Abstract
We conducted a meta-analysis to analyse the effect of metformin on survival of pancreatic cancer patients at various stages. We performed a systematic search of PubMed, Embase, Cochrane, and Web of Science to identify all relevant studies. Summary hazard ratios (HR) of survival and 95% confidence intervals (95% CI) were calculated with a fixed or random effects model according to inter-study heterogeneity. Nine retrospective cohort studies and two randomized controlled trials (RCTs) were eligible. There was a significant improvement in survival (HR = 0.86, 95% CI 0.76–0.97; P < 0.05) in the metformin group compared with control. Subgroup analysis indicated that metformin improved survival in patients with resection (HR = 0.79, 95% CI 0.69–0.91; P < 0.05) and patients with locally advanced tumors (HR = 0.68, 95% CI 0.55–0.84; P < 0.05) but not in patients with metastatic tumors, even when RCT data were included (HR = 0.99, 95% CI 0.70–1.40; P > 0.05), or were excluded (HR = 0.89, 95% CI 0.61–1.31; P > 0.05). This meta-analysis indicated that the effect of metformin does correlate with tumor stage but should be prudently considered given the limited and variable studies performed to data.
Introduction
Pancreatic cancer is among the most lethal of malignancies1–4. Each year, 53,000 new cases are diagnosed with this disease, which leads to approximately 42,000 deaths annually in the United States5. Limited advances have been made regarding treatment developments and the disease prognosis remains poor, with the 5-year overall survival rate ranging from 3–6% with a median survival of < six months6–9. Even in patients at early stages of the disease who receive surgical resection, the 5-year survival rate is no more than 24% due to a high rate of local recurrence and metastasis10–13.
Type 2 diabetes mellitus is considered to play important roles in tumorigenesis and development of pancreatic cancer. Insulin resistance and up-regulated insulin-like growth factor I in type 2 diabetes mellitus are proposed to be the mechanisms underpinning the disease onset. Metformin is an antidiabetic drug commonly prescribed for patients with type 2 diabetes mellitus. Recently, increasing evidence has indicated that metformin can decrease the risk of developing pancreatic cancer in patients with concurrent type 2 diabetes mellitus14, 15. However, this effect has not been observed with other antidiabetic agents. Moreover, subsequent experimental studies have also confirmed the antitumor effect of metformin both in vitro and in vivo, which provided a rationale for clinical use of metformin in cancer treatment. However, there is still no consensus relating to the use of metformin in pancreatic cancer.
Several meta-analyses have shown that metformin decreases the risk of pancreatic cancer16–21 but only one meta-analysis has been conducted, which explored the association between metformin and survival of pancreatic cancer patients22. In the meta-analysis, four studies were included and the results showed no evidence for metformin being either harmful or beneficial with regard to survival of pancreatic cancer patients (HR = 0.80, 95% CI 0.62–1.03; P > 0.05). However, some studies have suggested that the effect of metformin delivery varied among pancreatic cancer patients with different tumor stage, and therefore analysis of subgroups by tumor stage was performed23–27. In addition, all studies involved in the meta-analysis were cohort studies, and the results of two RCTs28, 29 have been published recently. Consequently, further meta-analysis is required. To this end, we performed a meta-analysis on nine cohort studies23–27, 30–33 and two RCTs in order to determine the effect of metformin on survival of pancreatic cancer patients. In addition, subgroup analysis was performed to determine whether tumor stage affected the response to metformin.
Results
Literature search
The selection process for the literature search is summarized in Fig. 1. Initially, a total of 1134 articles were identified form the search of the four databases. After reviewing titles and abstracts for relevance, 32 articles were evaluated further for eligibility. Of the 32 articles, 21 were excluded: nine articles were conference abstracts without detailed data; five articles pertained to the same study; three articles did not report the outcomes of HR, and only provided the mean survival time; and in another four articles, there were no control groups. Thus, nine cohort studies and two RCTs were included in this systematic review. The blinding method, randomization, generation of random sequences, and allocation concealment of the two RCTs are shown in Supplementary Table S1 and Figure S1.
Figure 1.
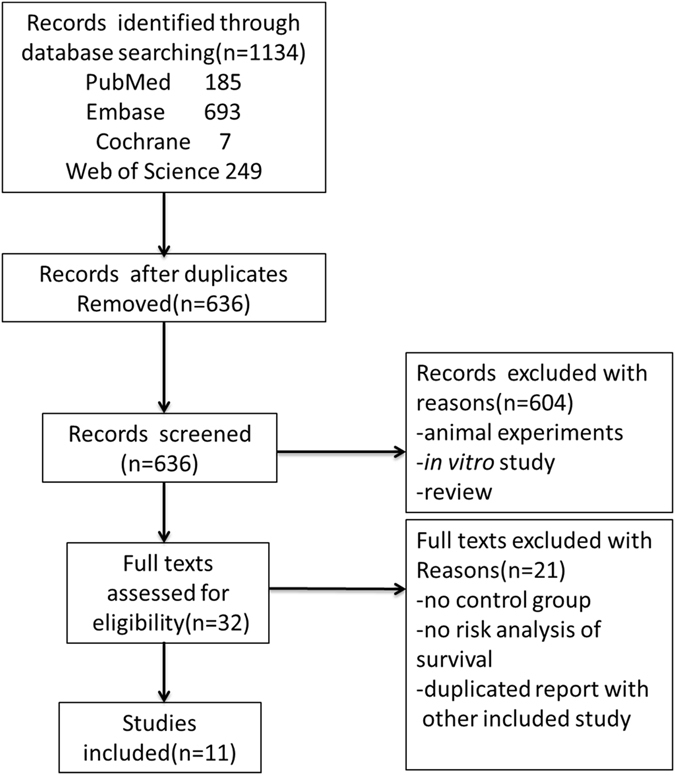
PRISMA flowchart summarizing the study identification and selection.
Quality of studies
The Jadad score34 of the two RCTs was 5 and 2, respectively. The trial of Reni et al. was of low quality (≤2), and the trial of Kordes et al. was of high quality (>2), according to the Jadad score. The risks of bias in nine retrospective cohort studies are shown in Supplementary Table S2. Of the nine retrospective cohort studies, the NOS scores of five studies were seven, and the remaining four studies were eight. All of the retrospective cohort studies were of high quality (≥7).
Study characteristics
The characteristics of 11 included studies are shown in Table 1. In the two RCTs included in this meta-analysis, a total of 181 patients with locally advanced or metastatic pancreatic cancer that could not be treated with resection, were studied. Patients in the study group of Reni et al. were treated with cisplatin, epirubicin, capecitabine, gemcitabine (PEXG) and metformin. In the study of Kordes et al., patients in the study group were treated with gemcitabine, erlotinib, and metformin given orally as a step-up dose. Meanwhile, patients in the control group received gemcitabine, erlotinib, and placebo.
Table 1.
Characteristics of included studies.
| study | design | tumor stage | size | treatment or metformin exposure in study group | intervention in control group | HR(95% CI) |
|---|---|---|---|---|---|---|
| Ambe 2015 | RCS | resectable | 44 | ongoing metformin use | never used metformin | 0.54(0.16–1.86) |
| Amin 2016 | RCS | all stages | 1916 | use in the 6 months prediagnosis. | diabetic medications other than metformin | 0.88(0.81–0.96) |
| Cerullo 2016 | RCS | resectable | 3396 | use following surgery | no use after surgery | 0.79(0.67–0.93) |
| Chaiteerakij 2016 | RCS | all stages | 980 | ever use | ever use | 0.93(0.81–1.07) |
| Choi 2016 | RCS | LA; metastatic | 349 | unclear | unclear | 0.70(0.49–1.99) |
| Hwang 2013 | RCS | LA; metastatic | 516 | use at any time in the peridiagnosis period | no use during peridiagnosis period | 1.11(0.89–1.38) |
| Kordes 2015 | RCT | LA; metastatic | 121 | GE + metformin | GE + placebo | 1.05(0.72–1.55) |
| Kozak 2016 | RCS | resectable | 171 | continuous use from first consult to discharge postsurgery | never use or discontinued use | 0.60(0.21–1.67) |
| Lee 2016 | RCS | all stages | 237 | cumulative use ≥ 1 month post-diagnosis | use < 1 month or never use | 0.61(0.46–0.81) |
| Reni 2016 | RCT | metastatic | 60 | PEXG + metformin | PEXG | 1.56(0.87–2.80) |
| Sadeghi 2012 | RCS | all stages | 302 | ever use | never use | 0.64(0.48–0.86) |
Abbreviations: RCS = retrospective cohort study; PA = pancreatic adenocarcinoma; peridiagnosis period = between 6 months prediagnosis and one month postdiagnosis; GE = gemcitabine + erlotinib; PEXG = cisplatin + epirubicin + capecitabine + gemcitabine; all stages = resectable + locally advanced + metastatic; LA = locally advanced.
In the nine retrospective cohort studies, one trial did not offer clear definition of metformin exposure, and the other eight trials gave different definitions for metformin exposure. Two trials defined metformin exposure as ever having used it, while one trial defined exposure as ongoing metformin use. In addition, one trial defined metformin exposure as use in the six months before diagnosis, while another trail defined exposure as ever having used the drug in the peri-diagnosis period. Moreover, one trial did not define metformin exposure clearly, yet the entire cohort of patients in the study group was recorded as having been treated with the drug following surgery. The study of Kozak et al. defined metformin exposure as continuous use from the first consultation to discharge after surgery, and the study by Lee et al. defined exposure as cumulative use at one month or longer after diagnosis.
The studies were conducted in four countries: the USA, Italy, Korea, and the Netherlands. In addition, most of these were performed in the last two years. In total, 8089 participants were included in these studies. Among the 8089 participants, 2792 were included in the metformin use group, while 5267 cases were included in the non-metformin use group. Three studies included patients at the resectable stage; one RCT included patients at the metastatic stage; another three studies included patients at a locally advanced or metastatic stage; and four studies included patients at either resectable, locally advanced, or metastatic stage.
Meta-analysis
The patient survival data are shown in Fig. 2A. All nine cohort studies and two RCTs, including 2792 patients in the metformin use group, reported patient survival as an outcome. The metformin group had significantly better survival compared with the control group (HR = 0.86, 95% CI: 0.76–0.97, P = 0.01). In addition, there was significant heterogeneity between the studies (χ2 = 23.61, df = 10; P < 0.01; I 2 = 57.6%). Sensitivity analysis was conducted by excluding one study at a time. The study by Lee et al. was identified as largely contributing to heterogeneity. After excluding that particular study, the inter-study heterogeneity weakened (χ2 = 17.23, df = 9; P < 0.05; I 2 = 47.8%), and the overall result was not affected (pooled HR = 0.89, 95% CI 0.83–0.94; P < 0.01, Fig. 2B).
Figure 2.
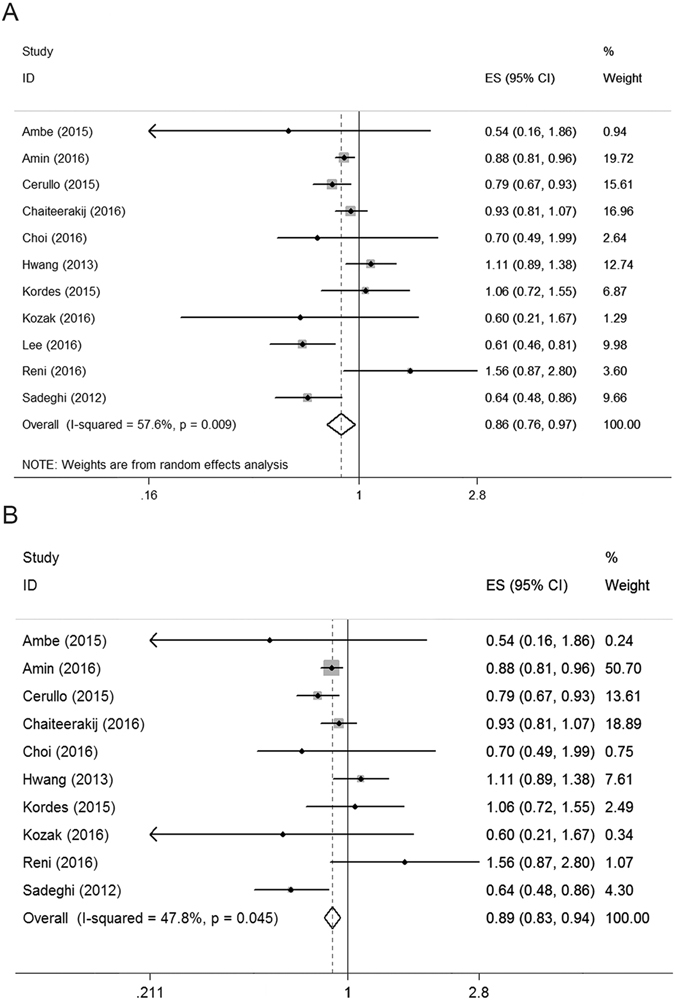
Forest plot for overall survival in all included studies (A) and sensitivity analysis excluded one study (B).
Subgroup analysis by tumor stage
Analysis of subgroups by tumor stage was performed. In the subgroup of patients at a resectable stage that included six retrospective cohort studies (4012 patients), a significantly better survival was observed in the metformin group compared to the non-metformin group (HR = 0.79, 95% CI: 0.69–0.91, P < 0.01, Fig. 3A). In the subgroup of patients with local advanced tumors, which included three retrospective cohort studies (548 patients), a significantly better survival was also observed in the metformin group compared to the control group (HR = 0.68, 95% CI: 0.55–0.84, P < 0.01, Fig. 3B). In the subgroup of metastasis patients, which included three retrospective cohort studies and one RCT (319 patients), the difference between the metformin and control group was not significant when the RCT was included (HR = 0.99, 95% CI: 0.70–1.40, P = 0.95, Fig. 4A) or not (HR = 0.89, 95% CI: 0.61–1.31, P = 0.56, Fig. 4B).
Figure 3.
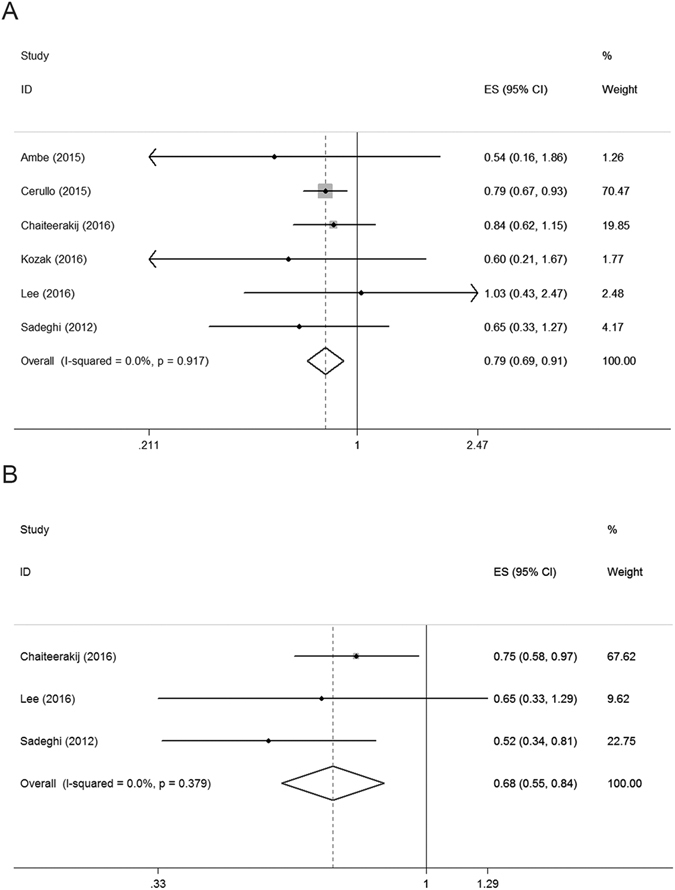
Forest plot for survival in patients at resectable stage (A) and locally advanced stage (B).
Figure 4.
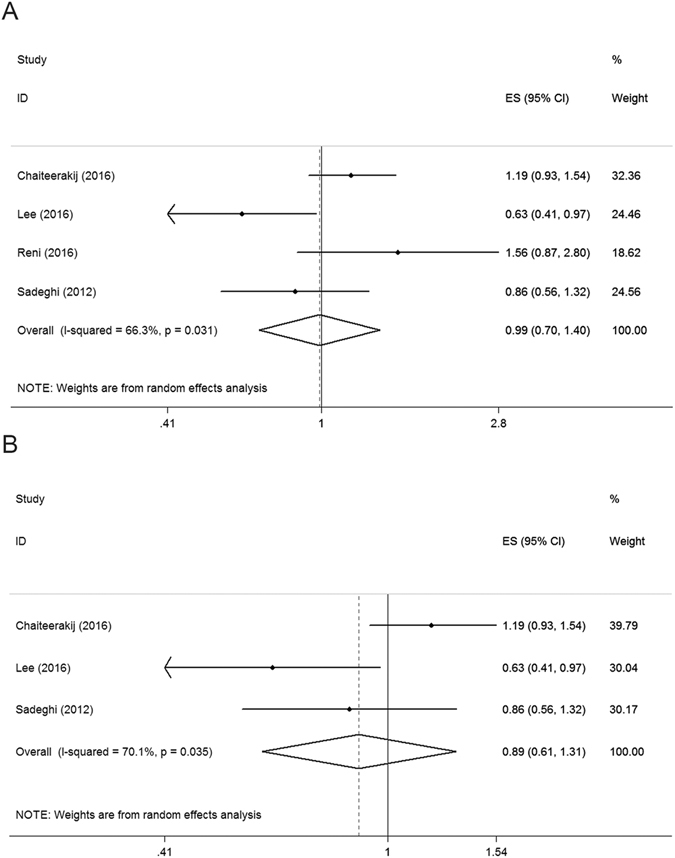
Forest plot for survival in patients at metastatic stage (A) with RCT, (B) without RCT).
Publication bias
No significant publication bias was found by funnel plots and Begg’s test (P = 0.64, Supplementary Figure S2).
Discussion
In this meta-analysis of cohort studies and RCTs investigating metformin in patients with pancreatic cancer, we demonstrated that metformin significantly benefited patients with pancreatic cancer with regard to survival. However, the later the tumor stage, the more obscure the effect of metformin with respect to its efficacy.
The antitumor effect of metformin has been suggested both by epidemiological data and laboratory studies, which provided the rationale for the clinical use of metformin in cancer treatment. Zhang et al. reported a meta-analysis of four cohort studies with significant heterogeneity, and the source of heterogeneity was not further analyzed. In recent years, a number of cohort studies and two RCTs have been conducted on the same subject, and the RCTs showed a different trend with respect to the effect of metformin on pancreatic cancer patients compared with the cohort studies. Therefore, we conducted this new meta-analysis on all nine cohort studies and two RCTs with particular focus on the source of the heterogeneity. The results indicate that tumor stage may contribute greatly to the heterogeneity of the results.
Although the results of the two RCTs were inconsistent with a meta-analysis of previous cohort studies i.e., the benefit of metformin on pancreatic cancer demonstrated in the meta-analysis was not corroborated in the RCTs, this can be explained by the heterogeneity for assigned patients. As shown in Figs 3 and 4, the meta-analysis showed that metformin only benefits patients without metastatic disease depending on whether the RCTs were included, but the assigned patients in both of the RCTs included metastatic pancreatic cancer. The trial of Reni et al. included only metastatic pancreatic cancer patients and the trial of Kordes et al. included both locally advanced and metastatic pancreatic cancer patients. We tentatively postulate that the involvement of metastatic pancreatic cancer patients contributed greatly to the results. Due to the significant heterogeneity between subgroups and the lack of RCTs with resectable pancreatic cancer patients included, we also retrieved clinical trials focusing on the effect of metformin on other solid tumors from PubMed to serve as a reference. Four trials showed that metformin benefited patients with resectable cancer in colorectal cancer35, gastric cancer36, endometrial adenocarcinoma37, and bladder cancer38, while one RCT showed no beneficial evidence for metformin on metastatic cancer in non-small cell lung cancer39. These findings also suggest that tumor stage plays an important role in the heterogeneity between studies.
The exact reason for the heterogeneity between different stages of tumors is unknown. We tentatively speculate that the concentration of metformin in the tumor tissue may play an important role. In patients with metastatic disease, tumor per se was always dense irrespective of large tumor burden, thus the concentration of metformin in neoplastic tissue meant that it was difficult to accumulate drug at a therapeutic level. Therefore, the treatment effect on patients in this group is difficult to explain. However, in patients with resection, plasma metformin might act directly on circulating cancer cells and micro-lesions, which are not macroscopic. Hence, patients in this group had an improved survival with metformin. Furthermore, Kordes et al. and Cerullo et al. also observed an improved survival in patients treated with metformin in a dose-dependent manner, which means that survival benefit increased with a rising daily dose. The improved survival of patients with a higher daily dose may indirectly reflect the influence of insufficient concentration of metformin in neoplastic tissue and its subsequent antitumor effect.
The two RCTs, which are more robust than cohort studies in terms of methodology, showed no beneficial effect of metformin. Due to these disappointing results, which were inconsistent with cohort studies and laboratory studies, the impact of metformin on the survival of pancreatic cancer patients seems very limited. However, the tumor stage varied in clinical trials. Moreover, results of cohort studies were consistent with RCTs, if only metastasis cancer patients were involved. Therefore, the results of the two RCTs are not sufficient to infer that metformin has no beneficial effect on the treatment of patients with pancreatic cancer, especially resectable cancer. Furthermore, perhaps what is more important in the trial by Reni and colleagues is that they evaluated not only the effect of metformin on survival but also the drug’s toxicity to patients. Their study demonstrated that metformin combined with chemotherapeutic agents was well tolerated, which has paved the way for subsequent studies.
The use of metformin in pancreatic cancer is similar to that of immunomodulatory treatment of critical illnesses. Both treatments have been documented to be beneficial in animal experiments and primary clinical studies but subsequent randomized clinical trials have demonstrated no beneficial effect or adverse effects, which made the treatments questionable40. However, subsequent studies using immunomodulators have indicated that certain populations of patients do benefit from these treatments41, 42. Similarly, since the trials were heterogeneous with regards to the tumor stage of pancreatic cancer, it is still possible that patients with resectable or locally advanced pancreatic cancer can benefit from metformin. According to the present meta-analysis and systematic review, metformin has the potential to benefit pancreatic cancer patients, especially those patients with tumor at an early stage. Therefore, further clinical trials should focus on patients with resectable cancer.
In interpreting the results of this meta-analysis, both the limited number of available studies and the methodological quality of the included trials should be noted. Of the 11 studies included, only two studies were RCTs, and the methodological quality of one trial was relatively low. Considerable heterogeneity was observed among these trials with regards to the effect of metformin. However, the limited number of studies hampered the power of the subgroup analysis conducted due to heterogeneity. Therefore, there is clearly a need for further investigations that include robust methods and analysis.
Materials and Methods
Systematic literature search
A systematic electronic search was conducted in the PubMed, Embase, Cochrane, and Web of Science databases. The searches were restricted to human disease published before July 23, 2016. The keywords “metformin” and “pancreatic cancer” were used but language restriction was not imposed. Search results were imported into a database and the duplicate records were removed. The titles and abstracts were then screened for relevance. If more than one article was published by the same research team on the same topic, only the most recent article was selected in our study. Next, the full-text papers were checked for eligibility. If the paper was not published in English, we sent an e-mail to the author to ask for an English edition. If an English edition was provided, the study was included. If not, it was eliminated. There was no need for participant consent in this review, since studies included were published without personal identifiable information.
Inclusion and exclusion criteria
We included all human cohort studies and RCTs that investigated the effects of metformin on survival of pancreatic cancer patients. We excluded: (1) cohorts that had no control group, (2) studies that did not report the essential outcomes (survival with HR and 95% CI), and (3) cohorts that included patients with other types of pancreatic tumor (e.g., pancreatic neuroendocrine tumors) and those that did not report the results for pancreatic cancer (adenocarcinoma) separately. The literature retrieval and screening were conducted respectively by two authors (LXG and LT), and a third author (GSM) was consulted when there was uncertainty or disagreement.
Data extraction
From the included studies, the following information was extracted: design of study, tumor stage, sample size, treatment strategy or exposure to metformin in study group, intervention in the control group, and survival. If the original data for the meta-analysis were not provided directly in the text, then we sent an e-mail to the corresponding author or extracted data from Kaplan-Meier curves in a previously described way43, 44.
Quality assessment
The internal validity for RCTs was determined using the Jadad scale and risk bias tool of the Cochrane Collaboration. The Newcastle-Ottawa Scale (NOS), including eight quality criteria, was applied to evaluate the quality of retrospective cohort studies.
Statistical analysis
All summarized data were analyzed using Stata, Windows version 12.0. The HR with 95% CI was used as a surrogate for survival effect. Heterogeneity between different trials was assessed by the chi-square test, and the extent of inconsistency was evaluated by the I 2 statistic. A Mantel-Haenszel random-effects model was used for data if heterogeneity was significant; otherwise, the fixed-effects model was applied. Sensitivity analysis was conducted to assess the impact of each study on the pooled HR and heterogeneity by removing one study at a time. Publication bias was investigated by funnel plot analysis using Begg’s regression test if sufficient studies were included. Two-tailed P-values < 0.05 were considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
The authors thank Dr. Donghui Li at the University of Texas MD Anderson Cancer Center for kindly providing unpublished data of her study. This work was supported by a grant from the National Natural Science Foundation of China (No. 81472309).
Author Contributions
X.G.L., T.L. and S.M.G. designed the experiments. X.G.L., T.L., Z.Q.L., S.M.G., and C.Y.W. carried out the experiments and calculations. X.G.L., T.L., S.M.G., and C.Y.W. wrote and edited the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06207-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shanmiao Gou, Email: gousm@163.com.
Chunyou Wang, Email: chunyouwang52@126.com.
References
- 1.Andriulli A, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–1662. doi: 10.1245/s10434-011-2110-8. [DOI] [PubMed] [Google Scholar]
- 2.Davis JL, Pandalai PK, Ripley RT, Langan RC, Avital I. Expanding surgical treatment of pancreatic cancer: the role of regional chemotherapy. Pancreas. 2012;41:678–684. doi: 10.1097/MPA.0b013e318249955a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragovich T. Is there a case for personalized therapy of pancreatic cancer? Clin Adv Hematol Oncol. 2012;10:344–345. [PubMed] [Google Scholar]
- 4.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17–26. doi: 10.1097/SLA.0b013e31825ffbfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Kampen JG, et al. Epigenetic targeting in pancreatic cancer. Cancer Treat Rev. 2014;40:656–664. doi: 10.1016/j.ctrv.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995-1999. Am J Epidemiol. 2011;174:1373–1381. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet (London, England) 2015;385:1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 10.Howard, T. J. et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract10, 1338–1345; discussion 1345–1336 (2006). [DOI] [PubMed]
- 11.Johnston WC, et al. Total pancreatectomy for pancreatic ductal adenocarcinoma: review of the National Cancer Data Base. HPB (Oxford) 2016;18:21–28. doi: 10.1016/j.hpb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 13.Oettle H, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 14.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, et al. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:510–519. doi: 10.1038/ajg.2013.7. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Soranna D, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 20.Decensi, A. et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 3 (2010). [DOI] [PubMed]
- 21.Wang, Z. et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 106 (2014). [DOI] [PubMed]
- 22.Zhang JW, Sun Q. Metformin may improve the prognosis of patients with pancreatic cancer. Asian Pac J Cancer Prev. 2015;16:3937–3940. doi: 10.7314/APJCP.2015.16.9.3937. [DOI] [PubMed] [Google Scholar]
- 23.Cerullo M, Gani F, Chen SY, Canner J, Pawlik TM. Metformin Use Is Associated with Improved Survival in Patients Undergoing Resection for Pancreatic Cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2016;20:1572–1580. doi: 10.1007/s11605-016-3173-4. [DOI] [PubMed] [Google Scholar]
- 24.Chaiteerakij R, et al. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. J Clin Oncol. 2016;34:1898–1904. doi: 10.1200/JCO.2015.63.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozak MM, et al. Statin and Metformin Use Prolongs Survival in Patients With Resectable Pancreatic Cancer. Pancreas. 2016;45:64–70. doi: 10.1097/MPA.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, et al. Can metformin change the prognosis of pancreatic cancer? Retrospective study for pancreatic cancer patients with pre-existing diabetes mellitus type 2. Dig Liver Dis. 2016;48:435–440. doi: 10.1016/j.dld.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kordes S, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 29.Reni M, et al. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22:1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 30.Ambe CM, Mahipal A, Fulp J, Chen L, Malafa MP. Effect of Metformin Use on Survival in Resectable Pancreatic Cancer: A Single-Institution Experience and Review of the Literature. PLoS One. 2016;11:e0151632. doi: 10.1371/journal.pone.0151632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin S, et al. Metformin Improves Survival in Patients with Pancreatic Ductal Adenocarcinoma and Pre-Existing Diabetes: A Propensity Score Analysis. Am J Gastroenterol. 2016;111:1350–1357. doi: 10.1038/ajg.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi Y, et al. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res Treat. 2016;48:171–179. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang AL, Haynes K, Hwang WT, Yang YX. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42:1054–1059. doi: 10.1097/MPA.0b013e3182965a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 35.Spillane S, Bennett K, Sharp L, Barron TI. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1364–1373. doi: 10.1158/1055-9965.EPI-13-0347. [DOI] [PubMed] [Google Scholar]
- 36.Lee CK, et al. Cumulative Metformin Use and Its Impact on Survival in Gastric Cancer Patients After Gastrectomy. Ann Surg. 2016;263:96–102. doi: 10.1097/SLA.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 37.Hawkes AL, et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device +/− metformin +/− weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials. 2014;39:14–21. doi: 10.1016/j.cct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Nayan, M. et al. The effect of metformin on cancer-specific survival outcomes in diabetic patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Urol Oncol33, 386 e387–313 (2015). [DOI] [PubMed]
- 39.Sayed R, Saad AS, El Wakeel L, Elkholy E, Badary O. Metformin Addition to Chemotherapy in Stage IV Non-Small Cell Lung Cancer: an Open Label Randomized Controlled Study. Asian Pac J Cancer Prev. 2015;16:6621–6626. doi: 10.7314/APJCP.2015.16.15.6621. [DOI] [PubMed] [Google Scholar]
- 40.Bower RH, et al. Early enteral administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med. 1995;23:436–449. doi: 10.1097/00003246-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Montejo JC, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr. 2003;22:221–233. doi: 10.1016/S0261-5614(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm SM, Kale-Pradhan PB. Combination of arginine and omega-3 fatty acids enteral nutrition in critically ill and surgical patients: a meta-analysis. Expert Rev Clin Pharmacol. 2010;3:459–469. doi: 10.1586/ecp.10.29. [DOI] [PubMed] [Google Scholar]
- 43.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).


