Abstract
Aim: It is not clear whether elevated serum non-high-density lipoprotein cholesterol (non-HDL-C) levels are a risk factor for cardiovascular disease (CVD) in subjects with chronic kidney disease (CKD) in the general population.
Methods: A total of 2,630 community-dwelling Japanese subjects (1,107 men and 1,523 women) without history of CVD and aged ≥ 40 years were prospectively followed up for an average of 19 years, and the association between serum non-HDL-C levels and the incidence of type-specific CVD was estimated using a Cox proportional hazards model. CKD was defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2 or proteinuria (≥ 1 + on dipstick).
Results: At baseline, 357 subjects had CKD. During the follow up, 186 coronary heart disease (CHD) and 277 stroke events occurred. The age- and sex-adjusted incidence of CHD was significantly higher in subjects with higher non-HDL-C levels, both in those with and without CKD. In the CKD group, the risk of CHD was significantly higher in those with non-HDL-C levels of 150–189 mg/dL [adjusted hazard ratio (HR), 2.23; 95% confidence interval (CI), 1.04–4.77] and those with levels ≥ 190 mg/dL (adjusted HR, 3.20; 95% CI, 1.46–7.03) than in those with levels < 150 mg/dL. In the non-CKD group, the risk of CHD was significantly higher only in those with non-HDL-C levels ≥ 190 mg/dL (adjusted HR, 2.12; 95% CI, 1.33 –3.38). However, no such association was observed for the risk of stroke.
Conclusions: Our findings suggest that higher serum non-HDL-C levels are associated with greater risk of CHD in subjects with and without CKD and that this association is greater in subjects with CKD than in those without CKD.
Keywords: Cardiovascular disease, Chronic kidney disease, Cohort study, Non-high-density lipoprotein cholesterol, Risk factors
Introduction
Chronic kidney disease (CKD) is a major public health concern worldwide because the prevalence of CKD has increased in many countries1), and subjects with CKD are at an increased risk of not only end-stage renal disease but also cardiovascular disease (CVD)2, 3). Therefore, it is clinically important to reduce the risk of CVD through the management of cardiovascular risk factors in subjects with CKD. Dyslipidemia is a well-known risk factor for CVD. Because dyslipidemia is common in subjects with CKD, lipid management and treatment would be important for the risk reduction of CVD in those with CKD4). However, the pattern of dyslipidemia in subjects with CKD differs from that observed in the general population; there is a greater tendency for elevation of triglyceride levels and reduction of high-density lipoprotein cholesterol (HDL-C) levels in subjects with CKD5–7). High serum low-density lipoprotein cholesterol (LDLC) levels are one of the established risk factors for CVD in the general population, but the importance of measuring LDL-C levels to assess prognosis in subjects with CKD remains controversial8). Therefore, it would be of clinical value to identify a lipid indicator for cardiovascular risk in subjects with CKD.
A serum non-HDL-C level, which is obtained from a routine lipid panel, is a comprehensive measure of atherogenic lipids, including LDL-C, lipoprotein (a), intermediate-density lipoprotein (IDL), and very-LDL (VLDL) remnants. Growing evidence suggests that elevated serum non-HDL-C levels are related to an increased risk of CVD events in the general population9–14). Serum non-HDL-C levels has also been reported to be a better index for future coronary heart disease (CHD) than other lipids such as LDL-C15). With regard to subjects with CKD, several prospective studies conducted in Western countries have reported that elevated non-HDL cholesterol levels is a significant risk factor for CHD in the general population and subjects with CKD16, 17). However, it has not been fully determined whether serum non-HDL-C levels are involved in CVD events in Asian subjects with early-stage CKD, although significant positive associations between serum non-HDL-C levels and CVD morbidity and mortality have been shown among Japanese patients undergoing hemodialysis18–20). In addition, there is limited information on the combined effect of non-HDL-C levels and CKD on the risk of subtypes of CVD. Herein, we present the findings from a prospective cohort study investigating the association between serum non-HDL-C levels and the risk of type-specific CVD among subjects with and without CKD in the general Japanese population.
Methods
Study Population
The Hisayama Study is a population-based cohort study of CVD that was established in 1961 in the town of Hisayama21). Hisayama is a suburban community adjacent to the city of Fukuoka on Kyushu Island in southern Japan. The design and characteristics of the study have been described elsewhere22–24). In 1988, a total of 2,742 ≥ 40-year-old residents consented to participate in the screening examination (participation rate: 80.9%). After excluding 106 residents with a history of CHD or stroke, two who died before the start of follow up, and four with missing data, the remaining 2,630 subjects (1,107 men and 1,523 women) were enrolled in this study. The study protocol was approved by the Kyushu University Institutional Review Board for Clinical Research. The participants provided written informed consent.
Follow-Up Survey
The subjects were prospectively followed up for 19 years from December 1988 to November 2007. The health status was checked either by annual health examination, mail, or telephone every year. We also established a daily monitoring system among the study team, local physicians, and the members of the town's Health and Welfare Office to collect information on new events or suspected cases of CVD. Physicians in the study team examined subjects who had or were suspected of having CHD or stroke, and evaluated their detailed clinical information. When a resident died, an autopsy was performed at the Department of Pathology of the Kyushu University. During the follow-up period, no subject was lost to follow up, and 842 subjects died, of whom 605 (71.9%) underwent autopsy.
Definition of CKD
Serum creatinine levels were measured by the non-compensated Jaffé method. The Jaffé method value was converted to an enzymatic method value using the following conversion equation25):
serum creatinine [enzymatic method (mg/dL)] = 0.9754 × serum creatinine [Jaffé method (mg/dL)] – 0.2802
Estimated glomerular filtration rate (eGFR) was calculated according to the modified Japanese Society of Nephrology Chronic Kidney Disease Initiative (JSNCKDI) equation26). Other formulas for calculating eGFR, including the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, were used for the sensitivity analysis27). CKD was defined as proteinuria (≥ 1 + on dipstick) or eGFR < 60 mL/min/1.73 m2 according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline28).
Risk Factors
In 2,525 subjects, blood samples were drawn after an overnight fast of at least 12 h. In the remaining 105 subjects, blood samples were collected in the postprandial state. Serum total cholesterol and HDL-C levels were measured enzymatically. Serum non-HDL-C levels were calculated by subtracting HDL-C from total cholesterol values. Diabetes mellitus was defined as a fasting plasma glucose level of ≥ 126 mg/dL, 2-h post-loaded or casual glucose level of ≥ 200 mg/dL, or current use of insulin or oral glucose-lowering agents. Serum albumin levels were measured using the bromocresol green dye-binding method. Serum specimens were stored at −20°C until they were used in 2002 for the measurement of high-sensitivity C-reactive protein (hs-CRP) levels24). Blood pressure was measured three times using a standard mercury sphygmomanometer in the sitting position after resting for at least 5 min. The mean of three measurements was used for the analysis. Body height and weight were measured in light clothing without shoes, and the body mass index (kg/m2) was calculated. Electrocardiogram (ECG) abnormalities were defined as left-ventricular hypertrophy (Minnesota code 3-1), ST depression (Minnesota code 4-1, 2, 3), or atrial fibrillation (Minnesota code 8-3). Each participant completed a self-administered questionnaire covering medical history, treatment for hypertension and diabetes, smoking habits, alcohol intake, and exercise. Smoking habits and alcohol intake were classified into currently habitual or not. The subjects who engaged in sports or other forms of exertion at least three times a week during their leisure time were included in the regular exercise group.
Definition of CVD Events
CVD was defined as first-ever development of CHD or stroke. CHD was defined as acute myocardial infarction, silent myocardial infarction, sudden cardiac death within 1 h of the onset of acute illness, or included those who had undergone coronary intervention (coronary artery bypass surgery or angioplasty). Acute myocardial infarction was diagnosed using the criteria described elsewhere29). Stroke was defined as an acute onset of nonconvulsive and focal neurological deficit lasting for more than 24 h. All CVD events were adjudicated on the basis of physical examination, a review of all available clinical data, including medical records and brain imaging, and autopsy findings by a panel of the study members.
Statistical Analysis
Subjects were categorized into three groups on the basis of serum non-HDL-C levels; < 150, 150–189, and ≥ 190 mg/dL, according to the Japan Atherosclerosis Society Guidelines, in which the treatment goals for serum non-HDL-C levels are set at < 190 and < 150 mg/dL for those with low and high cardiovascular risk30). The adjusted mean values of possible risk factors were calculated using an analysis of covariance. The frequencies of risk factors were adjusted for age and sex using a logistic regression model. The linear trends in the mean values and frequencies of risk factors across serum non-HDL-C levels were tested using linear regression analysis and logistic regression analysis, respectively. The incidence of CVD was calculated by a person-year method and adjusted for age and sex distribution of the overall study population using a direct method with 10-year age and sex groupings. The hazard ratios (HRs) with their 95% confidence intervals (CIs) and linear trends of risk estimates across serum non-HDL-C levels were estimated using a Cox proportional hazards model. Heterogeneity in the relationship between subgroups according to the presence of CKD was tested by adding a multiplicative interaction term to the relevant model. All statistical analyses were performed using the SAS program package version 9.3 (SAS Institute Inc., Cary, NC, USA). Two-tailed p values < 0.05 were considered statistically significant.
Results
Among all subjects, 357 (13.6%) had CKD. The age- and sex-adjusted mean values and frequencies of risk factors for CVD are listed according to serum non-HDL-C levels by CKD status in Table 1. In the non-CKD group, the mean values of age, systolic and diastolic blood pressures, serum total cholesterol levels, body mass index, serum albumin levels, serum hs-CRP levels, and the frequencies of antihypertensive medication and diabetes mellitus increased with elevating serum non-HDL-C levels. In contrast, the mean values of serum HDL-C levels and eGFR and the proportions of men, ECG abnormalities, and alcohol intake decreased with higher serum non-HDL-C levels. Similar associations were observed for the CKD group, except for mean age and systolic and diastolic blood pressure levels.
Table 1. Age- and sex-adjusted means and frequencies of risk factors for cardiovascular disease according to non-HDL-C levels by CKD status.
| CKD (−) |
CKD (+) |
|||||
|---|---|---|---|---|---|---|
| Serum non-HDL-C levels, mg/dL |
Serum non-HDL-C levels, mg/dL |
|||||
| < 150 | 150–189 | ≥ 190 | < 150 | 150–189 | ≥ 190 | |
| (n = 1,089) | (n = 753) | (n = 431) | (n = 141) | (n = 118) | (n = 98) | |
| Age, years | 57 (11) | 58 (12) | 59 (12) | 68 (11) | 66 (11) | 66 (11) |
| Men, % | 48.3 | 38.9 | 29.0 | 59.4 | 38.6 | 34.0 |
| Systolic blood pressure, mmHg | 132 (20) | 133 (20) | 135 (20) | 142 (20) | 140 (20) | 143 (20) |
| Diastolic blood pressure, mmHg | 77 (11) | 78 (11) | 79 (11) | 82 (11) | 81 (11) | 82 (11) |
| Antihypertensive medication, % | 9.5 | 11.3 | 15.9 | 22.0 | 25.1 | 30.0 |
| Diabetes mellitus, % | 8.1 | 11.4 | 19.5 | 13.1 | 17.2 | 19.4 |
| Serum total cholesterol, mg/dL | 172 (22) | 218 (22) | 263 (22) | 177 (22) | 218 (22) | 271 (22) |
| Serum HDL cholesterol, mg/dL | 51 (12) | 50 (12) | 49 (12) | 51 (12) | 50 (12) | 48 (12) |
| Body mass index, kg/m2 | 22.2 (3.1) | 23.1 (3.1) | 23.7 (3.1) | 22.8 (3.1) | 23.9 (3.1) | 23.5 (3.1) |
| Serum albumin, g/dL | 4.2 (0.2) | 4.3 (0.2) | 4.3 (0.2) | 4.2 (0.2) | 4.3 (0.2) | 4.3 (0.2) |
| Serum hs-CRP, mg/L | 0.45 (0.04–5.24) | 0.51 (0.04–5.98) | 0.66 (0.05–7.81) | 0.59 (0.05–7.59) | 0.68 (0.06–8.21) | 0.72 (0.06–8.45) |
| eGFR, mL/min/1.73 m2 | 84 (16) | 82 (16) | 81 (16) | 66 (16) | 65 (16) | 62 (16) |
| Proteinuria, % | 0.0 | 0.0 | 0.0 | 54.9 | 54.3 | 50.1 |
| ECG abnormalities, % | 17.3 | 15.5 | 10.2 | 21.0 | 18.8 | 17.2 |
| Smoking habits, % | 23.4 | 19.6 | 20.9 | 18.1 | 21.0 | 16.1 |
| Alcohol intake, % | 31.2 | 23.5 | 22.0 | 33.7 | 26.0 | 15.7 |
| Regular exercise, % | 16.4 | 16.4 | 19.9 | 15.7 | 19.5 | 14.9 |
Data are the means (SD) or percentages. Means of age are sex-adjusted and frequencies of sex are age-adjusted.
Geometric mean values (95% confidence interval) of serum hs-CRP are shown due to skewed distribution.
CKD was defined as proteinuria (dipstick ≥ 1 +) or eGFR < 60 mL/min/1.73 m2, which was calculated by using the modified Japanese Society of Nephrology Chronic Kidney Disease Initiative (JSN-CKDI) equation.
Abbreviations: CKD, chronic kidney disease: non-HDL-C, non-high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; ECG, electrocardiogram; SD, standard deviation.
During the follow-up period, a total of 422 subjects experienced CVD; 186 had CHD and 277 had stroke. Table 2 shows the age- and sex-adjusted incidences of CVD, CHD, and stroke according to serum non-HDL-C levels by CKD status. There were no significant associations between serum non-HDL-C levels and the age- and sex-adjusted incidence of CVD in the group with or without CKD. With respect to CVD subtypes, the age- and sex-adjusted incidence of CHD increased significantly with elevating serum non-HDL-C levels in the groups with and without CKD (with or without CKD subjects p for trend < 0.05), but no such associations were observed for ischemic or hemorrhagic stroke. These associations were not substantially altered after adjusting for age, sex, systolic blood pressure, antihypertensive medication, diabetes, body mass index, serum albumin and hs-CRP levels, ECG abnormalities, smoking habits, alcohol intake, and regular exercise (Table 3). The multivariable-adjusted HRs of CHD increased significantly with higher serum non-HDL-C levels in the groups with and without CKD (both p for trend < 0.01). In the CKD group, the risk of CHD became significantly higher in subjects with non-HDL-C levels of 150–189 mg/dL (adjusted HR, 2.23; 95% CI, 1.04–4.77) and in those with levels of ≥ 190 mg/dL (adjusted HR, 3.20; 95% CI, 1.46–7.03) than in those with levels of < 150 mg/dL, whereas the risk of CHD was significantly higher only in those with non-HDL-C levels of ≥ 190 mg/dL (adjusted HR, 2.12; 95% CI, 1.33–3.38) in the non-CKD group. There was no evidence of significant heterogeneity in the association of serum non-HDL-C levels with the risk of CHD between the CKD groups (p for heterogeneity = 0.6).
Table 2. Age- and sex-adjusted incidence (per 1000 person-years) of cardiovascular disease according to non-HDL-C levels by CKD status.
| CKD (−) |
CKD (+) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Serum non-HDL-C levels, mg/dL |
Serum non-HDL-C levels, mg/dL |
|||||||
| < 150 | 150–189 | ≥ 190 | p for trend | < 150 | 150–189 | ≥ 190 | p for trend | |
| (n = 1,089) | (n = 753) | (n = 431) | (n = 141) | (n = 118) | (n = 98) | |||
| Cardiovascular disease | ||||||||
| No. of events | 145 | 100 | 74 | 38 | 32 | 33 | ||
| Person-years | 17,207 | 12,367 | 7,162 | 1,691 | 1,552 | 1,248 | ||
| Age- and sex-adjusted incidence | 10.9 | 11.5 | 13.4 | 0.1 | 16.8 | 20.2 | 22.2 | 0.1 |
| Coronary heart disease | ||||||||
| No. of events | 53 | 45 | 36 | 16 | 17 | 19 | ||
| Person-years | 17,743 | 12,794 | 7,326 | 1,795 | 1,637 | 1,341 | ||
| Age- and sex-adjusted incidence | 3.8 | 4.3 | 7.7 | 0.002 | 6.3 | 11.1 | 12.5 | 0.02 |
| Stroke | ||||||||
| Ischemic stroke | ||||||||
| No. of events | 76 | 54 | 34 | 16 | 19 | 13 | ||
| Person-years | 17,324 | 12,480 | 7,296 | 1,717 | 1,594 | 1,301 | ||
| Age- and sex-adjusted incidence | 5.7 | 7.1 | 5.4 | 0.8 | 6.5 | 11.4 | 7.7 | 0.7 |
| Hemorrhagic stroke | ||||||||
| No. of events | 27 | 14 | 9 | 10 | 1 | 4 | ||
| Person-years | 17,324 | 12,480 | 7,296 | 1,717 | 1,594 | 1,301 | ||
| Age- and sex-adjusted incidence | 1.9 | 1.4 | 1.2 | 0.3 | 4.9 | 0.4 | 2.5 | 0.3 |
Abbreviations: CKD, chronic kidney disease; non-HDL-C, non-high-density lipoprotein cholesterol.
Table 3. Age- and sex-adjusted and multivariable-adjusted HR for the development of cardiovascular disease according to non-HDL-C levels by CKD status.
| CKD (−) |
CKD (+) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Serum non-HDL-C levels, mg/dL |
Serum non-HDL-C levels, mg/dL |
|||||||
| < 150 | 150–189 | ≥ 190 | p for trend | < 150 | 150–189 | ≥ 190 | p for trend | |
| (n = 1,089) | (n = 753) | (n = 431) | (n = 141) | (n = 118) | (n = 98) | |||
| Cardiovascular disease | ||||||||
| No. of events | 145 | 100 | 74 | 38 | 32 | 33 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.00 (0.78–1.30) | 1.30 (0.97–1.73) | 0.1 | 1.00 | 1.14 (0.70–1.85) | 1.48 (0.91–2.41) | 0.1 |
| Multivariable-adjusted HR (95% CI) a | 1.00 | 1.00 (0.77–1.31) | 1.33 (0.98–1.81) | 0.1 | 1.00 | 1.17 (0.70–1.97) | 1.52 (0.89–2.58) | 0.1 |
| Coronary heart disease | ||||||||
| No. of events | 53 | 45 | 36 | 16 | 17 | 19 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.32 (0.88–1.97) | 2.04 (1.32–3.16) | 0.002 | 1.00 | 1.69 (0.84–3.41) | 2.36 (1.18–4.72) | 0.02 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 1.31 (0.86–1.99) | 2.12 (1.33–3.38) | 0.002 | 1.00 | 2.23 (1.04–4.77) | 3.20 (1.46–7.03) | 0.004 |
| Stroke | ||||||||
| Ischemic stroke | ||||||||
| No. of events | 76 | 54 | 34 | 16 | 19 | 13 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.01 (0.71–1.44) | 1.06 (0.70–1.60) | 0.8 | 1.00 | 1.38 (0.70–2.73) | 1.14 (0.53–2.42) | 0.7 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 1.03 (0.72–1.48) | 1.06 (0.68–1.65) | 0.8 | 1.00 | 1.28 (0.60–2.73) | 1.11 (0.49–2.54) | 0.8 |
| Hemorrhagic stroke | ||||||||
| No. of events | 27 | 14 | 9 | 10 | 1 | 4 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 0.70 (0.37–1.34) | 0.75 (0.35–1.62) | 0.3 | 1.00 | 0.14 (0.02–1.08) | 0.68 (0.20–2.28) | 0.3 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 0.67 (0.35–1.31) | 0.74 (0.33–1.66) | 0.3 | 1.00 | 0.17 (0.02–1.45) | 0.72 (0.19–2.73) | 0.5 |
Adjusted for age, sex, systolic blood pressure, antihypertensive medication, diabetes, body mass index, serum albumin, serum high-sensitivity C-reactive protein, electrocardiogram abnormalities, smoking habits, alcohol intake, and regular exercise.
Abbreviations: CKD, chronic kidney disease; non-HDL-C, non-high-density lipoprotein cholesterol; HR, hazard ratio; CI, confidence interval.
Fig. 1 shows the influence of serum non-HDL-C levels and CKD on the risk of CHD. Compared with subjects without CKD and serum non-HDL-C levels of < 150 mg/dL, the multivariable-adjusted risk of CHD was significantly higher by 2.18 fold (95% CI: 1.38–3.43) than that in those without CKD and serum non-HDL-C levels of ≥ 190 mg/dL, whereas it increased by 3.03 fold (95% CI: 1.70–5.38) and 4.02 fold (95% CI: 2.28–7.09) in subjects with CKD and serum levels of 150–189 and ≥ 190 mg/dL, respectively. There was no evidence of significant difference in the risk of CHD between subjects without and with CKD and serum non-HDL-C levels of < 150 mg/dL (p = 0.7).
Fig. 1.
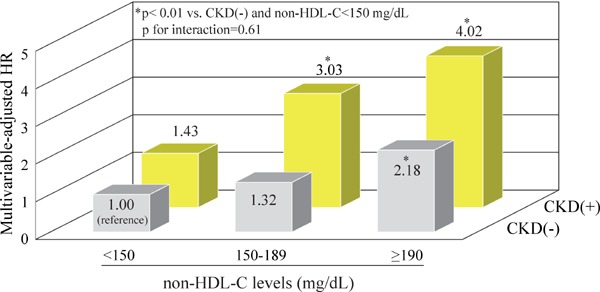
Multivariable-adjusted HR for the development of coronary heart disease according to the CKD status and serum non-HDL-C levels
CKD was defined as proteinuria (dipstick ≥ 1+) or estimated glomerular filtration rate < 60 mL/min/1.73 m2, which was calculated using the modified Japanese Society of Nephrology Chronic Kidney Disease Initiative (JSNCKDI) equation.
Adjusted for age, sex, systolic blood pressure, antihypertensive medication, diabetes, body mass index, serum albumin and high-sensitivity C-reactive protein levels, electrocardiogram abnormalities, smoking habits, alcohol intake, and regular exercise
Abbreviations: CKD, chronic kidney disease; non-HDL-C, non-high-density lipoprotein cholesterol; HR, hazard ratio.
For sensitivity analysis, all the analyses were repeated after determining the CKD status using the CKD-EPI equation. Therefore, the association between serum non-HDL-C levels and the risk of CVD and its subtypes was not altered substantially in either CKD group (Supplementary Table 1 and Supplementary Fig. 1).
Supplementary Table 1. Age- and sex-adjusted and multivariable-adjusted HR for the development of cardiovascular disease according to non-HDL-C levels by CKD status (CKD defined by eGFR using the CKD-EPI equation).
| CKD (−) |
CKD (+) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Serum non-HDL-C levels, mg/dL |
Serum non-HDL-C levels, mg/dL |
|||||||
| < 150 | 150–189 | ≥ 190 | p for trend | < 150 | 150–189 | ≥ 190 | p for trend | |
| (n = 1,150) | (n = 809) | (n = 487) | (n = 80) | (n = 62) | (n = 42) | |||
| Cardiovascular disease | ||||||||
| No. of events | 163 | 119 | 89 | 20 | 13 | 18 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.05 (0.83–1.33) | 1.29 (0.99–1.69) | 0.08 | 1.00 | 0.94 (0.46–1.92) | 2.10 (1.08–4.08) | 0.04 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 1.05 (0.82–1.35) | 1.32 (0.99–1.75) | 0.08 | 1.00 | 0.90 (0.40–2.01) | 2.62 (1.23–5.61) | 0.02 |
| Coronary heart disease | ||||||||
| No. of events | 63 | 54 | 43 | 6 | 8 | 12 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.33 (0.92–1.92) | 1.92 (1.29–2.87) | 0.002 | 1.00 | 2.09 (0.70–6.27) | 4.75 (1.71–13.23) | 0.002 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 1.38 (0.94–2.03) | 2.03 (1.32–3.13) | 0.001 | 1.00 | 2.59 (0.75–8.96) | 5.36 (1.62–17.71) | 0.005 |
| Stroke | ||||||||
| Ischemic stroke | ||||||||
| No. of events | 84 | 66 | 41 | 8 | 7 | 6 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 1.08 (0.78–1.49) | 1.04 (0.71–1.53) | 0.8 | 1.00 | 1.22 (0.43–3.45) | 1.63 (0.55–4.83) | 0.4 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 1.09 (0.77–1.53) | 1.05 (0.70–1.58) | 0.8 | 1.00 | 0.89 (0.26–3.08) | 2.72 (0.72–10.28) | 0.2 |
| Hemorrhagic stroke | ||||||||
| No. of events | 30 | 14 | 10 | 7 | 1 | 3 | ||
| Age- and sex-adjusted HR (95% CI) | 1.00 | 0.62 (0.33–1.19) | 0.71 (0.34–1.48) | 0.2 | 1.00 | 0.21 (0.03–1.79) | 0.97 (0.24–3.96) | 0.7 |
| Multivariable-adjusted HR (95% CI)a | 1.00 | 0.60 (0.31–1.17) | 0.69 (0.32–1.49) | 0.2 | 1.00 | 0.23 (0.02–2.87) | 1.21 (0.25–5.86) | 0.9 |
Adjusted for age, sex, systolic blood pressure, antihypertensive medication, diabetes, body mass index, serum albumin, serum high-sensitivity C-reactive protein, electrocardiogram abnormalities, smoking habits, alcohol intake, and regular exercise.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; non-HDL-C, non-high-density lipoprotein cholesterol; HR, hazard ratio.
Supplementary Fig. 1.
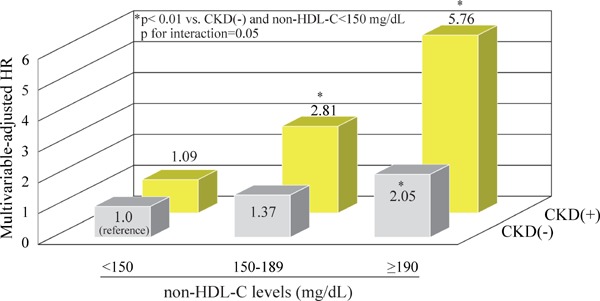
Multivariable-adjusted HR for the development of coronary heart disease according to CKD status and serum non-HDL-C levels (CKD defined by eGFR using the CKD-EPI equation)
Adjusted for age, sex, systolic blood pressure, antihypertensive medication, diabetes, body mass index, serum albumin, serum high-sensitivity C-reactive protein, electrocardiogram abnormalities, smoking habits, alcohol intake, and regular exercise.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; non-HDL-C, non-high-density lipoprotein cholesterol; HR, hazard ratio.
Discussion
This study demonstrated that in the general Japanese population, higher serum non-HDL-C levels were associated with greater risk of CHD regardless of the CKD status. To the best of our knowledge, this is the first study addressing the association between serum non-HDL-C levels and cardiovascular risk among subjects with early-stage CKD in an Asian community-based population. Although the proportional increase in the risk of CHD with elevating serum non-HDL-C levels was similar between subjects with and without CKD, the risk of CHD was higher in subjects with lower serum non-HDL-C levels and CKD than in those with lower serum non-HDL-C levels but without CKD. These findings underscore the clinical significance of serum non-HDL-C levels as a lipid indicator for the risk of CHD in subjects with CKD. Serum non-HDL-C levels may be used more broadly for the prevention of cardiovascular events in subjects with CKD.
Several longitudinal studies have investigated the influence of dyslipidemia, including the dyslipidemia-induced increases in serum total cholesterol and LDL-C levels, on the development of CVD among subjects with moderate-to-severe CKD31, 32). Nonetheless, the prognostic role of LDL-C in subjects with CKD is still undefined33). The Alberta Kidney Disease Network study in Canada showed that higher serum LDL-C levels were significantly associated with a higher risk of myocardial infarction, but the association was weaker for subjects with lower baseline eGFR levels8). Similar findings were observed in a randomized control trial conducted in moderate-to-severe subjects with CKD. The Study of Heart and Renal Protection (SHARP) trial demonstrated that reduced serum LDL-C levels were associated with lower risk of CVD in patients with CKD, but this association appeared to be attenuated with lower eGFR levels34). Subjects with CKD, particularly moderate-to-severe CKD, have a different pattern of serum lipid profile from subjects without CKD. Subjects with CKD have significantly lower serum total cholesterol, LDL-C, normal-size LDL, and HDL-C levels and higher triglycerides, VLDL, IDL, and small-dense LDL levels than subjects without CKD35). Therefore, the serum non-HDL-C level may be a better and more comprehensive indicator of dyslipidemia than the serum LDL-C level for subjects with CKD.
Non-HDL-C was a predictor of CHD in subjects with early-stage CKD in the Apolipoproteinrelated MOrtality RISk (AMORIS) cohort and Atherosclerosis Risk in Communities (ARIC) study16, 17). In the Modification of Diet in Renal Disease (MDRD) study, however, no association was observed between non-HDL-C levels and CVD mortality in subjects with stage 3 and 4 CKD36). Several prospective studies conducted in patients undergoing hemodialysis have reported that higher serum non-HDL-C levels were significantly associated with a greater cardiovascular risk18–20, 37). In a nationwide prospective registry of 45,390 Japanese patients undergoing hemodialysis, higher serum non-HDL-C levels were positively associated with higher incidences of myocardial and brain infarction18). Increased cardiovascular mortality linked with higher serum non-HDL-C levels was also found in small cohorts of Japanese patients undergoing hemodialysis19, 20). The Choices for Healthy Outcomes In Caring for ESRD (CHOICE) study conducted in 823 American patients undergoing hemodialysis demonstrated that mortality due to CVD increased significantly with elevated serum non-HDL-C levels among subjects without malnutrition and/or inflammation37).
The presence of a lipid-rich plaque in coronary atherosclerotic lesions is an important determinant of acute coronary events38). In our previous study, the risk of CHD significantly increased in subjects with high serum non-HDL-C levels29). The present analysis found that elevated serum non-HDL-C levels were significantly associated with an increased risk of CHD in subjects with CKD, whereas there was no significant association with the risk of stroke. We previously reported that higher serum non-HDL-C levels were associated with the development of atherothrombotic brain infarction, which is caused by atherosclerosis of large cerebral arteries, but no significant associations were observed for total ischemic or hemorrhagic stroke29). It is plausible that the influence of high serum non-HDL-C levels might be different in different subtypes of stroke. In the present study, we could not assess the association between serum non-HDL-C and stroke subtypes according to the CKD status because of the insufficient numbers of stroke events. Further large-scale prospective studies are needed to clarify this association and its underlying mechanism.
In our study, we assessed the combined influence of serum non-HDL-C levels and CKD status on the risk of CHD. Subjects with CKD had significantly higher risk of CHD than those without CKD in the group with a serum non-HDL-C of ≥ 150 mg/dL, even if it is at the same level. This finding suggests it may be necessary to control serum non-HDL-C levels more strictly in subjects with CKD than in those without CKD to reduce the cardiovascular risk.
Several limitations in our study should be noted. First, the baseline serum non-HDL-C levels were determined on the basis of a single measurement of the serum lipid level. The subsequent use of lipid-modifying medication could have altered lipid levels in some subjects and might have led to some misclassifications. However, such misclassifications would weaken the association observed in this study, biasing the results toward a null hypothesis. Second, because there were only four subjects (0.2%) with eGFR < 30 mL/min/1.73 m2, the results may not be applicable to subjects with advanced CKD stage. Third, the subjects were followed up for an extended period, and risk factor levels could have been changed by subsequent lifestyle modification and medical treatment, which might affect the outcome. Further studies are warranted to determine whether our findings from this study are applicable to other populations.
In conclusion, elevated serum non-HDL-C levels were shown to be a significant risk factor for the development of CHD not only among subjects without CKD but also among those with CKD in a general Japanese population. The serum non-HDL-C level is easily obtained from a routine lipid panel by subtracting the HDL-C level from total cholesterol level. Thus, the serum non-HDL-C level is a useful biomarker of CHD in subjects with CKD. Routine measurement of serum non-HDL-C levels would be clinically valuable for the effective prevention of cardiovascular events in subjects with CKD who are at high risk of CVD.
Acknowledgements
The authors thank the staff of the Division of Health and Welfare of Hisayama for their cooperation in this study.
This study was supported in part by grants-inaid for Scientific Research (A) (16H02644 and 16 H02692), (B) (16H05850), and (C) (26350895, 264 60748, 15K09267, 15K08738, 15K09835, and 16K 09244) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (H25-Junkankitou [Seishuu]-Sitei-022, H26-Junkankitou [Seisaku]-Ippan-001, and H27-Shokuhin-[Sitei]-017); and by the Japan Agency for Medical Research and Development (AMED) (16dk0207025h0001, 16ek02 10042h0002, and 16gm0610007h0204 (CREST)).
Conflict of Interest
TK has received lecture fees from MSD K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., Bayer Yakuhin Ltd., Chugai Pharmaceutical Co., Ltd., and Bristol-Myers Squibb K.K., and research funding from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., Eizai Co., Ltd., MSD K.K., Astellas Pharma Inc., Novartis Pharma K.K., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Torii Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Sumitomo Dainippon Pharma Co., Ltd., Sanofi-Aventis K.K., Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Ono Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Fuso Pharmaceutical Industries Ltd., Novo Nordisk Pharm Ltd., Teijin Pharma Ltd., Toray Medical Co., Ltd., AbbVie GK., AstraZeneca K.K., and Asahi Kasei Medical Co., Ltd. TN has received lecture fees from Mochida Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and research funding from Mochida Pharmaceutical Co., Ltd. TU, MN, JH, NM, YH, DY, HK, and YK have nothing to disclose.
References
- 1). Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med 2010; 268: 456-467 [DOI] [PubMed] [Google Scholar]
- 2). Ninomiya T, Kiyohara Y, Tokuda Y, Doi Y, Arima H, Harada A, Ohashi Y, Ueshima H, Japan Arteriosclerosis Longitudinal Study Group Impact of kidney disease and blood pressure on the development of cardiovascular disease: an overview from the Japan Arteriosclerosis Longitudinal Study. Circulation 2008; 118: 2694-2701 [DOI] [PubMed] [Google Scholar]
- 3). Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 2006; 290: F262-F272 [DOI] [PubMed] [Google Scholar]
- 5). Grützmacher P, März W, Peschke B, Gross W, Schoeppe W. Lipoproteins and apolipoproteins during the progression of chronic renal disease. Nephron 1988; 50: 103-111 [DOI] [PubMed] [Google Scholar]
- 6). Shimizu R, Torii H, Yasuda D, Hiraoka Y, Kitada N, Hashida T, Yoshimoto A, Kita T, Kume N. Serum lipid goal attainment in chronic kidney disease (CKD) patients under the Japan Atherosclerosis Society (JAS) 2012 guidelines. J Atheroscler Thromb 2015; 22: 949-957 [DOI] [PubMed] [Google Scholar]
- 7). Honda H, Hirano T, Ueda M, Kojima S, Mashiba S, Hayase Y, Michihata T, Shibata T. High-density lipoprotein subfractions and their oxidized subfraction particles in patients with chronic kidney disease. J Atheroscler Thromb 2016; 23: 81-94 [DOI] [PubMed] [Google Scholar]
- 8). Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, James M, Hemmelgarn B, Alberta Kidney Disease Network Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol 2013; 24: 979-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipo protein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med 2001; 161: 1413-1419 [DOI] [PubMed] [Google Scholar]
- 10). Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation 2005; 112: 3375-3383 [DOI] [PubMed] [Google Scholar]
- 11). Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 2005; 294: 326-333 [DOI] [PubMed] [Google Scholar]
- 12). Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M, Grundy SM. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care 2005; 28: 1916-1921 [DOI] [PubMed] [Google Scholar]
- 13). Arsenault BJ, Rana JS, Stroes ES, Després JP, Shah PK, Kastelein JJ, Wareham NJ, Boekholdt SM, Khaw KT. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol 2009; 55: 35-41 [DOI] [PubMed] [Google Scholar]
- 14). Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H, Japan Arteriosclerosis Longitudinal Study Group Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC -. Circ J 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 15). Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol 2006; 98: 1363-1368 [DOI] [PubMed] [Google Scholar]
- 16). Holzmann MJ, Jungner I, Walldius G, Ivert T, Nordqvist T, Ostergren J, Hammar N. Dyslipidemia is a strong predictor of myocardial infarction in subjects with chronic kidney disease. Ann Med 2012; 44: 262-270 [DOI] [PubMed] [Google Scholar]
- 17). Lamprea-Montealegre JA, Sharrett AR, Matsushita K, Selvin E, Szklo M, Astor BC. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis 2014; 234: 42-46 [DOI] [PubMed] [Google Scholar]
- 18). Shoji T, Masakane I, Watanabe Y, Iseki K, Tsubakihara Y, Committee of Renal Data Registry, Japanese Society for Dialysis Therapy Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol 2011; 6: 1112-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Nishizawa Y, Shoji T, Kakiya R, Tsujimoto Y, Tabata T, Ishimura E, Nakatani T, Miki T, Inaba M. Non-high-density lipoprotein cholesterol (non-HDL-C) as a predictor of cardiovascular mortality in patients with end-stage renal disease. Kidney Int Suppl 2003; 84: S117-S120 [DOI] [PubMed] [Google Scholar]
- 20). Echida Y, Ogawa T, Otsuka K, Ando Y, Nitta K. Serum non-high-density lipoprotein cholesterol (non-HDL-C) levels and cardiovascular mortality in chronic hemodialysis patients. Clin Exp Nephrol 2012; 16: 767-772 [DOI] [PubMed] [Google Scholar]
- 21). Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 22). Ninomiya T, Kiyohara Y, Kubo M, Yonemoto K, Tanizaki Y, Doi Y, Hirakata H, Iida M. Metabolic syndrome and CKD in a general Japanese population: the Hisayama Study. Am J Kidney Dis 2006; 48: 383-391 [DOI] [PubMed] [Google Scholar]
- 23). Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Rahman M, Arima H, Tsuryuya K, Iida M, Kiyohara Y. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: the Hisayama study. Stroke 2007; 38: 2063-2069 [DOI] [PubMed] [Google Scholar]
- 24). Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama Study. Arterioscler Thromb Vasc Biol 2008; 28: 1385-1391 [DOI] [PubMed] [Google Scholar]
- 25). Nagata M, Ninomiya T, Doi Y, Yonemoto K, Kubo M, Hata J, Tsuruya K, Iida M, Kiyohara Y. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant 2010; 25: 2557-2564 [DOI] [PubMed] [Google Scholar]
- 26). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 27). Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1-150 [Google Scholar]
- 29). Imamura T, Doi Y, Ninomiya T, Hata J, Nagata M, Ikeda F, Mukai N, Hirakawa Y, Yoshida D, Fukuhara M, Kitazono T, Kiyohara Y. Non-high-density lipoprotein cholesterol and the development of coronary heart disease and stroke subtypes in a general Japanese population: the Hisayama Study. Atherosclerosis 2014; 233: 343-348 [DOI] [PubMed] [Google Scholar]
- 30). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Japan Atherosclerosis Society Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 31). Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005; 293: 1737-1745 [DOI] [PubMed] [Google Scholar]
- 32). Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol 2007; 18: 304-311 [DOI] [PubMed] [Google Scholar]
- 33). Tonelli M, Wanner C, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members KDIGO 2013 Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 259-305 [DOI] [PubMed] [Google Scholar]
- 34). Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet 2011; 377: 2181-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Chu M, Wang AY, Chan IH, Chui SH, Lam CW. Serum small-dense LDL abnormalities in chronic renal disease patients. Br J Biomed Sci 2012; 69: 99-102 [PubMed] [Google Scholar]
- 36). Chawla V, Greene T, Beck GJ, Kusek JW, Collins AJ, Sarnak MJ, Menon V. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 1582-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004; 291: 451-459 [DOI] [PubMed] [Google Scholar]
- 38). Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004; 90: 1385-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]


