Abstract
The oral cavity harbours a complex microbiome that is linked to dental diseases and serves as a route to other parts of the body. Here, the aims were to characterize the oral microbiota by deep sequencing in a low-caries population with regular dental care since childhood and search for association with caries prevalence and incidence. Saliva and tooth biofilm from 17-year-olds and mock bacteria communities were analysed using 16S rDNA Illumina MiSeq (v3-v4) and PacBio SMRT (v1-v8) sequencing including validity and reliability estimates. Caries was scored at 17 and 19 years of age. Both sequencing platforms revealed that Firmicutes dominated in the saliva, whereas Firmicutes and Actinobacteria abundances were similar in tooth biofilm. Saliva microbiota discriminated caries-affected from caries-free adolescents, with enumeration of Scardovia wiggsiae, Streptococcus mutans, Bifidobacterium longum, Leptotrichia sp. HOT498, and Selenomonas spp. in caries-affected participants. Adolescents with B. longum in saliva had significantly higher 2-year caries increment. PacBio SMRT revealed Corynebacterium matruchotii as the most prevalent species in tooth biofilm. In conclusion, both sequencing methods were reliable and valid for oral samples, and saliva microbiota was associated with cross-sectional caries prevalence, especially S. wiggsiae, S. mutans, and B. longum; the latter also with the 2-year caries incidence.
Introduction
The most prevalent oral diseases, dental caries and periodontal diseases, are complex infectious diseases where life style and host factors, including behavioural, socioeconomic factors, medical status and genetics, interact1, 2. The oral cavity is reported to harbour more than 700 species/phylotypes, among which some species have been cultured3, 4. The bacteria are organized in site-specific biofilm communities5, with dysbiosis in the tooth surface or gingival pocket communities preceding caries and periodontal diseases6.
For dental caries, which result from the demineralization of tooth tissues by acids produced by bacterial fermentation of dietary carbohydrates, the aciduric and acidophilic mutans streptococci (Streptococcus mutans and Streptococcus sobrinus) have been specifically linked to disease development6. However, any acidogenic species, including the mutans streptococci, aciduric non-mutans streptococci, Bifidobacterium, Lactobacillus, Actinomyces, and Scardovia 7–10, may contribute to disease development. The relative impact of a species from these genera may vary between populations and within a population over time. For example, it was recently reported that in Romanian adolescents, in whom dental care is limited and disease activity is high, the frequency of both S. mutans and S. sobrinus was very high compared to in Swedish adolescents, who are exposed to life-long disease prevention and treatment programmes11. In 1973, S. mutans infection was as prevalent among Swedish adolescents (96%)12 as that reported for Romanian adolescents in 201311. Eleven years later (1984), the prevalence had decreased to 77% in Swedish adolescents12, and it had decreased to 50% in 201311.
Non-targeted methods are warranted to characterize a disease-associated bacterial community, i.e., methods not limited by present knowledge or expectations. Over recent decades, non-targeted multiplex DNA sequencing of the 16S rRNA gene and taxonomic determination from gene databases have commonly been used13. To date, most studies searching for caries-associated microbial patterns have been conducted in young children with severe caries. Only a limited number of studies have targeted adolescents or adults14, 15. Currently, Illumina technology is most commonly used for the microbiome characterization of clinical samples. This platform generates many sequences, but taxonomic resolution is limited. Recently, multiplex amplicon sequencing was launched for the Single-Molecule Real-Time (PacBio SMRT, Pacific Biosciences of California, USA) platform, which produces long reads, i.e., up to nine variable regions of the 16S rDNA, and improved taxonomic resolution16.
The above described results indicate systematic differences in the oral microbiota between populations by socio-economic status and that shifts occur over time not only at the individual but also at the population level. Therefore, studies targeting various well-characterized populations are required to understand determinants of health and disease under given conditions. The aims of the present study were to (i) characterize and compare saliva and tooth biofilm microbiota via deep sequencing using the Illumina MiSeq and the PacBio SMRT platforms and the Human Oral Microbiome Database (HOMD, www.HOMD.org) for taxa resolution17 in a population with long-term caries prevention and low disease activity, (ii) compare saliva and tooth biofilm microbiota in caries-affected and caries-free adolescents cross-sectionally and longitudinally, and (iii) evaluate the relative validity between and within the two sequencing methods. The study will add knowledge concerning saliva and tooth biofilm microbiota in adolescents, representing the most typical situation in Western countries18. The study will also be the first to employ the PacBio SMRT amplicon technique for determination of oral microbiota.
Results
Illumina MiSeq global sequencing results
Summary data from saliva (n = 64) and tooth biofilm (n = 49) sequencing using the Illumina MiSeq platform are presented in Table 1. The average read length of these sequences was 427 bp (v3-v4), and the average number of cleaned sequences per sample was 71,047 for saliva and 20,056 for tooth biofilm samples. Taxa were found in 10 phyla in both sample types and in 98 and 86 genera in saliva and tooth biofilm, respectively (Table 1). ProbeSeq (http://homings.forsyth.org/index2.html) and QIIME19 processing of raw sequences produced similar results (Table 1). In the following sections, data are presented for ProbeSeq processing unless stated otherwise.
Table 1.
Overview of Illumina MiSeq sequencing by bioinformatic processing and PacBio SMRT sequencing.
| Illumina Miseq | PacBio SMRT | ||
|---|---|---|---|
| saliva | tooth biofilm | tooth biofilm | |
| Sample number | 64 | 49 | 42 |
| Mean sequence length (bp) | 429 | 424 | 1,360 |
| ProbeSeq/SMRT specific pipeline | |||
| Total number of reads | 7,858,135 | 1,328,722 | 158,705 |
| Quality filtered reads | 4,547,033 | 982,980 | 142,912 |
| Reads per sample, mean (min-max) | 71,047 (46,109–156,263) | 20,056 (8,242–34,339) | 3,367 (667–8,162) |
| Phyla1 | 10 | 10 | 10 |
| Genera1 | 98 | 86 | 77 |
| Species/phylotypes/Genus probes1 | 395 | 373 | 345 |
| QIIME | |||
| Total number of reads after filtering2 | 8,245,243 | 1,582,139 | — |
| Quality filtered reads | 5,501,208 | 1,299,986 | — |
| Reads per sample, mean (min-max) | 85,952 (60,035–180,901) | 26,526 (12,157–49,024) | — |
| Phyla1 | 10 | 10 | — |
| Genera1 | 97 | 80 | — |
| Species/phylotypes1 | 405 | 368 | — |
1For ProbeSeq and QIIME, OTUs with <10 sequences per OUT were excluded and for PacBio SMRT those with <2 sequences per OUT. 2After split library using default QIIME parameters.
Determination of phyla in saliva and tooth biofilm samples using Illumina MiSeq
Firmicutes dominated in saliva (48% abundance, % of all sequences) followed by Actinobacteria (20%), whereas the abundances of Firmicutes and Actinobacteria were more similar (32% and 24%, respectively) in tooth biofilms. Bacteroidetes, Fusobacteria, and Proteobacteria, which were also represented in all adolescents and both sample types, together constituted 32% and 41% in saliva and tooth biofilm, respectively (Supplementary Tables S1 and S2 ).
Determination of genera in saliva and tooth biofilm using Illumina MiSeq
In total, 11 genera in saliva and 11 genera in tooth biofilm each represented 2% or more of all sequences (Fig. 1A,B, Supplementary Tables S1 and S2). Of these, 7 genera were detected in both sample types (i.e., Actinomyces, Fusobacterium, Leptotrichia, Prevotella, Rothia, Streptococcus, and Veillonella) (Fig. 1A,B). Although these genera were represented in all adolescents, the individual abundance was highly varied, as exemplified for saliva in Fig. 2. In total, 16 genera were found in both sample types and all adolescents.
Figure 1.
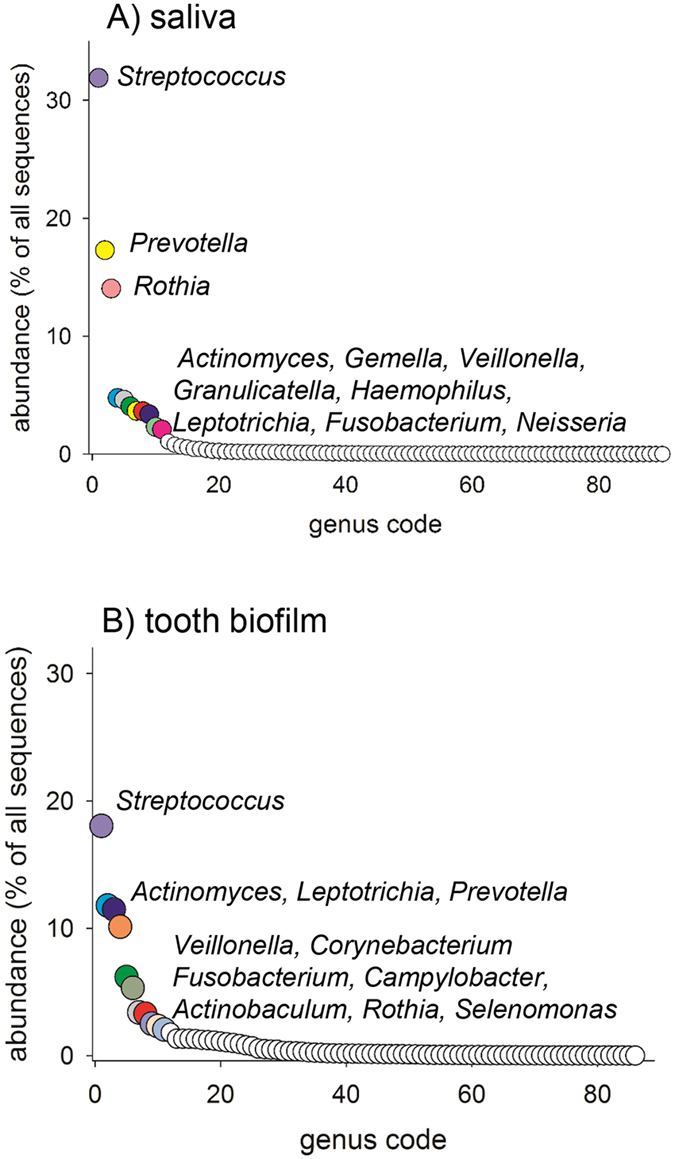
Abundance plot of genera in (A) saliva and (B) tooth biofilm.
Figure 2.
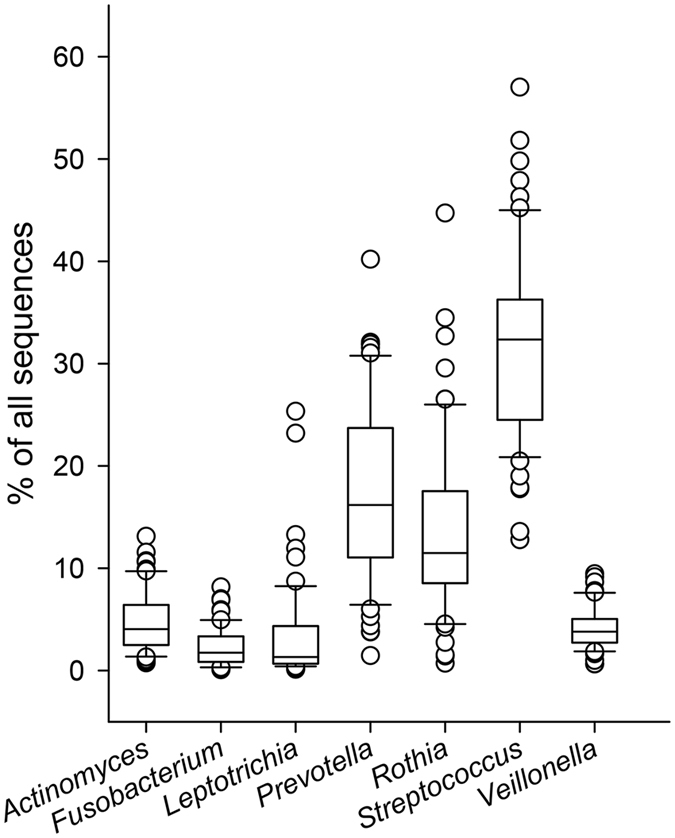
Box plot of genera present in both saliva and tooth biofilm and in all participants. Data are % of all sequences (abundance) in saliva. The bottom and top of the box indicate the first and third quartiles, the line inside the box the median, and the ends of the whiskers the 10th and the 90th percentile values. Outliers are plotted as individual circles.
At the genus level, Streptococcus dominated (32% abundance) in saliva, followed by Prevotella (17%), Rothia (14%), and 8 additional genera (5-2%) (Fig. 1A). In tooth biofilm, Streptococcus, Actinomyces, Leptotrichia, and Prevotella prevalence rates were more similar (18%, 12%, 11%, and 10%, respectively) as well as 7 additional genera (6-2%) (Fig. 1B). The abundances of all identified genera are presented in Supplementary Tables S1 and S2.
Species/phylotypes/Genus probes in saliva and tooth biofilm using Illumina MiSeq
In saliva, 30% of all sequences were recognized by the Streptococcus Genus probe 4 (recognizing 32 different Streptococcus species/phylotypes). Other prevalent species/phylotypes/Genus probes (>2% abundance) in saliva included, in descending order, Rothia mucilaginosa, Prevotella melaninogenica, Haemophilus parainfluenzae, species recognized by the Granulicatella Genus probe, and Gemella sanguinis (Supplementary Table S3). A full list of abundances is shown in Supplementary Table S3, and the species recognized by the Genus probes are listed at http://homings.forsyth.org/Genus%20probe%20list%20for%20website_v2.0.pdf.
In tooth biofilm, 11% of all sequences were recognized by the Streptococcus Genus probe 4. Other prevalent species/phylotypes/Genus probes (>2% abundance) in tooth biofilm included Corynebacterium matruchotii (4%), Actinobaculum sp. HOT183, Actinomyces gerencseriae, Actinomyces sp. HOT448, Campylobacter gracilis, species recognized by Fusobacterium Genus probe 4, Leptotrichia wadei, Prevotella melaninogenica, Prevotella nigrescens, Veillonella dispar, and species recognized by Veillonella Genus probe 2 (all between 2 and 3%). A full list of abundances is given in Supplementary Table S4.
PacBio SMRT sequencing of tooth biofilm samples
The average read length of the PacBio SMRT sequences was 1,360 bp (v1-v8), and the average number of cleaned sequences per sample was 3,367 (Table 1). Thus, the PacBio SMRT read length was significantly longer, but the sequencing depth was significantly lower than that of Illumina MiSeq sequencing. In total, 345 species/phylotypes in 10 phyla and 77 genera were detected.
Similar to the Illumina MiSeq sequencing of tooth biofilm, the abundances of sequences in the Firmicutes and Actinobacteria phyla were similar (23 and 26%, respectively), followed by Bacteroidetes, Fusobacteria and Proteobacteria (in total 39% of all sequences). Actinomyces was the most abundant genus (19%), followed by Streptococcus (10%), Prevotella (9%), Leptotrichia (8%), and Fusobacterium, Corynebacterium, and Veillonella (all 6%). The most frequently detected species was Corynebacterium matruchotii (5% abundance). The full lists of abundances are given in Supplementary Tables S5 and S6.
Core microbiome in saliva and tooth biofilm samples
Using Illumina MiSeq, 50 species/phylotypes/Genus probes were detected in all saliva samples, and 23 were detected in all tooth biofilm samples (100% prevalence). Nineteen of these “core species” were shared by saliva and tooth biofilm (Supplementary Tables S3 and S4). The PacBio SMRT analyses confirmed that Campylobacter gracilis, Corynebacterium matruchotii, Streptococcus mitis, Veillonella dispar, and Veillonella parvula were present in all of the analysed tooth biofilm samples (Supplementary Table S6).
Tooth biofilm microbiota in adolescents with or without caries
Study group characteristics
The characteristics of the 17-year-old adolescents in the caries-affected versus caries-free group are presented in Table 2. In addition to higher caries scores, the former group had a higher BMI (p = 0.033), more colony forming units (CFUs) of mutans streptococci in saliva (cultivation) (p < 0.001), and a higher S. mutans prevalence by PCR (p = 0.001). Additionally, they brushed their teeth less frequently (p = 0.012). Caries, caries-associated variables, and detected taxa were not systematically different between boys and girls.
Table 2.
Participant characteristics.
| Caries prevalence at baseline | Caries increment | |||||
|---|---|---|---|---|---|---|
| Caries free (n = 26) | Caries (n = 37) | P- value | No (n = 38) | Yes (n = 17) | P- value | |
| Boys/Girls, % | 46.2/53.6 | 45.9/54.1 | 0.987 | 52.6/47.4 | 35.3/64.7 | 0.232 |
| Number of teeth, mean (95% CI) | 28.0 (27.6–28.4) | 27.6 (27.2–28.0) | 0.109 | 28.1 (27.6–28.5) | 27.9 (27.3–28.6) | 0.7094 |
| Caries status | ||||||
| DFS, mean (95% CI) | 0 | 5.8 (4.5–7.2) | <0.001 | 0 | 2.3 (1.5–3.1) | <0.0015 |
| DS, mean (95% CI) | 0 | 1.2 (0.7–1.6) | <0.001 | 0 | 0.53 (0.12–0.94) | <0.0015 |
| Smoking, % | 7.7 | 2.7 | 0.360 | 2.6 | 17.6 | 0.0484 |
| Swedish snuff, % | 0 | 10.8 | 0.083 | 10.5 | 11.8 | 0.8924 |
| BMI, kg/m2, mean (95% CI) | 21.4 (20.3–22.4) | 23.3 (21.8–24.9) | 0.033 | 22.3 (21.2–23.5) | 23.5 (20.5–26.6) | 0.3316 |
| Saliva flow rate, ml/min, mean (95% CI) | 1.3 (1.0–1.6) | 1.4 (1.2–1.7) | 0.523 | 1.3 (1.1–1.5) | 1.4 (1.0–1.7) | 0.6826 |
| Tooth brushing, %1 | 92.3 | 64.9 | 0.012 | 78.9 | 64.7 | 0.2636 |
| Additional fluoride, %2 | — | — | 23.7 | 58.8 | 0.0114 | |
| Sweet snacks/day, mean (95% CI) | 2.5 (1.6–3.4) | 3.8 (2.2–5.3) | 0.186 | 3.0 (2.1–4.0) | 3.1 (1.0–5.3) | 0.9266 |
| Mutans streptococci by culture, CFU/ml3 | 1.3 (0.6–2.0) | 3.1 (2.6–3.7) | <0.001 | 2.4 (1.7–3.1) | 2.8 (1.9–3.6) | 0.4896 |
| S. mutans by PCR saliva, % | 44.0 | 83.8 | 0.001 | 64.9 | 82.4 | 0.1916 |
| S. mutans by PCR tooth biofilm, % | 36.0 | 64.9 | 0.025 | 51.4 | 64.7 | 0.3596 |
Participants were 17 years old at recruitment with follow-up 2 years later. Caries was defined as DFS >0 and caries-free as DFS = 0. Caries increment was defined as DFS at follow-up - DFS at baseline. Differences in subject distributions were tested with Chi2 test among groups, and differences between group means were tested with Student’s t-test. Adjustment for sex or number of teeth did not alter the relation between groups for any variable. 1Proportion brushing twice a day or not are compared. All brushed at least 3–4 times a week. 2Additional fluoride refers to rinsing, varnish, tablets, gel treatments. Information only available in 19-year olds. All used fluoridated tooth paste. 3Mean (95% CI) for log 10 of colony forming units (CFU) per ml chewing stimulated saliva. 4Information at 19-years of age. 52-year caries increment. In adolescent with no 2-year increment, 52.2% were caries-free and 47.4 had caries at baseline, whereas those 94.1% with 2-year increment had caries at baseline. 6Data are based on information at 17-years of age.
Microbiome profile by caries status
Microbiota richness. The species richness in saliva and tooth biofilm samples did not differ between subjects with or without caries (Fig. 3A).
Figure 3.
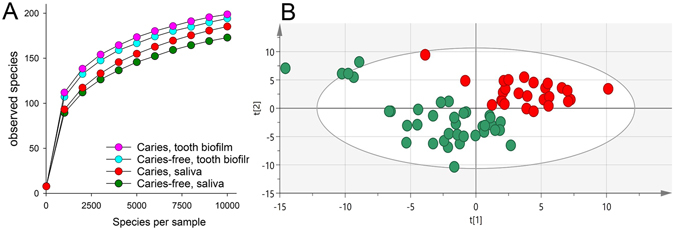
Species richness in saliva and tooth biofilm samples from 17-year old Swedish adolescents and subject clustering. (A) Rarefaction curves showing species richness based on number of species by species per sample in saliva and tooth biofilm samples. (B) Principal component analysis (PCA) scatter plot separating the caries-affected (red dots) from caries-free adolescents (green dots) based on microbial taxa detection in saliva and lifestyle confounders.
Microbiota diversity. PCA modelling with species/phylotypes/Genus probes in saliva (Illumina MiSeq ProbeSeq processing), tooth brushing, BMI, sugar intake, and tobacco use included as potential confounders separated caries-affected adolescents from those who were caries free (Fig. 3B). Similarly, PCoA modelling by QIIME identified Operational Taxonomic Units (OTUs) tended to separate most caries affected subjects from caries free subjects (Supplementary Fig. 1).
A linear discriminant analysis (LDA) effect size-based cladogram, in which successive circles represent a phylogenetic level (phylum, class, family, genus) indicated that species in the Synergistetes phylum, Synergistia class, Clostridiales [F-1] and Synergistaceae families, and Dialister, Scardovia, Clostridiales [F-1][G-1], Fretibacterium, Shuttleworthia, Peptostreptococcaceae [11] [G-6] and [11][G-9], and Veillonellaceae [G-1] genera were enriched in subjects with caries, whereas taxa in the Fusobacteria phylum, Fusobacteriia class, Actinomycetaceae and Ruminococcaceae families, and Actinomyces, Ruminococcaceae [G-1], and [G-2] genera were enriched in caries-free subjects (Fig. 4A). These results are also illustrated in an LDA bar graph (Fig. 4B).
Figure 4.
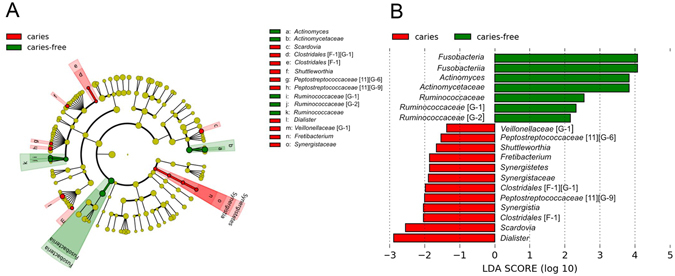
LEfSe analysis of saliva microbiota in caries versus caries-free adolescents. (A) Cladogram generated by LEfSe indicating differences at phylum, class, family and genus levels between the two groups. Each successive circle represents a phylogenetic level. Regions in red indicate taxa enriched in caries affected while regions in green indicate taxa enriched in caries-free subjects. Differing taxa are listed on the right side of the cladogram. (B) Bar graph showing LDA scores.
Partial least squares (PLS) modelling with caries status (DFS yes/no) at 17 years of age as the dependent variable and an independent block of species/phylotypes/Genus probes (yes/no) in saliva along with the same set of confounders as in PCA yielded a model with four significant components and a cross-validated predictive capacity (Q2) of 37% of the two first components. The strongest associations (PLS correlation coefficients >0.1) with the presence of caries were found for Scardovia wiggsiae, Streptococcus mutans, Selenomonas Genus probe 1, Bifidobacterium longum, and Leptotrichia sp. HOT498, whereas Mycoplasma orale and Porphyromonas sp. HOT278 were most strongly (PLS correlation coefficients >0.1) associated with being caries free. In addition, BMI was significantly associated with having caries, and tooth brushing was significantly associated with being caries free (Fig. 5).
Figure 5.
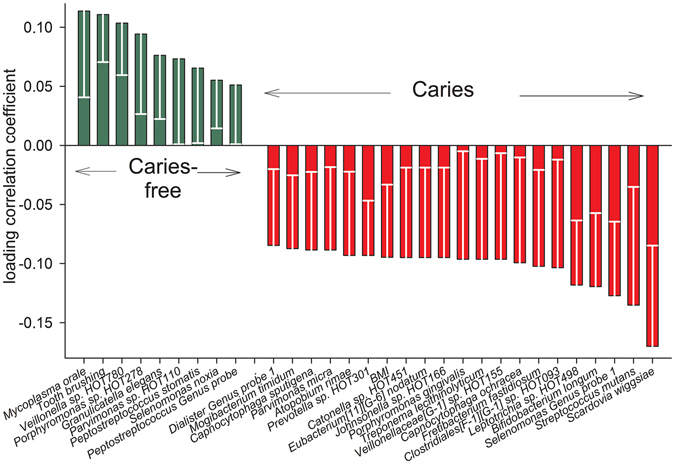
PLS loading column plot. Mean (95% CI) PLS correlation coefficients for the top 20 taxa associated with having caries and all taxa associated with being caries-free. The PLS model included caries as dependent variable and saliva detected species level taxa, sex, BMI, oral hygiene, and sweet snacking, as the independent block. w describes the PLS weights from the combination of the original variables in the X-swarm and c the same for the Y-swarm. A variable is considered statistical significant when the 95% CI of the correlation coefficient does not include zero.
The findings of the multivariate PLS model were consistent with univariate comparisons in which S. wiggsiae was found in the saliva of 100% of individuals with caries compared to 65.4% of caries-free adolescents (p < 0.0001, which was significant after false discovery correction) (Supplementary Table S3). Other PLS identified caries-associated species prevalence rates were also higher in univariate comparisons (p ≤ 0.008) but did not reach significance after false discovery correction, i.e., S. mutans, Selenomonas Genus probe 1, B. longum, and Leptotrichia sp. HOT498. In addition, the mean percent of all sequences (abundance) of S. mutans was significantly higher (p < 0.0001) in caries-affected than in caries-free adolescents and tended to be higher (p ≤ 0.008) for Bifidobacterium longum, Fusobacterium nucleatum subsp. nucleatum, and Selenomonas Genus probe 1, whereas the opposite was found for Mycoplasma orale and Porphyromonas sp. HOT278 (both p = 0.009) (Supplementary Table S3).
For tooth biofilm microbiota (Illumina MiSeq, ProbeSeq processing), PCA did not indicate any clustering of subjects, and PLS modelling was not applied. Similarly, neither the prevalence nor abundance displayed a significant difference between the two caries groups, although the prevalence of the Capnocytophaga Genus probe 1 and Veillonella rogosae tended to be higher for caries-affected than caries-free adolescents (p ≤ 0.008) as did the abundance of S. mutans (Supplementary Table 4).
Similarly, PCA modelling of PacBio SMRT sequences did not indicate the clustering of subjects by tooth biofilm taxa, and no significant differences were found in univariate analyses between the two groups. However, caries-diseased adolescents tended to have a higher prevalence of Actinobaculum sp. HOT183, Actinomyces sp. HOT171, Granulicatella adiascens, Rothia aeria, and Veillonellaceae [G-1] sp. HOT150 (p ≤ 0.008). Actinobaculum sp. HOT183 and Veillonellaceae [G-1] sp. HOT150 also tended to be more abundant in caries-affected than caries-free adolescents, whereas Fusobacterium nucleatum subsp. polymorphum tended to be more abundant in the caries-free group (p ≤ 0.008) (Supplementary Table 6).
Saliva microbiota and the 2-year caries increment from 17 to 19 years of age
PCA did not reveal any systematic clustering of subjects by 2-year caries increment but a higher proportion of adolescents who had developed caries over the 2-year follow-up period were smokers and had received additional fluoride treatments (Table 2). However, when 2-year mean caries increments were compared in adolescents with or without B. longum in saliva (means adjusted for sex, oral hygiene, sugar intake, BMI, and tobacco use by general linear modelling (glm)), the former group had a significantly higher increment (2.1 versus 0.5 new caries lesions, p = 0.005). Adolescents with S. mutans or S. wiggsiae had a numerically higher 2-year mean caries increment (both 0.8 versus 0.3), but the differences did not reach significance (p > 0.05).
Method validations
Reliability of sequencing analysis
Nine tooth biofilm DNA samples in triplicate and saliva samples from 5 subjects collected one day apart during one week were analysed using the Illumina MiSeq platform. Triplicate samples clustered close together, and samples were distinctly separated from each other (Fig. 6A). The repeated saliva samples clustered together for three subjects, whereas the sample from one day deviated for one subject and for all three samples in one subject (Fig. 6B).
Figure 6.
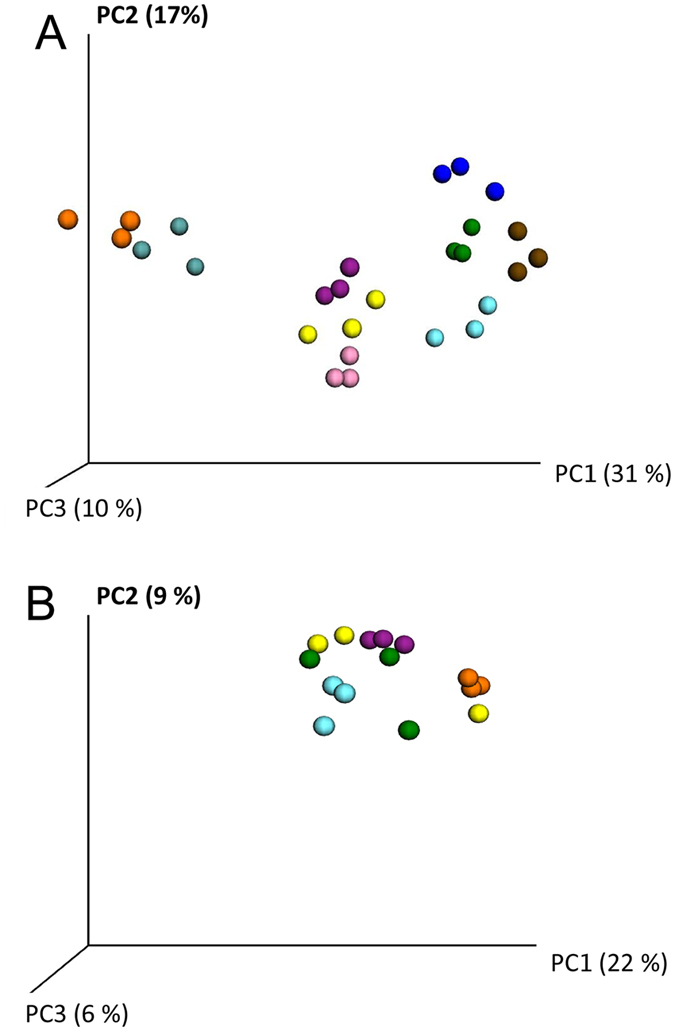
Reproducibility of Illumina Miseq sequencing of saliva samples. PCoA plots of (A) same samples run in triplicate, and (B) three independent samples collected from five subjects every second day during a week. Same colour indicates same sample or subject.
Validity
Illumina MiSeq sequencing detected all 10 species in the Streptococcus mock correctly among OTUs with ≥10 sequences but also suggested 5 OTUs as unnamed Streptococcus phylotypes. Of the 20 Lactobacillus species in that mock, 16 were correctly identified among OTUs with ≥10 sequences, and 4 could not be detected because they were not included in the HOMD database (L. curvatus, L. colehominis, L. gallinarium, and L. graminis). Similarly, with ≥10 sequences/OTU as the cut-off, 25 (of 32) species from the mixed genera mock were correctly identified. For the 8 non-identified species, 1 was represented by 2 sequences only, and 7 species of different genera were not detected at all. The specificity was low for OTUs with fewer than 10 sequences. The number of sequences for the detected species varied greatly, i.e., 38,862 sequences for S. mutans and 3,721 for S. sanguinis in the streptococcus mock.
The sensitivity and specificity for S. mutans detection by both Illumina MiSeq and PCR were calculated. The sensitivity values for both saliva and tooth biofilm were high (0.98 and 0.90, respectively). ROC curves were calculated (Supplementary Fig. 2), and the area under the curve was 0.94 for saliva and 0.74 for tooth biofilm.
PacBio SMRT sequences of the Streptococcus mock identified 9 of 10 species with >1 sequence. The 10th was present with 1 sequence. In the Lactobacillus mock, 16 of 20 species were detected, with the same undetected species described above, i.e., 4 species not in the HOMD database. Similarly, a more than 10-fold variation in the number of detected sequences per species was observed, i.e., for streptococci, from 553 for S. sobrinus to 49 for S. sanguinis (in addition to S. mitis with 1 sequence); for lactobacilli, from 343 for L. paracasei to 10 for L. crispatus.
In addition, a negative control sample (ultra-pure water) subjected to Illumina MiSeq sequencing yielded 620 reads, which was far below the acceptance criterion for this method, i.e., ≥5,000 reads per sample.
Correlation between saliva and tooth biofilm taxa
Spearman correlation coefficients between abundances in the saliva and tooth biofilm by Illumina MiSeq sequencing varied from 0.9 to −0.1 for various genera, reflecting the similarities and differences between the hard- and soft-tissue niches. The Spearman correlation coefficients between saliva and tooth biofilm abundances for the caries-associated taxa were as follows: S. wiggsiae and Leptotrichia sp. HOT498 r = 0.6 (p < 0.001), S. mutans r = 0.7 (p < 0.001), and B. longum and Selenomonas Genus probe 1 r = 0.3 (p < 0.05).
Discussion
In the present study, saliva and tooth biofilm samples were analysed using Illumina MiSeq, and tooth biofilm samples were also analysed using PacBio SMRT sequencing. This study is the first to report the use of the latter technology on oral samples. PacBio SMRT sequencing confirmed the results from Illumina MiSeq sequencing at the phylum and genus levels and partially at the species level, including the predominance of C. matruchotii in tooth biofilms. C. matruchotii was overlooked in many previous studies using PCR-based or metagenomic sequencing but was recognized as a prominent species in metatranscriptome20, metaproteome21, and spectral imaging fluorescence hybridization5 analyses. Saliva microbiota, but not tooth biofilm microbiota, distinguished adolescents with caries from caries-free adolescents, with S. wiggsiae, S. mutans, B. longum, Leptotrichia sp. HOT498 and species recognized by Selenomonas Genus probe 1 (S. noxia and Selenomonas sp. HOT140) as the most influential taxa for group distinction.
The dominant taxa in saliva and tooth biofilms largely coincided with those reported in previous studies, especially at the phylum and genus levels14, 20–23. Thus, according to the Illumina MiSeq results, phylum Firmicutes and genus Streptococcus dominated in saliva, whereas abundances of the phyla Firmicutes and Actinobacteria were similar in tooth biofilm samples, and the Streptococcus genus was only slightly more abundant than Actinomyces. PacBio SMRT sequencing also identified similar levels of Firmicutes and Actinobacteria but a considerably higher abundance of Actinomyces than Streptococcus. Generally, studies using PCR-based 454 FLX+ pyrosequencing or Illumina MiSeq sequencing report a higher abundance of Streptococcus than Actinomyces, but the opposite has been indicated in newer metatranscriptome20 and metaproteome21 studies. The reason for the discrepancy between the two sequencing methods observed in the present study cannot be determined but likely relates to method-specific biases/strengths and possibly the difference in sequencing depth between the two methods. Five samples were placed per PacBio SMRT cell, but fewer would have been preferential making cost of PacBio SMRT sequencing compared to Illumina MiSeq a hindrance. However, comparisons with other studies must be performed with caution because the sequencing methods, age of the study subjects, dentition (primary versus permanent teeth), and socio-economic conditions of the target populations differed between most studies. Although the PacBio SMRT method has been used for samples from non-oral sites and mock communities24, 25, the fact that the present study was the first to report tooth biofilm microbiota limits comparisons with other studies. Thus, in accordance with results from metaproteomic profiling21, PacBio SMRT identified Actinomyces, Streptococcus, Corynebacterium, Leptotrichia, and Veillonella as the five most abundant genera, whereas Rothia was less abundant by PacBio SMRT than by both metaproteomic profiling21 and Illumina MiSeq sequencing in the present study.
The structure of tooth biofilm has recently been elegantly illustrated by spectral imaging fluorescence in situ hybridization5. The arrangement was referred to as a “hedgehog structure” with clustering of species in Corynebacterium, Streptococcus, Porphyromonas, Haemophilus/Aggregatibacter, Neisseriaceae, Fusobacterium, Leptotrichia, Capnocytophaga, and Actinomyces, with dominance of the 3 first genera and a central role of C. matruchotii. In the present study, most of these (Corynebacterium, Streptococcus, Porphyromonas, Fusobacterium, Leptotrichia, Capnocytophaga, and Actinomyces) were found in all or nearly all of the adolescents regardless of the sequencing method, and Neisseria and Haemophilus/Aggregatibacter were found by the more extensive Illumina MiSeq sequencing. C. matruchotii, which was also detected in all adolescents by both sequencing methods, was the most abundant species by PacBio SMRT and ranked second by Illumina MiSeq, reflecting its central position in the hedgehog or so-called “corn-cob formation”5.
Overall, saliva sequences represented more genera and species than tooth biofilm sequences. This may reflect that saliva is in contact with various surfaces in the mouth, i.e., mucosal surfaces, tongue epithelium, teeth, and gingival margins, rather than transient bacteria or the effect of a higher sequencing depth in the saliva than in tooth biofilm samples. By contrast, bacteria on teeth are selectively recruited by discriminatory attachment to tooth tissue adhering saliva proteins/peptides and interactions with neighbouring bacteria4, 5, 22, 26. Among the earliest saliva proteins/peptides that adhere to a clean tooth surface are the acidic proline-rich proteins, statherin and cystatins27, which unfold their binding epitopes for Streptococcus and Actinomyces upon tooth attachment22. Yet, other explanations cannot be ruled out, such as an influence of total amount of DNA captured in saliva versus tooth biofilm samples and systematic species differences in DNA retrieval. Notably, a mixture of enzymes were used in the present study to break the resistant cell walls of Gram positive bacteria, and Gram positive species in the mock with mixed genera, i.e. species in Bifidobacteria, Scardovia and Rothia, were detected in numbers that were similar to Gram negative species.
Some validations were performed in the present study, including negative and positive controls and repeated analyses. It was concluded that false positives due to contamination were negligible, and reproducibility was high for Illumina MiSeq sequencing; however, the sample composition may vary daily in some individuals. The latter is consistent with results reported by David et al.28. The detection specificity at the species level was acceptable for both the Illumina MiSeq and PacBio SMRT methods when the appropriate cut-offs were applied for taxa allocation. Unexpectedly, the numbers of sequences per species in the mock varied largely, even though equal volumes of bacterial suspensions of the same OD were added; therefore, this finding should be considered in the data interpretation and modelling. We chose to use a more conservative dichotomous variable (yes/no) in statistical models of taxa in the independent data block. The present study population was characterized by a significant caries reduction over the last five decades29, regular high-standard dental care, including compulsory preventive strategies, from early childhood18, and generally slow development of caries symptoms. The selected conservative approach better addressed time fluctuations in microbiota abundances. Use of the ProbeSeq or QIIME approach for the bioinformatics of Illumina sequences did not confer any substantial difference in the interpretation of results. Because the ProbeSeq approach has the advantage of clustering species with very high 16S rDNA identity into a Genus probe group, this approach was preferred. Thus, for the present clinical research question, we found the ProbeSeq processing of oral samples and dichotomous measures most suitable and that our results supported the previously reported validity of ProbeSeq-processed Illumina MiSeq data30.
In the present study group, saliva microbiota separated caries-affected from caries-free subjects, whereas microbiota from tooth biofilm did not separate these groups. There are several plausible explanations for this finding, including that tooth biofilm samples only represent sampled surfaces, whereas saliva is the pool from which tooth colonizers are recruited and reflects most surfaces in the mouth. In contrast to our findings, it has been claimed that saliva does not reflect tooth biofilm microbiota and variations among single tooth surfaces31. In the present study, the caries-associated bacterial species in saliva were consistent with those found in previous studies that used various techniques (cultivation, PCR, DNA hybridization chips) on tooth biofilm samples, and the abundances of the caries-associated taxa in saliva were significantly correlated with the corresponding abundance in tooth biofilms. This result supports the use of saliva; however, as recommended32, a combination with more traditional techniques, such as culturing and PCR, should be used for optimal identification. Thus, based on the present findings, we suggest that chewing-stimulated saliva is valid for the characterization of caries-associated microbiota in large-scale epidemiological studies, but this finding has been contradicted by others32, 33.
The species that were most strongly associated with caries were S. wiggsiae, S. mutans, B. longum, Leptotrichia sp. HOT498, and species detected by Selenomonas Genus probe 1. Selenomonas Genus probe 1 targets S. noxia and Selenomonas sp. HOT140, of which S. noxia has been found to be increased in patients with gingivitis34. Thus, a higher prevalence of Selenomonas Genus probe 1 likely reflected less frequent tooth brushing and gingivitis in caries-affected adolescents rather than an aetiological role in the caries process. Consistent with the present finding of Leptotrichia sp. HOT498 being more prevalent in the saliva of caries-affected than caries-free adolescents, it was recently reported that this species was more prevalent in severely caries-affected Romanian adolescents than in Swedish adolescents with a low caries prevalence11. This difference was found in tooth biofilms and for sequences obtained by the LibL 454 FLX+ pyrosequencing option covering the first four variable regions of the 16S rRNA gene. However, S. wiggsiae, S. mutans, and B. longum are well-documented aciduric and acidophilic species that have been associated with caries in many earlier studies6–10, 35, 36. Among these three species, B. longum was significantly associated with the 2-year caries incidence; however, because the number of subjects carrying B. longum was low, the association must be evaluated in a larger sample. In addition, smoking and having topical fluoride treatment besides a fluoride toothpaste were associated with 2-year caries increment. Smoking is well documented to be associated with caries prevalence and even in this small study sample the association was evident. In contrast, fluoride is a well documented caries protective compound. It may therefore seem contradictory to find such treatment more prevalent among adolescents with disease development. However, in the study population persons with a high risk to develop caries and/or high caries activity have annual dental visits and preventive measures are required. For this reason fluoride treatment in this population reflects caries treatment in high caries subjects and not the effect of fluoride per se.
The strengths of the present study include that (i) the study groups were larger than those of previous studies, and the study extended knowledge beyond early childhood caries and caries in deciduous teeth, (ii) the criteria for sequence inclusion were based on results from simultaneously analysed mock samples, (iii) the sparsely used PacBio SMRT method with improved taxa resolution was applied, (iv) the study population represented populations with a substantial caries decline in the 20th century, and (v) prospective caries incidence was evaluated. The weaknesses include that (i) tooth biofilm could not be analysed in all participants due to limitations in DNA accessibility, (ii) it cannot be assured that every single tooth surface was sampled, (iii) the sequencing depth was low for PacBio SMRT, and (iv) saliva was not analysed using PacBio SMRT.
Conclusions
Based on the present findings, we conclude that saliva microbiota can separate adolescents with caries from caries-free adolescents in a low-caries population with daily tooth brushing using fluoridated tooth paste18. The three most influential species were S. wiggsiae, S. mutans, and B. longum. We also showed that C. matruchotii is enriched in tooth biofilms relative to saliva and that overall PacBio SMRT sequencing covering most of the 16S rDNA supported the findings of Illumina sequencing. However, some discrepancies need to be followed up in future studies.
Methods
Study subjects
Seventeen-year-old adolescents, who were caries-free (no present or previous caries) or had present caries activity and a high caries risk37, were invited to participate. Subjects were consecutively recruited from three Public Dental Health Care Clinics in the city of Umeå, Sweden. The exclusion criteria were that the adolescent (or caregiver) did not consent, had a chronic disease or was on medication, had taken antibiotics during the latest 6 months, or was unable to communicate in Swedish or English. The caries status was evaluated at 17 and 19 years of age. Baseline data were collected in 2013. Twenty-six caries-free and 37 caries-affected adolescents were recruited, and 55 of these subjects were re-evaluated at 19 years of ages. The population from which the participants were recruited has an overall low caries prevalence, as well as annual dental care, including preventive measures, provided since 2–3 years of age (http://www.socialstyrelsen.se/nationalguidelines).
Sampling for microbiota analyses
Whole saliva (5 ml) was collected into ice-chilled sterile test tubes while chewing on a 1-g piece of paraffin wax. Next, 100 μl of fresh saliva was transferred to a transport medium for cultivation, the remaining saliva centrifuged at 4 °C (13,000 rpm for 5 min), and the pellets stored at −80 °C until DNA extraction and amplicon sequencing by Illumina MiSeq (http://www.illumina.com).
Supragingival tooth biofilm was collected using sterile wooden toothpicks, pooled by subject in 100 µl TE-buffer (10 mM Tris, 1 mM EDTA, pH 7.6), and stored at −80 °C until DNA extraction and 16S rDNA amplicon sequencing by Illumina MiSeq and PacBio RS II SMRT (Pacific Biosciences, Menlo Park, CA, USA) was conducted.
Caries scoring and lifestyle information
The number of teeth, cavitated carious lesions (D), fillings (F), and missing (M) tooth surfaces were scored from visual and radiograph examinations in dental clinics. The sum of the decayed and filled tooth surfaces (DFS) was calculated (caries prevalence). The M-component was not included in the caries index because losses of teeth were due to orthodontic or tooth hypomineralization reasons. The caries incidence (increment) was calculated as the DFS increase from 17 to 19 years of age. Information on the general health status, medication, oral hygiene, dietary habits, and tobacco use (smoking and snuff) was obtained by a questionnaire when the adolescent was 17 years old.
Mock communities
Three mock communities of oral species were created. These included (i) 20 Lactobacillus species (L. acidophilus, L. brevis, L. buchneri, L. casei, L. coleohominis, L. crispatus, L. curvatus, L. fermentum, L. gallinarium, L. gasseri, L. graminis, L. jensenii, L. johnsonii, L. panis, L. paracasei, L. pentosus, L. reuteri, L. rhamnosus, L. salivarius, and L. vaginalis); (ii) 10 Streptococcus species, (S. gordonii, S. intermedius, S. mitis, S. mitis bv 2, S. mutans, S. oralis, S. parasanguinis I, S. salivarius, S. sanguinis, and S. sobrinus); and (iii) 25 species of mixed genera (Actinomyces gerencseriae, Actinomyces meyeri, Actinomyces odontolyticus, Bifidobacterium longum, Escherichia coli, Fusobacterium nucleatum, L. acidophilus, L. casei, L. coleohominis, Leptotrichia buccalis, Porphyromonas gingivalis, Prevotella denticola, Prevotella oris, Rothia dentocariosa, Scardovia wiggsiae, S. gordoni, S. intermedius, S. mitis, S. mutans, S. oralis, S. parasanguinis I, S. salivarius, S. sanguinis, S. sobrinus, and Veillonella parvula). Equal aliquots were obtained from each bacterial suspension (OD600 = 2.0), resulting in a total volume of 1 ml. All 3 mocks were used for Illumina MiSeq sequencing, and the Streptococcus and Lactobacillus mocks for PacBio SMRT sequencing.
DNA extraction
Genomic DNA was extracted from the saliva pellets, tooth biofilm samples, 3 mock communities, and a sample of ultra-pure water (negative control) with a Gene elute™ Bacterial Genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA) as described previously11. Briefly, samples were (i) centrifuged for 5 min at 13,000 rpm, (ii) lysed in buffer with lysozyme and mutanolysin for 30 min at 37 °C, (iii) treated with RNase for 2 min at room temperature followed by Proteinase K for 10 min at 55 °C, (iv) mixed with ethanol and transferred to the binding column, and (v) washed and eluted in 100 µl of elution buffer. The DNA quality and quantity were evaluated using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) to meet the standard set by the sequencing facilities (OD 260/280 ratio ≥1.8). DNA was extracted from undivided samples, and after the DNA content had been determined, the volumes at concentrations requested by the sequencing facilities were subjected to further preparation.
Sequencing
Multiplex 16S rDNA amplicon sequencing was performed using two different platforms, Illumina MiSeq (http://www.illumina.com) and PacBio RS II SMRT (http://www.pacb.com/smrt-science/smrt-sequencing/). The former analyses were performed at Forsyth Research Institute (Cambridge, MA, USA), and the latter at GATC Biotech AG (Konstanze, Germany).
For Illumina MiSeq sequencing, the HOMINGS protocol was applied as described previously30. Briefly, the V3-V4 hypervariable regions of the 16S rDNA were PCR amplified using the forward 341F (AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTCCT ACGGGAGGCAGCAG) and reverse 806R (CAAGCAGAAGACGGCATACGAGATNNNNNNNNNNNNAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT) primers (primer sequences are in bold, and sample-specific sequence tags (barcodes) are indicated by NNNNNNNNNNNN). Amplicons were purified (AMPure beads; Beckman Coulter Genomics, Danvers, MA); 100 ng of each library was pooled, gel purified, and quantified; and 20% PhiX was added to 12 pmol of the library mixture and run on the MiSeq system. Pair-end reads were fused; barcodes, primers, and ambiguous and chimeric sequences were removed; and taxa were identified by the ProbeSeq customized BLAST programme (HOMINGS, http://homings.forsyth.org/index2.html) for the recognition of 538 species by 638 probes of 17 to 40 bases followed by the detection of 129 genus-level probes for closely related species. Species/phylotypes targeted by the Genus probes are described at http://homings.forsyth.org/index2.html. Taxa represented by <10 sequences were excluded.
In parallel, the QIIME pipeline (version 1.8.0) was applied to raw sequences for comparisons, i.e., sequences were quality filtered according to QIIME default values and binned into clusters (OTUs) using UCLUST at 97.0% similarity. Potential chimaeras were removed (USEARCH), and taxonomic determination was performed by BLAST against the HOMD database for oral bacteria (www.HOMD.org). The named or unnamed species or phylotypes were identified by their HOMD Human Oral Taxon (HOT) identity. OTUs with <10 sequences were excluded.
For PacBio SMRT amplicon sequencing, hairpin adaptors were ligated to V1-V8 hypervariable regions of the 16S rDNA, sample barcodes were added to the end of the amplicons, and standard PacBio SMRT bell adapters were ligated to the barcoded amplicon24. The obtained sequences were quality filtered as described above, and potential chimaera sequences were removed. These steps were performed at GATC. Chimaera-free OTU clusters were taxonomically identified by BLAST against the HOMD database at 98.5% similarity.
The original sequencing data are available from the Figshare (accession link: 10.6084/m9.figshare.4552849).
Complementary PCR and cultivation
The presence of S. mutans in saliva and tooth biofilm was evaluated by PCR using the KAPA2G Robust HotStart PCR Ready Mix (2´) kit (Kapa Biosystems, Boston, MA, USA) as described previously11. In addition, colony forming units (CFUs) per ml of saliva for mutans streptococci (S. mutans and S. sobrinus) were assessed in fresh saliva by cultivation on mitis salivarius sucrose agar supplemented with 0.2 U of bacitracin (Becton, Dickinson and Company, Stockholm, Sweden) and incubated at 37 °C in 5% CO2 for 48 h.
Statistical analyses
Normally distributed variables are presented as means with 95% confidence intervals, and group differences were analysed using ANOVA or unpaired t-tests. The bacterial taxa mean percentage of all sequences (abundance) and the proportion of adolescents in whom the taxa were identified (prevalence) were calculated and tested with non-parametric tests (Mann-Whitney U and Chi-squared/Fisher’s exact test, respectively). Correlations were evaluated by Spearman correlation coefficients. The mean caries increment was adjusted by sex, BMI, sugar intake, and oral hygiene in general linear modelling (glm). These analyses were performed using SPSS version 23 (IBM Corporation, Armonk, NY, USA).
P-values of taxa prevalence comparisons were evaluated with Benjamini-Hochberg corrections for multiple testing, i.e., p-values ≤ 0.001 were considered significant. For other comparisons, a p-value <0.05 was considered significant. Tests were two-sided.
Rarefaction curves plotting the number of observed species as a function of the number of sequences per sample were established to compare the microbial richness (α diversity) among the samples. Principal component analysis (PCA) was used to explore the clustering of subjects by the ProbeSeq taxa, and principal coordinate analysis (PCoA) was used to define clustering by QIIME identified OTUs.
The Linear Discriminant Analysis Effect Size (LEfSe) algorithm was used to identify taxa (genus level or higher) that differed in relative abundance between the two groups38. The online Galaxy Version 1.0 interface (http://huttenhower.sph.harvard.edu) was used, and the threshold for the logarithmic LDA score was set at 1.0. The results are displayed in a cladogram and a bar graph.
Partial least square (PLS) regression was used to identify taxa associated with the caries status. The PLS models included taxa prevalence and potential lifestyle confounders, i.e., sex, BMI, oral hygiene, sugar intake, and tobacco use. The results are presented as PLS correlation coefficients from a column loading plot. The variables for which the 95% CI did not include zero were considered significant. SIMCA P+ version 12.0 (Umetrics AB, Umeå, Sweden) was used for PCA and PLS.
Ethical approval
The study was approved by the Regional Ethical Review Board in Umeå, Sweden (Dnr 2012-111-31 M) with an addendum for the 2-year follow up (Dnr 2015-389-32 M). All experiments and data collections were performed in accordance with relevant guidelines and regulations, including that all adolescents and their caregivers provided informed consent and data collection and handling followed the Helsinki declaration and the Swedish Law on personal data act (PuL).
Electronic supplementary material
Acknowledgements
The present study was supported by grants from The Swedish Patent Revenue Foundation and TUA, the County Council of Västerbotten, Sweden. None of the funding bodies had any influence on the design, data collection, analysis, interpretation or writing of the manuscript. Carina Öhman and Agnetha Rönnlund are acknowledged for skilful laboratory work.
Author Contributions
I.J. and P.L.H. designed the study, granted various permissions, and provided laboratory supplies, L.E. and I.J. compiled and analysed data, L.E. and I.J. drafted the manuscript and all authors contributed to the final article.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06221-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Chapple IL, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017;44(Suppl 18):S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst FE, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilian M, et al. The oral microbiome - an update for oral healthcare professionals. Br. Dent. J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 5.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J. Dent. Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 7.Ruoff KL. Nutritionally variant streptococci. Clin. Microbiol. Rev. 1991;4:184–190. doi: 10.1128/CMR.4.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma C, et al. Comparison of Oral Microbial Profiles between Children with Severe Early Childhood Caries and Caries-Free Children Using the Human Oral Microbe Identification Microarray. PLoS One. 2015;10:e0122075. doi: 10.1371/journal.pone.0122075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanner AC, et al. Microbiota of severe early childhood caries before and after therapy. J. Dent. Res. 2011;90:1298–1305. doi: 10.1177/0022034511421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantzourani M, et al. The isolation of bifidobacteria from occlusal carious lesions in children and adults. Caries Res. 2009;43:308–313. doi: 10.1159/000222659. [DOI] [PubMed] [Google Scholar]
- 11.Johansson I, Witkowska E, Kaveh B, Lif Holgerson P, Tanner AC. The Microbiome in Populations with a Low and High Prevalence of Caries. J. Dent. Res. 2016;95:80–86. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klock B, Krasse B. Caries status and microbial conditions in children in 1973 and 1984. Scand. J. Dent. Res. 1987;95:13–17. doi: 10.1111/j.1600-0722.1987.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012;6:1–10. doi: 10.1038/ismej.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belstrom D, Paster BJ, Fiehn NE, Bardow A, Holmstrup P. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J. Oral Microbiol. 2016;8:30170. doi: 10.3402/jom.v8.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, J. et al. Influences of pH and iron on the salivary microbiome in individuals with and without caries. Appl. Environ. Microbiol. Epub ahead of print (2016). [DOI] [PMC free article] [PubMed]
- 16.Franzen O, et al. Improved OTU-picking using long-read 16S rRNA gene amplicon sequencing and generic hierarchical clustering. Microbiome. 2015;3:43. doi: 10.1186/s40168-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, T. et al. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford), baq013 (2010). [DOI] [PMC free article] [PubMed]
- 18.Splieth CH, Christiansen J, Foster Page LA. Caries Epidemiology and Community Dentistry: Chances for Future Improvements in Caries Risk Groups. Outcomes of the ORCA Saturday Afternoon Symposium, Greifswald, 2014. Part 1. Caries Res. 2016;50:9–16. doi: 10.1159/000442713. [DOI] [PubMed] [Google Scholar]
- 19.Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benitez-Paez A, Belda-Ferre P, Simon-Soro A, Mira A. Microbiota diversity and gene expression dynamics in human oral biofilms. BMC Genomics. 2014;15:311. doi: 10.1186/1471-2164-15-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belda-Ferre P, et al. The human oral metaproteome reveals potential biomarkers for caries disease. Proteomics. 2015;15:3497–3507. doi: 10.1002/pmic.201400600. [DOI] [PubMed] [Google Scholar]
- 22.Wade WG. The oral microbiome in health and disease. Pharmacol. Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, et al. Exploration of Human Salivary Microbiomes–Insights into the Novel Characteristics of Microbial Community Structure in Caries and Caries-Free Subjects. PLoS One. 2016;11:e0147039. doi: 10.1371/journal.pone.0147039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Jenior ML, Koumpouras CC, Westcott SL, Highlander SK. Sequencing 16S rRNA gene fragments using the PacBio SMRT DNA sequencing system. PeerJ. 2016;4:e1869. doi: 10.7717/peerj.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner J, et al. Evaluation of PacBio sequencing for full-length bacterial 16S rRNA gene classification. BMC Microbiol. 2016;16:274. doi: 10.1186/s12866-016-0891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolenbrander PE. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu. Rev. Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- 27.Heller, D., Helmerhorst, E. J. & Oppenheim, F. G. Saliva and Serum Protein Exchange at the Tooth Enamel Surface. J. Dent. Res. Epub ahead of print (2016). [DOI] [PMC free article] [PubMed]
- 28.David LA, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norderyd O, et al. Oral health of individuals aged 3–80 years in Jonkoping, Sweden during 40 years (1973–2013). II. Review of clinical and radiographic findings. Swed. Dent. J. 2015;39:69–86. [PubMed] [Google Scholar]
- 30.Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J. Endod. 2015;41:1975–1984. doi: 10.1016/j.joen.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon-Soro A, et al. Microbial geography of the oral cavity. J. Dent. Res. 2013;92:616–621. doi: 10.1177/0022034513488119. [DOI] [PubMed] [Google Scholar]
- 32.Nyvad B, Crielaard W, Mira A, Takahashi N, Beighton D. Dental caries from a molecular microbiological perspective. Caries Res. 2013;47:89–102. doi: 10.1159/000345367. [DOI] [PubMed] [Google Scholar]
- 33.Foxman B, et al. The effects of family, dentition, and dental caries on the salivary microbiome. Ann. Epidemiol. 2016;26:348–354. doi: 10.1016/j.annepidem.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner AC. Anaerobic culture to detect periodontal and caries pathogens. J. Oral Biosci. 2015;57:18–26. doi: 10.1016/j.job.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henne K, Rheinberg A, Melzer-Krick B, Conrads G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe. 2015;35:60–65. doi: 10.1016/j.anaerobe.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Valdez RM, et al. Comparative in vitro investigation of the cariogenic potential of bifidobacteria. Arch. Oral Biol. 2016;71:97–103. doi: 10.1016/j.archoralbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Soderstrom U, Johansson I, Sunnegardh-Gronberg K. A retrospective analysis of caries treatment and development in relation to assessed caries risk in an adult population in Sweden. BMC Oral Health. 2014;14:126. doi: 10.1186/1472-6831-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


