, n (%) | 56 (6.4) | 50 (12.1) | 0.001 |
| Cardiac, n (%) | 30 (3.4) | 33 (8.0) | 0.001 |
| Non-cardiac, n (%) | 26 (3.0) | 17 (4.1) | 0.318 |
| Non-fatal MI, n (%) | 31 (3.5) | 18 (3.8) | 0.450 |
| Target lesion revascularization, n (%) | 87 (9.9) | 46 (11.2) | 0.494 |
| New lesion stenting, n (%) | 83 (9.5) | 56 (13.6) | 0.034 |
| Coronary bypass surgery, n (%) | 15 (1.7) | 9 (2.2) | 0.659 |
| Non-fatal stroke, n (%) | 19 (2.2) | 9 (2.2) | 1.00 |
| MACE, n (%) | 224 (25.6) | 137 (33.3) | 0.005 |
MACE: major adverse cardiac events; MI: myocardial infarction
Fig. 2.
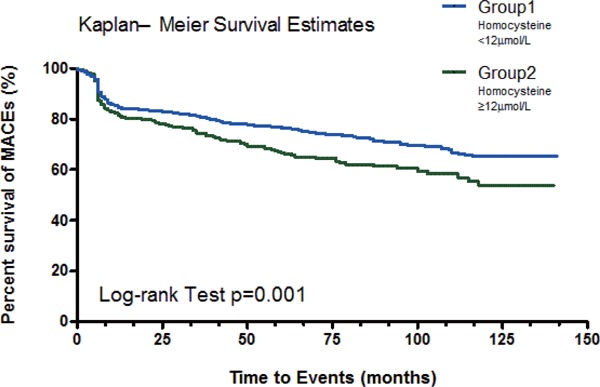
The MACEs-free survival by Kaplan–Meier analysis between groups
The event-free survival rate was significantly higher in the group I patients with lower homocysteine levels at admission (< 12 µmol/L) (Log-rank test, p = 0.001).
MACE: major adverse cardiac event.
Table 5. Predictors of long-term major cardiac events.
| Cox Proportional Hazards Regression | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Predictors | HR | 95% CI | P value | HR | 95%CI | P value |
| Age (every 10 years) | 1.08 | 0.99∼1.17 | 0.090 | 1.00 | 0.99∼1.01 | 0.658 |
| Female | 0.99 | 0.76∼1.30 | 0.974 | 1.09 | 0.79∼1.50 | 0.598 |
| Diabetes mellitus | 1.39 | 1.12∼1.72 | 0.003 | 1.24 | 0.97∼1.59 | 0.085 |
| Hypertension | 1.15 | 0.93∼1.42 | 0.427 | 1.01 | 0.80∼1.27 | 0.954 |
| Hyperlipidemia | 1.08 | 0.88∼1.33 | 0.463 | 1.07 | 0.84∼1.35 | 0.594 |
| ACS | 1.07 | 0.87∼1.32 | 0.515 | |||
| LVEF < 40% | 1.54 | 1.15∼2.07 | 0.004 | 1.52 | 1.11∼2.09 | 0.010 |
| Cardiogenic shock | 0.97 | 0.59∼1.59 | 0.892 | |||
| Multi-vessel disease | 1.63 | 1.29∼2.06 | < 0.001 | 1.51 | 1.17∼1.95 | 0.002 |
| Type B2 or C lesions | 1.50 | 1.09∼2.05 | 0.012 | 1.34 | 0.95∼1.89 | 0.092 |
| Post-procedural MLD (mm) | 0.64 | 0.52∼0.79 | < 0.001 | 0.66 | 0.53∼0.83 | < 0.001 |
| Creatinine (mg/dL) | 1.94 | 1.23∼3.05 | 0.004 | 1.69 | 1.06∼2.71 | 0.027 |
| eGFR (ml/min/1.73 m2) | 0.99 | 0.99∼1.01 | 0.109 | 1.01 | 1.00∼1.12 | 0.065 |
| HCY (≥ 12 µmol/L) | 1.43 | 1.15∼1.77 | 0.001 | 1.29 | 1.02∼1.64 | 0.036 |
ACS: acute coronary syndrome; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; MLD: minimal luminal diameter; HCY: plasma total homocysteine level.
Table 6. Long-term outcomes by homocysteine quintiles.
| Patient number | HCY range | Event number | Event rate | * Adjusted HR | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| Quintile 1–3 | 789 | < 11.3 | 201 | 25.4 | 1 | – | – |
| Quintile 4 | 293 | 11.3–13.4 | 84 | 28.7 | 1.06 | 0.78~1.44 | 0.697 |
| Quintile 5 | 206 | > 13.4 | 75 | 36.4 | 1.37 | 1.03~1.82 | 0.032 |
Adjusted HR: hazard ratio adjusted for age, sex, hypertension, diabetes mellitus, hyperlipidemia, serum creatinine, multi-vessel disease, post procedure MLD, and LVEF < 40%
HCY: plasma total homocysteine level. LVEF: left ventricular ejection fraction; MLD: minimal luminal diameter.
Discussion
In our retrospective analysis of a single center registry, 1,307 patients with coronary artery diseases after bare metal stent implantation were included for nearly 5 years follow-up. The patients with hyperhomocysteinemia (≥ 12 µmol/L) had a higher long-term MACE rate, mainly from cardiac mortality and new lesion stenting. After adjusting for multiple clinical, angiographic, and PCI-related variables, the association remained significant (HR, 1.29; 95% CI: 1.02–1.64). There is a dose dependent association between HCY levels and risks for long-term MACE, where the adjusted HRs in quintile 4 (HCY range 11.3–13.4 µmol/L) and quintile 5 (HCY range > 13.4 µmol/L) were 1.06 (95% CI: 0.78–1.44) and 1.37 (95% CI: 1.03–1.82), respectively.
Since McCully et al.13) described vascular diseases in patients with severe hyperhomocysteinemia (> 100 µmol/L) in 1969, subsequent case control and epidemiologic studies have shown a strong association between plasma HCY levels and the risks of atherosclerotic diseases. As observational studies have shown, even mildly elevated plasma HCY levels (between 11.4 and 14.3 µmol/L) increase the risk of cardiovascular events14, 15). However, the pathophysiological mechanisms were not fully understood. As implied from basic research on the mechanism of HCY associated with vascular injury16–19), HCY produced reactive oxygen species and caused lipid peroxidation, platelets and leukocytes activation, and increased prothrombotic factors, which resulted in vascular inflammation, thrombus formation. Furthermore, HCY causes endothelial dysfunction with impaired release of nitric oxide and vasodilatation in response to shear stress in vascular wall, which predisposes to vulnerable plagues rupture, contributes to atheroma progression, and ultimately gives rise to vascular events. Case control studies showed HCY level was significantly higher in patients with acute coronary syndrome (ACS)20, 21). In regard to the role of HCY in PCI, researchers also reported that patients with coronary slow flow phenomenon had higher HCY levels22), increased oxidative stress markers and impaired endothelial cell function23). Although hyperhomocysteinemia was considered to be associated with more ACS and periprocedural acute thrombotic events in previous reports, there was no significant difference in incidence of initial ACS presentation and in-hospital acute events between two groups in our study. We thought it could be explained by two main reasons. Frist, insufficient numbers of in-hospital thrombotic events and relatively mild to moderate elevated HCY levels in both groups are not enough to detect the difference in the prespecified clinical outcomes. Second, the thrombotic events in CAD caused from a complex interaction between numerous factors from patient-related, pharmacologic-related, procedural-related, or device-related related factors that might be unequal distribution between two groups in a nonrandomized trial.
With respect of the issue of increased cardiac mortality in patients with hyperhomcysteinemia, some relevant clinical observation results might explain this background issue. First, HCY levels correlated to the severity and extent of coronary atherosclerosis24) and associated with vascular calcification25, 26) and unstable atherosclerotic plagues27). These anatomic factors increased the difficulties of coronary intervention and associated with acute complications and worse long-term clinical outcomes. Second, the prothrombotic and proinflammatory effects of HCY are associated with coronary slow flow during intervention and may impair angiogenesis and collateral vessels development28), resulting in inadequate myocardial reperfusion, increased extent of myocardial necrosis, and finally ventricular pumping failure and worse long-term outcomes. However, we did not prospectively include the prespecified variables, such as vulnerability of atherosclerotic plagues or quantification of coronary slow flow during PCI in our initial study protocol and thus, there was no complete clinical data to analysis this association. Nevertheless, this issue is sufficiently noteworthy to merit additional well-designed studies.
In human studies, Kosokabe et al.29) observed a positive correlation between plasma HCY levels and atherosclerotic plaque areas in reference segments of coronary arteries, which were measured by intravascular ultrasound 6 months after the intervention. This evidence supports the atherogenic propensity of HCY and also could explain our study results, which more numbers of new lesions stenting and worse long-term outcomes in our patients with hyperhomocystinemia.
However, the results in past studies were inconsistent. In the late 1990s, Nygard et al.30) reported a strong and graded relationship between plasma HCY levels and the overall mortality in 587 patients with angiographically confirmed CAD after a median follow-up of 4.6 years. In their study, patients with a homocysteine level > 15 mmol/L had a 24.7% total mortality rate, compared with 3.8% for those with a homocysteine level < 9 mmol/L (p = 0.02). Later, Stubbs et al.31) also stated the similar association of plasma HCY levels and the long-term prognosis (median 2.5 years) in 440 patients with acute coronary syndrome, in which patients with a HCY level (> 12.2 µmol/L) had a 2.6-fold higher risk of cardiac death and myocardial re-infarction. In contrast, a prospective observational study by Zairis et al.12), including 483 patients with either stable angina or acute coronary syndrome after successful coronary stenting, found no significant association between plasma HCY levels and the composite endpoint of cardiac death, MI, or re-hospitalization for angina after a median follow-up of 22 months. The disparate results may be attributed to the shorter follow-up duration and different enrolled patient population and study endpoints in later study. In Zairis et al. study, patients with CAD and LVEF < 35%, implicating severe and extensive disease may have a higher plasma HCY level and poor prognosis4), were excluded. Patients with ST segment elevation myocardial infarction (STEMI) or non-ST segment elevation myocardial infarction (NSTEMI) presented more than 6 h or 24 h of index pain, respectively, were excluded. The endpoint of rehospitalization for angina may not be objectively and difficult to differentiate in some clinical situation. These enrolled criteria and study endpoints were also different from our study.
Even though evidence has shown an association between elevated plasma HCY levels and increased risks of late cardiac events and poor prognosis in patients with CAD, in randomized controlled trials, secondary prevention management with folic acid and vitamins failed to prevent or decrease cardiovascular events, even decreasing homocysteine levels32–34). However, it seems too early to make a conclusion before further well-designed clinical trials with different dosages or agents were performed. A longer follow-up period and specific patient population with moderately elevated homocysteine levels may be necessary to observe impacts of HCY on progression of coronary atherosclerosis and cause late stent failure and clinical events. In fact, a recent meta-analysis on patients with chronic kidney disease found significant benefits of reducing risks of cardiovascular disease from folate treatment, especially in those with a higher baseline homocysteine level (mean > 25 µmol/L, relative risk 0.87, p = 0.049)35). The further research to investigate the pathogenesis of HCY in atherosclerotic disease is important to contribute a newly potential therapeutic target to reduce homocysteine levels and improve outcomes.
Limitations
There were several limitations in this study. First, we used a cutoff value of HCY levels 12 µmol/L make uneventful distribution of the study population. Second, the optimal timing for blood collection and testing methods for plasma HCY levels have not been well defined. We sample blood in early morning after fasting for more than 12 h and within 24 h after admission. HCY levels may influence by numerous environmental factors such as dietary contents, which we could not estimate and adjust. Third, the methylene tetrahydrofolate reductase (MTHFR) enzyme, folic acid, vitamin B6, and vitamin B12 are required for metabolizing homocysteine to methionine or cysteine. Gene polymorphism of MTHFR C677T and dietary deficiency of folic acid, vitamin B6, or vitamin B12 are associated with hyperhomocysteinemia. In our study protocol, we did not routinely measure serum folate, vitamin B12, and gene polymorphism. Thus, we could not analyze the interaction between folic acid, vitamin B12, gene polymorphism, and risks of cardiovascular events. However, the association of MTHFR genotype and cardiovascular events is still controversial, and it could be modified by the folate status, where high folate levels reduced the effect of MTHFR C677T on HCY levels7, 36). Thus, we suggested that HCY levels reflect the influence of the gene–environment interactions and should be a more relevant factor to predict cardiovascular outcomes. Fourth, this is a single center prospective study and the results may not be applicable to other racial and ethnic groups.
Conclusions
An elevated HCY level (≥ 12 µmol/L) increased the risk of long-term cardiovascular events in patients who undergo intracoronary BMS implantations. After adjusting for clinical, angiographic, and PCI-related variables, an elevated homocysteine level remained a robust independent predictor for long-term clinical outcomes. Even randomized trials failed to demonstrate clinical benefits of HCY reducing therapies, HCY levels remain a useful clinical predictive marker for early identification of high-risk patients with worse long-term outcomes after coronary artery stenting.
Acknowledgments
Thanks for Victor Chien-Chia Wu contributed towards the study by English editing.
COI
There is no conflict of interest to declare in relation to this work.
Source of Funding
This work was supported by the grant from Chang Gung Memorial Hospital [grant number: CORPG 3C0162]
References
- 1). Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, Neumann FJ, Schömig A: Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998; 32: 1866-1873 [DOI] [PubMed] [Google Scholar]
- 2). Abizaid A, Kornowski R, Mintz GS, Hong MK, Abizaid AS, Mehran R, Pichard AD, Kent KM, Satler LF, Wu H, Popma JJ, Leon MB: The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998; 32: 584-589 [DOI] [PubMed] [Google Scholar]
- 3). Wald DS, Law M, Morris JK: Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002; 325: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Schaffer A, Verdoia M, Cassetti E, Marino P, Suryapranata H, De Luca G, Novara Atherosclerosis Study Group (NAS) : Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014; 134: 288-293 [DOI] [PubMed] [Google Scholar]
- 5). Homocysteine Studies Collaboration: Homocysteine and risk of ischemic heart disease and stroke. JAMA. 2002; 288: 2015-2022 [DOI] [PubMed] [Google Scholar]
- 6). Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD: Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet. 2005; 365: 224-232 [DOI] [PubMed] [Google Scholar]
- 7). Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, MTHFR Studies Collaboration Group MTHFR 677 C → T polymorphism and risk of coronary heart disease. JAMA. 2002; 288: 2023-2031 [DOI] [PubMed] [Google Scholar]
- 8). den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, Vandenbroucke JP, Rosendaal FR: Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996; 334: 759-762 [DOI] [PubMed] [Google Scholar]
- 9). Morita H, Kurihara H, Yoshida S, Saito Y, Shindo T, Oh-Hashi Y, Kurihara Y, Yazaki Y, Nagai R: Diet-induced hyperhomocysteinemia exacerbates neointima formation in rat carotid arteries after balloon injury. Circulation. 2001; 103: 133-139 [DOI] [PubMed] [Google Scholar]
- 10). Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM: Association of plasma homocysteine with restenosis after percutaneous coronary angioplasty. Eur Heart J. 2002; 23: 726-733 [DOI] [PubMed] [Google Scholar]
- 11). Schnyder G, Flammer Y, Roffi M, Pin R, Hess OM: Plasma homocysteine levels and late outcome after coronary angioplasty. J Am Coll Cardiol. 2002; 40: 1769-1776 [DOI] [PubMed] [Google Scholar]
- 12). Zairis MN, Ambrose JA, Manousakis SJ, Stefanidis AS, Papadaki OA, Bilianou HI, DeVoe MC, Fakiolas CN, Pissimissis EG, Olympios CD, Foussas SG: The impact of plasma levels of C-reactive protein, lipoprotein (a) and homocysteine on the long-term prognosis after successful coronary stenting. J Am Coll Cardiol. 2002; 40: 1375-1382 [DOI] [PubMed] [Google Scholar]
- 13). Mccully KS: Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969; 56: 111-128 [PMC free article] [PubMed] [Google Scholar]
- 14). Arnesen E, Refsum H, Bønaa KH, Ueland PM, Førde OH, Nordrehaug JE: Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995; 24: 704-709 [DOI] [PubMed] [Google Scholar]
- 15). Malinow MR, Nieto FJ, Szklo M, Chambless LE, Bond G: Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The atherosclerosis risk in communities study. Circulation. 1993; 87: 1107-1113 [DOI] [PubMed] [Google Scholar]
- 16). Ridker PM, Hennekens CH, Selhub J, Miletich JP, Malinow MR, Stampfer MJ: Interrelation of hyperhomocyst( e)inemia, factor v leiden, and risk of future venous thromboembolism. Circulation. 1997; 95: 1777-1782 [DOI] [PubMed] [Google Scholar]
- 17). Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA: Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997; 95: 1119-1121 [DOI] [PubMed] [Google Scholar]
- 18). Majors A, Ehrhart LA, Pezacka EH: Homocysteine as a risk factor for vascular disease: Enhanced collagen production and accumulation by smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997; 17: 2074-2081 [DOI] [PubMed] [Google Scholar]
- 19). Bellamy MF, McDowell IF: Putative mechanisms for vascular damage by homocysteine. J Inherit Metab Dis. 1997; 20: 307-315 [DOI] [PubMed] [Google Scholar]
- 20). Cheng M., Ho H., Lin J., Chen Y., Chan E., Chiu D. T.: Clinical relevance of plasma homocysteine levels in Taiwanese patients with coronary artery disease. Biofactors. 2008; 34: 125-134 [DOI] [PubMed] [Google Scholar]
- 21). Akyürek O., Akbal E., Güneş F.: Increase in the Risk of ST Elevation Myocardial Infarction Is Associated With Homocysteine Level. Arch. Med. Res. 2014; 45: 501-506 [DOI] [PubMed] [Google Scholar]
- 22). Tang O., Wu J., Qin F.: Relationship between methylenetetrahydrofolate reductase gene polymorphism and the coronary slow flow phenomenon. Coron. Artery Dis. 2014; 25: 653-657 [DOI] [PubMed] [Google Scholar]
- 23). Tanriverdi H., Evrengul H., Enli Y., Kuru O., Seleci D., Tanriverdi S., Tuzun N., Kaftan H. A., Karabulut N.: Effect of homocysteine-induced oxidative stress on endothelial function in coronary slow-flow. Cardiology. 2007; 107: 313-320 [DOI] [PubMed] [Google Scholar]
- 24). Chao C. L., Tsai H. H., Lee C. M., Hsu S. M., Kao J. T., Chien K. L., Sung F. C., Lee Y. T.: The graded effect of hyperhomocysteinemia on the severity and extent of coronary atherosclerosis. Atherosclerosis. 1999; 147: 379-386 [DOI] [PubMed] [Google Scholar]
- 25). Hirose N., Arai Y., Ishii T., Tushima M., Li J.: Association of mild hyperhomocysteinemia with aortic calcification in hypercholesterolemic patients. J. Atheroscler. Thromb. 2001; 8: 91-94 [DOI] [PubMed] [Google Scholar]
- 26). Li J., Chai S., Tang C., Du J.: Homocysteine potentiates calcification of cultured rat aortic smooth muscle cells. Life Sci. 2003; 74: 451-461 [DOI] [PubMed] [Google Scholar]
- 27). Xu H., Liu C., Wang Q.: Plaque image characteristics, hyperhomocysteinemia, and gene polymorphism of homocysteine metabolism-related enzyme (MTHFR C677T) in acute coronary syndrome. Cell Biochem. Biophys. 2013; 2: 403-407 [DOI] [PubMed] [Google Scholar]
- 28). Duan J. L., Murohara T., Ikeda H., Sasaki K., Shintani S., Akita T., Shimada T., Imaizumi T.: Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 2000; 20: 2579-2585 [DOI] [PubMed] [Google Scholar]
- 29). Kosokabe T, Okumura K, Sone T, Kondo J, Tsuboi H, Mukawa H, Tomida T, Suzuki T, Kamiya H, Matsui H, Hayakawa T: Relation of a common methylenetetrahydrofolate reductase mutation and plasma homocysteine with intimal hyperplasia after coronary stenting. Circulation. 2001; 103: 2048-2054 [DOI] [PubMed] [Google Scholar]
- 30). Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE: Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997; 337: 230-236 [DOI] [PubMed] [Google Scholar]
- 31). Stubbs PJ, Al-Obaidi MK, Conroy RM, Collinson PO, Graham IM, Noble IM: Effect of plasma homocysteine concentration on early and late events in patients with acute coronary syndromes. Circulation. 2000; 102: 605-610 [DOI] [PubMed] [Google Scholar]
- 32). Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J, Jr, Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators : Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006; 354: 1567-1578 [DOI] [PubMed] [Google Scholar]
- 33). Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K, NORVIT Trial Investigators : Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006; 354: 1578-1588 [DOI] [PubMed] [Google Scholar]
- 34). Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M: Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004; 291: 565-575 [DOI] [PubMed] [Google Scholar]
- 35). Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X: Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr. 2013; 32: 722-727 [DOI] [PubMed] [Google Scholar]
- 36). Holmes M. V., Newcombe P., Hubacek J. A., Sofat R., Ricketts S. L., Cooper J., Breteler M. M., Bautista L. E., Sharma P., Whittaker J. C., Smeeth L., Fowkes F. G. R., Algra A., Shmeleva V., Szolnoki Z., Roest M., Linnebank M., Zacho J., Nalls M. A., Singleton A. B., Ferrucci L., Hardy J., Worrall B. B., Rich S. S., Matarin M., Norman P. E., Flicker L., Almeida O. P., Van Bockxmeer F. M., Shimokata H., Khaw K. T., Wareham N. J., Bobak M., Sterne J. A. C., Smith G. D., Talmud P. J., Van Duijn C., Humphries S. E., Price J. F., Ebrahim S., Lawlor D. A., Hankey G. J., Meschia J. F., Sandhu M. S., Hingorani A. D., Casas J. P.: Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet. 2011; 378: 584-594 [DOI] [PMC free article] [PubMed] [Google Scholar]


