Abstract
Haploidentical transplantation performed with post-transplantation cyclophosphamide (PTCy)-based graft-versus-host disease (GVHD) prophylaxis has been associated with favorable outcomes for patients with acute myeloid leukemia and lymphomas. However, it remains unclear if such approach is effective for patients with acute lymphoblastic leukemia (ALL). We analyzed outcomes of 109 consecutively treated ALL patients 18 years of age and older at 5 institutions. The median age was 32 years and the median follow-up for survivors was 13 months. Thirty-two patients were in first complete remission (CR1), while the rest were beyond CR1. Neutrophil engraftment occurred in 95% of the patients. The cumulative incidence (CI) of grades II–IV and III–IV acute GVHD at day 100 post-transplant was 32% and 11%, while chronic GVHD, non-relapse mortality, relapse rate and disease-free survival (DFS) at 1 year post-transplant were 32%, 21%, 27% and 51%, respectively. Patients in CR1 had 52% DFS at 3 years. These results suggest that haploidentical transplants performed with PTCy-based GVHD prophylaxis provide an excellent alternative to HLA matched transplants for patients with ALL.
Keywords: Acute lymphoblastic leukemia, haploidentical transplant
Introduction
Acute lymphoblastic leukemia (ALL) has an age-adjusted incidence rate of 1.73 per 100,000 person-years in United States (US) with a median age of 14 years.1 Approximately 6,590 new cases and 1,430 deaths are estimated for 2016.2 There have been notable improvements in cure rates of childhood ALL over past several decades with 5-year overall survival (OS) rates exceeding 80% in children, but only approximately 40% among adults.1, 3, 4 However, over 60% of adults will relapse;5, 6 these patients usually have poor long-term outcomes with median OS of <10 months.7, 8
There has been contradictory evidence about the role of frontline allogeneic hematopoietic stem-cell transplant (ASCT) for patients in first complete remission (CR1).9 Two recent meta-analyses showed potential benefit.10, 11 In contrast, for patients with relapsed/refractory ALL, ASCT remains the only potential cure.6, 7, 12, 13 Gokbuget et al. reported outcomes of 547 ALL patients in first relapse where the 3-year OS was 38% for patients who underwent ASCT, while none of the non-transplant patients survived beyond one year.7
The preferred donor for transplantation is an HLA-matched sibling (MSD), while a matched unrelated donor (MUD) is considered a suitable alternative;12 however, MUD availability varies widely with recipient’s race.14 Recently, haploidentical donors have emerged as an important alternative donor source due to the use of post-transplantation cyclophosphamide (PTCy) for prevention of graft-versus-host disease (GVHD).15, 16 Several recent disease-specific studies showed favorable outcomes for patients with acute myeloid leukemia (AML) and lymphoma using PTCy GVHD prophylaxis,17, 18 but remains unclear if patients with ALL would benefit from this approach as well. In a large cancer registry database study, Ruggeri et al. showed no significant differences in ALL outcomes between cord transplant and haploidentical donor alternatives.19 However, in this study different GVHD prophylaxis regimens were used and not limited to PTCy-based. We seek from this report to present the largest multicenter observational study in adult ALL patients assessing the feasibility and efficacy of haploidentical stem cell transplantation (HSCT) with PTCy GVHD prophylaxis.
Methods
Between 10/2005–11/2015, 124 consecutive patients with ALL underwent HSCT with PTCy at five centers, 4 in US (MD Anderson Cancer Center, Houston, TX; City of Hope National Medical Center, Duarte, CA; Washington University School of Medicine, St. Louis, MO; Northside Hospital, Atlanta, GA) and 1 in Colombia (Instituto de Cancerologia, Medellin, Colombia). Patients have been followed through June 2016. Pediatric patients (age of <18 years) were excluded from this study (n=15 patients) while patients who had a haploidentical transplant as a second HSCT (n=13) were analyzed separately. Institutional Review Board from each institution approved this study.
The primary endpoint was disease-free survival (DFS). Secondary endpoints included OS, cumulative incidence (CI) of non-relapse mortality (NRM), relapse, acute GVHD (aGVHD), and chronic GVHD (cGVHD). Minimal residual disease (MRD) was defined as any evidence of detectable disease by cytogenetics, flow cytometry and/or polymerase chain reaction (PCR) for patients in morphologic remission at transplant; PCR was performed for the clonal immunoglobulin gene and/or T-cell receptor gene rearrangements. Acute and chronic GVHD were graded according to standard criteria.20, 21
Statistical methods
DFS was computed from date of transplant to date of disease progression or death (if died without disease progression) or the last evaluation date. Patients who were alive and did not experience progression of disease at the last follow-up date were censored. OS was computed from date of transplant to last known vital sign. Patients alive at the last follow-up date were censored. The Kaplan-Meier method was used to estimate OS and DFS. Differences in DFS between groups were assessed using the log-rank test. The association between DFS and patient subgroups was determined using Cox proportional hazards regression models. The cutoff p value used to include univariate risk factors in multivariate analyses was <0.1. The cumulative incidence (CI) of NRM, relapse, and GVHD were determined using the competing risks method. The competing risk for NRM included relapse, and for the CI for relapse included death; patients who were still alive at the last follow-up date were censored. For GVHD, the competing risks included relapse and death, while those patients who did not experience GVHD, did not relapse, and were still alive at the last follow-up date were censored. All statistical analyses were performed using SAS 9.3 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC). All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Patient, disease, and transplant characteristics are summarized in Table 1. Among the 109 patients included in the study, 96 patients had their first transplant, while 13 patients had a HSCT as a second transplant. The majority of patients (79%) had B-cell ALL. Median age at transplant was 31.9 years (range 18.2–66.4). Median time from diagnosis to transplant was 16.7 months (range 2.6–161.1), with 32 patients (29%) having their transplant in CR1, 36 (33%) in CR2, and 41 (38%) were beyond CR2 or had primary refractory disease. Details about stem cell source and conditioning regimens are listed in Table 1. Myeloablative conditioning was used in 70 patients (64%). All patients received PTCy (50mg/kg on day+3 and day+4) for GVHD prophylaxis, along with mycophenolate mofetil (MMF) (100%) and tacrolimus (79%) or cyclosporine. Thirty-one (28%) of the patients experienced disease progression and 51% of the patients died during the assessment period. The median follow up of the surviving patients was 12.8 months (range 0.2–55.9).
Table 1.
Patient, Transplant, and Disease Characteristics
| Measure | All Patients* (N=109) |
|---|---|
| Gender, n (%) | |
|
| |
| Male | 64 (59) |
| 45 (41) | |
|
| |
| Age category in years, n (%) | |
| 18–34 | 60 (55) |
|
| |
| 35–49 | 28 (26) |
|
| |
| ≥ 50 | 21 (19) |
| Ethnicity, n (%) | |
| White | 56 (52) |
|
| |
| Hispanic | 28 (26) |
|
| |
| Black | 15 (14) |
|
| |
| Asian | 8 (7) |
|
| |
| ALL Subtype, n (%) | |
|
| |
| B-cell ALL | 86 (79) |
|
| |
| T-cell ALL | 23 (21) |
| Philadelphia chromosome, n (%) (B-cell ALL patients only) | |
| Negative | 46 (71) |
|
| |
| Positive | 19 (29) |
|
| |
| Cytogenetic risk,** n (%) | |
|
| |
| Poor | 26 (40) |
| Not poor | 39 (60) |
|
| |
| Response before transplant, n (%) | |
| CR 1 | 32 (29) |
|
| |
| CR 2 | 36 (33) |
|
| |
| Other (PIF: n=9; second transplant: n=13) | 41 (38) |
| Minimal residual disease, n (%) (CR 1 and CR 2 patients only) | |
| Yes | 14 (26) |
|
| |
| No | 40 (74) |
|
| |
| WBC at presentation for B-Cell ALL, n (%) | |
|
| |
| ≤ 30 | 38 (68) |
| > 30 | 18 (32) |
|
| |
| WBC at presentation for T-Cell ALL, n (%) | |
| ≤ 100 | 9 (60) |
|
| |
| > 100 | 6 (40) |
|
| |
| Hematopoietic Cell Transplant-Comorbidity Index, n (%) | |
|
| |
| 0–1 | 56 (52) |
| 2–3 | 32 (30) |
| > 3 | 19 (18) |
|
| |
| Cell source, n (%) | |
|
| |
| Peripheral blood | 59 (54) |
| Bone marrow | 50 (46) |
|
| |
| Donor relation, n (%) | |
| Child | 19 (17) |
|
| |
| Parent | 37 (34) |
|
| |
| Sibling | 53 (49) |
| Extramedullary disease, n (%) | |
| Yes | 17 (16) |
|
| |
| No | 91 (84) |
|
| |
| Non-myeloablative regimen, n (%) | |
|
| |
| Yes | 39 (36) |
|
| |
| No | 70 (64) |
| Preparative regimen, n (%) | |
| Melphalan-based | 40 (37) |
|
| |
| Flu/Cy/TBI | 32 (29) |
|
| |
| Busulfan-based | 9 (8) |
|
| |
| TBI-based | 25 (23) |
|
| |
| Other | 3 (3) |
Abbreviations: ALL, acute lymphoblastic leukemia; CR1, patients in first complete remission; CR2, patients in second complete remission; Flu/Cy/TBI, fludarabine/cyclophosphamide/total body irradiation; PIF, primary induction failure.
Because of missing data for some covariates, numbers don't add up for a total of 109 patients in all subgroups.
Alvarnas JC, Brown PA, Aoun P, et al. Acute Lymphoblastic Leukemia, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1240–1279.
Engraftment occurred in 95%, 4 patients failed engraftment and 2 were not evaluable because of early death (<28 days). The median time to neutrophil engraftment (defined as the first of 3 consecutive days when the absolute neutrophil count was >500 cells/µL) was 17 days (range 12–42) and for platelet recovery (defined as the first day after transplantation when the platelet count was >20,000/µL independent of platelet transfusions) was 25 days (range 12–114). The CI of day 100 grades II–IV aGVHD and grades III–IV aGVHD were 32% and 11%, respectively, and the 1-year and 5-year CI of cGVHD were 32% and 36%, respectively (Figure 1A–B). The CI of NRM at day 100, 1 year, and 5 years was 11%, 21%, and 30%, respectively, and the CI of relapse at 1 and 5 years post-transplant was 27% and 40% (Figure 1C). Among patients with known cause of death (n=43), relapse accounted for majority of deaths (approximately 46%). The most common cause of NRM was infection (26%), and only 4 patients (9%) died of GVHD (3 acute and one chronic). Table 2 lists the details of different causes of NRM deaths. When grouped by time from transplant, 20 patients died within the first 6 months and infection was the commonest cause of death (n=8), and 3 each died from aGVHD and relapse. In contrast for patients who died after 6 months (n=23), relapse was the most common cause of death (n=17) with only 3 patients died of infection and 1 patient of GVHD. There was no significant association between stem cell source (peripheral blood versus bone marrow), disease status at transplant, or conditioning regimen (myeloablative versus nonmyeloablative) and NRM. We also examined the center effect and found no significant association with NRM (p=0.16).
Figure 1.
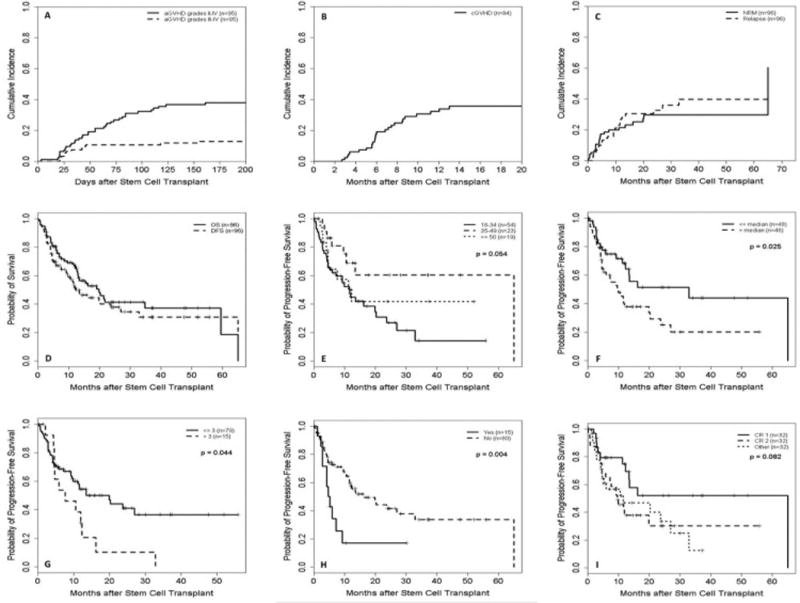
(A) Cumulative incidence (CI) of acute graft-versus-host disease (aGVHD); (B) CI of chronic GVHD (cGVHD); (C) CI of non-relapse mortality (NRM) and relapse rate; (D) Disease-free survival (DFS) and overall survival (OS); (E) DFS by age subgroup in years; (F) DFS by median time from diagnosis to transplantation; (G) DFS by comorbidity score; (H) DSF by presence (Yes) or absence (No) of extramedullary disease); and (I) DFS by disease status prior to transplant. Abbreviations: CR1, patients in first complete remission; CR2, patients in second complete remission; other, all other patients with primary refractory disease or beyond CR2.
Table 2.
Causes of death
| All Patients with known cause of death* (n=43) |
||
|---|---|---|
|
|
||
| Primary Cause of Death | No. | % |
| Relapse/Progressive disease | 20 | 46.50% |
|
| ||
| Non-relapse mortality | 23 | 53.50% |
|
| ||
| Infection** | 11 | |
| GVHD** | 4 | |
| Graft failure** | 1 | |
| Intracranial hemorrhage | 1 | |
| Other non-specified causesⱡ | 6 | |
|
| ||
| Total | 43 | 100.00% |
Abbreviations: GVHD, graft versus host disease; No., number of deaths; %, percentage of deaths per total number of deaths.
Two patients had no documented cause of death and not included in the total percentage calculation. One was attributed to non-relaspe mortality.
Documented infections included the following: 6 bacterial, 2 viral, 1 fungal, and 2 had no specification about type of infection. The patient with fungal infection (aspergillosis) has an underlying concomitant extensive GVHD. Three patients died from acute GVHD, 2 of which have infection listed as a contributing factor (one viral and the other is a non-specfied infection). The patient with graft failure had acute GVHD and non-specified infection listed as contributing causes of death.
These patients had various non-specified causes of deaths such as ARDS (n=2), cardiac failure (n=2), pulmonary failure (n=1), and liver failure (n=1).
The median DFS and OS for all patients receiving their first transplant were 12.3 and 19.9 months, respectively. For the entire group, the 1-year DFS and OS were 51% and 66%, and the 3-year rates were 31% and 37%, respectively (Figure 1D). The median DFS and OS for second transplants were 3.4 and 4.4 months, respectively. Subgroup univariate analyses were assessed for ALL subtype, disease status prior to transplant, age, gender, time from diagnosis to transplant, MRD, cytogenetic risk group, Philadelphia-chromosome status, white blood cell count at presentation, donor gender, stem cell source, comorbidity index, preparative regimen intensity, and extramedullary disease (Table 3). The measures with statistically significant effect on DFS included: time from diagnosis to transplant (3-year DFS of 44% and 20% for ≤median and >median, respectively; p=0.025), comorbidity score (3-year DFS of 37% and 0% for score ≤3 and >3, respectively; p=0.044), Philadelphia-chromosome status (3-year DFS of 25% and 43% for negative and positive, respectively; p=0.047), and extramedullary disease (3-year DFS of 34% and 17% for absence and presence, respectively; p=0.004) (Figures 1E–H). Differences in age (3-year DFS of 14%, 60%, and 42% for patients 18–34, 35–49, and ≥50 years old, respectively; p=0.054), disease status prior to transplant (3-year DFS of 52%, 30%, and 13% for patients with CR1, CR2, and all others, respectively; p=0.082) (Figure 1I), and preparative regimen (3-year DFS of 39% and 20% for patients receiving myeloablative and non-myeloablative conditioning, respectively; p=0.081) approached statistical significance. Additionally, we examined the center effect and found no significant association with DFS (p=0.58).
Table 3.
Univariate analysis identifying potential predictive factors for disease-free survival
| Covariate | Median in months (95% CI) | p-value | HR (95% CI) | p-value |
|---|---|---|---|---|
| Gender | ||||
|
| ||||
| Male | 11.6 (7.2, 27.0) | ref | ||
| Female | 13.5 (7.5, 65.1) | 0.59 | 0.86 (0.49, 1.51) | 0.59 |
|
| ||||
| Age in years | ||||
|
| ||||
| 18–34 | 11.5 (5.1, 19.9) | ref | ||
| 35–49 | 65.1 (9.6, 65.1) | 0.38 (0.17, 0.86) | 0.021 | |
| ≥ 50 | 12.3 (4.5, NR) | 0.054 | 0.74 (0.35, 1.54) | 0.41 |
|
| ||||
| Time from initial diagnosis to transplant | ||||
|
| ||||
| ≤ median | 32.9 (12.3, 65.1) | ref | ||
| > median | 9.8 (4.5, 19.9) | 0.025 | 1.92 (1.08, 3.43) | 0.027 |
|
| ||||
| Response before transplant | ||||
|
| ||||
| CR 1 | 65.1 (12.3, 65.1) | ref | ||
| CR 2 | 9.6 (4.5, 19.9) | 2.11 (1.01, 4.44) | 0.048 | |
| Other | 11.5 (4.5, 27.0) | 0.082 | 2.13 (1.01, 4.50) | 0.047 |
|
| ||||
| MRD(only CR1/CR2) | ||||
|
| ||||
| No | 19.9 (11.2, 65.1) | ref | ||
| Yes | 9.8 (3.4, NR) | 0.31 | 1.55 (0.66, 3.62) | 0.31 |
|
| ||||
| ALL Subtype | ||||
|
| ||||
| B-cell ALL | 16.2 (10.5, 32.9) | ref | ||
| T-cell ALL | 7.5 (4.5, NR) | 0.27 | 1.45 (0.75, 2.82) | 0.27 |
|
| ||||
| Cytogenetic risk | ||||
|
| ||||
| Poor | 23.7 (4.1, NR) | ref | ||
| Not poor | 10.5 (5.7, 20.2) | 0.41 | 1.36 (0.65, 2.87) | 0.41 |
|
| ||||
| Cell source | ||||
|
| ||||
| Peripheral blood | 12.3 (9.2, NR) | ref | ||
| Bone marrow | 13.4 (4.5, 27.0) | 0.67 | 1.13 (0.65, 1.98) | 0.67 |
|
| ||||
| Donor relation | ||||
|
| ||||
| Child | NR (4.1, NR) | ref | ||
| Parent | 9.2 (4.1, 20.2) | 2.00 (0.85, 4.70) | 0.11 | |
| Sibling | 13.4 (11.2, 65.1) | 0.11 | 1.17 (0.50, 2.76) | 0.72 |
|
| ||||
| Female-to-Male donor | ||||
|
| ||||
| No | 11.5 (7.2, 19.9) | ref | ||
| Yes | 23.7 (2.8, NR) | 0.97 | 0.99 (0.48, 2.04) | 0.97 |
|
| ||||
| Mother donor | ||||
|
| ||||
| No | 11.6 (7.5, 27.0) | ref | ||
| Yes | 5.9 (2.8, 23.7) | 0.22 | 1.53 (0.77, 3.03) | 0.22 |
|
| ||||
| Cell source donor group(only CR1/CR2) | ||||
|
| ||||
| PB female-to-male | NR (0.7, NR) | ref | ||
| PB other | 9.2 (4.3, NR) | 2.26 (0.28, 18.13) | 0.44 | |
| BM female-to-male | 4.0 (2.8, NR) | 2.83 (0.29, 27.58) | 0.37 | |
| BM other | 13.4 (5.7, NR) | 0.76 | 1.74 (0.22, 13.96) | 0.60 |
|
| ||||
| Comorbidity score | ||||
|
| ||||
| ≤ 3 | 19.9 (9.6, NR) | ref | ||
| > 3 | 7.5 (4.5, 12.3) | 0.044 | 1.93 (1.01, 3.71) | 0.048 |
|
| ||||
| WBC at presentation | ||||
|
| ||||
| < 30 | 11.6 (4.6, 65.1) | ref | ||
| 30–99 | 6.6 (4.1, NR) | 1.48 (0.61, 3.55) | 0.38 | |
| ≥ 100 | 9.2 (3.2, NR) | 0.68 | 1.10 (0.46, 2.60) | 0.84 |
|
| ||||
| Extramedullary disease | ||||
|
| ||||
| No | 16.2 (11.5, 32.9) | ref | ||
| Yes | 4.8 (2.8, 9.2) | 0.004 | 2.67 (1.34, 5.32) | 0.005 |
|
| ||||
| Philadelphia chromosome status | ||||
|
| ||||
| Negative | 11.5 (4.3, 16.2) | ref | ||
| Positive | 32.9 (4.1, NR) | 0.047 | 0.40 (0.16, 1.01) | 0.053 |
|
| ||||
| Non-myeloablative regimen | ||||
|
| ||||
| No | 19.9 (9.8, NR) | ref | ||
| Yes | 10.5 (3.9, 13.4) | 0.081 | 1.64 (0.94, 2.88) | 0.084 |
|
| ||||
| Preparative regimen | ||||
|
| ||||
| Flu/Cy/TBI | 11.2 (3.9, 32.9) | ref | ||
| Melphalan-based | 11.6 (5.7, NR) | 0.93 (0.48, 1.81) | 0.83 | |
| Busulfan-based | 2.8 (0.7, 19.9) | 1.89 (0.73, 4.90) | 0.19 | |
| TBI-based | NR (9.6, NR) | 0.10 | 0.51 (0.22, 1.21) | 0.13 |
|
| ||||
| Preparative regimena | ||||
|
| ||||
| Flu/Cy/TBI | 9.2 (3.2, 65.1) | ref | ||
| Other | 16.2 (9.8, NR) | 0.26 | 0.65 (0.31, 1.39) | 0.27 |
Abbreviation: ALL, acute lymphoblastic leukemia; BM, bone marrow; CI, confidence interval; CR1, patients in first complete remission; CR2, patients in second complete remission; Flu/Cy/TBI, fludarabine/cyclophosphamide/total body irradiation; HR, hazard ratio; MRD, minimal residual disease; NR, not reached; PB, peripheral blood; ref, reference group; TBI, total body irradiation.
In a multivariable analysis that included the aforementioned covariates (Table 4), intensity of conditioning regimen was the only statistically significant predictive factor (HR 2.65, 95% CI 1.09, 6.46; p=0.032). In a separate evaluation of pre-transplant response and MRD status, we found that patients with CR1 and negative MRD had the best DFS; however, this did not reach statistical significance probably because of small sample size [3-year DFS: CR1/MRD− (57%), CR1/MRD+ (50%), other/MRD− (32%), other/MRD+ (0%); p=0.12].
Table 4.
Multivariable analysis for significant covariates to predict disease-free survival
| Covariate | HR (95% CI) | p-value |
|---|---|---|
| Response before transplant | ||
|
| ||
| CR 1 | ref | |
| CR 2 | 3.13 (0.79, 12.30) | 0.10 |
| Other | 2.11 (0.60, 7.37) | 0.24 |
|
| ||
| Philadelphia chromosome status | ||
|
| ||
| Negative | ref | |
| Positive | 0.35 (0.12, 1.08) | 0.069 |
|
| ||
| Time from initial diagnosis to transplant | ||
|
| ||
| ≤ median | ref | |
| > median | 0.41 (0.13, 1.34) | 0.14 |
|
| ||
| Age in years | ||
|
| ||
| 18–34 | ref | |
| 35–49 | 0.78 (0.29, 2.08) | 0.61 |
| ≥ 50 | 0.76 (0.21, 2.73) | 0.67 |
|
| ||
| Comorbidity score | ||
|
| ||
| ≤ 3 | ref | |
| > 3 | 0.89 (0.27, 2.91) | 0.85 |
|
| ||
| Non-myeloablative regimen | ||
|
| ||
| No | ref | |
| Yes | 2.65 (1.09, 6.46) | 0.032 |
|
| ||
| Extramedullary disease | ||
|
| ||
| No | ref | |
| Yes | 2.25 (0.71, 7.18) | 0.17 |
Abbreviation: ALL, acute lymphoblastic leukemia; CI, confidence interval; CR1, patients in first complete remission; CR2, patients in second complete remission; HR, hazard ratio; ref, reference group.
Discussion
Allogeneic transplantation is standard of care for high-risk patients in CR1 and all relapsed ALL.22 The 5-year PFS, relapse and NRM ranged from 44%–60%, 24%–36% and 16%–18%, respectively, in three of the largest studies to-date for ALL patients receiving a MSD ASCT while in CR1.23–25 MUD is considered the best alternative donor source with 5-year PFS, relapse and NRM of 38%–63%, 15%–20% and 22%–43%, respectively.26–28 Our study is the first to document outcomes for patients with ALL with HSCT performed using PTCy-based GVHD prophylaxis.
The majority of patients in our cohort had advanced disease beyond CR1. Although did not reach statistical significance (p=0.082), patients in CR1 who received HSCT experienced better 3-year DFS of 52% which favorably compare with results from HLA matched transplants.23–25 Moreover, despite the inherent differences that limit fair comparison with other studies, it is notable that outcomes for those transplanted while in CR2 or beyond in our study also compared favorably to outcomes with MSD or MUD recipients.6, 9 Further, our results showed poor survival for ALL patients who had second HSCT (n=13 patients), similar to outcomes reported by other groups.29
The use of haploidentical transplants for ALL patients is limited to a few retrospective studies, majority were not disease-specific, and none of which specifically investigated the role of PTCy for these patients;19, 30, 31 no significant differences in acute leukemia outcomes were noted in the recent registry-based study between the two commonly used alternative donor sources, haploidentical and cord transplants.19 The finding of relatively higher incidence of cGVHD is comparable to recent findings by Ruggeri et al.19 When adjusted by conditioning regimen and center effect (results are not shown), we did not find an association between either the regimen intensity or the treatment center and cGVHD outcomes. Our findings demonstrate effective disease control and safety profile in concordance with outcomes of HLA matched transplants. Multiple pre-transplant and transplant-related prognostic factors (Table 3) were analyzed to determine their predictive effect on outcome and to identify susceptible groups that would most benefit from HSCT. Few prognostic factors were found of significance on univariate analysis as noted in Table 3; however only the myeloablative conditioning intensity effect was found to predict better outcomes on multivariate analysis (Table 4). The role of preparative regimens has been debated in ALL patients, but there have been an estimated overall favorable effect and preference for myeloablative conditioning especially for younger patients with less co-morbid conditions.32–34
Despite the study novelty and strengths driven by including all consecutive patients treated at five large transplant centers, we acknowledge the inherited limitations that are associated with retrospective observational studies. In an attempt to gain as much insight as possible from this analysis, we have included all possible risk factors that may potentially influence prognosis. However, we interpret the univariate and multivariate outcomes with caution given the relatively small sample size and heterogeneous disease population included. In addition to the lack of a comparator arm in our study, we were not able to assess accurately the GVHD severity for all patients. Further, there was insufficient data to collect about use of tyrosine kinase inhibitors (TKIs) for Philadelphia chromosome positive ALL patients. Future studies to better assess GVHD severity and include comparator groups are imperative. Despite the fact that TKIs are widely used for maintenance after transplant the evidence remains debatable,35 especially in regards to OS benefit, and this remains an unmet need for future studies. The concordance of our results for HSCT with PTCy with MSD or MUD prospective studies, showing at least same efficacy, is encouraging and warrants further investigation in prospective randomized studies. In conclusion, this novel multicenter retrospective study shows not only feasibility but potentially a very suitable alternative donor source for patients with high-risk ALL undergoing haploidentical transplants with PTCy-based GVHD prophylaxis. Prospective studies remain an unmet need to further examine safety and efficacy of haploidentical transplantation in patients with different diseases and to compare outcomes with other donor sources.
Highlights.
Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for prevention of GVHD is feasible for patients with ALL;
Patients in first complete remission have very good long term survival;
Haploidentical donors provide an excellent alternative to HLA matched transplants.
Acknowledgments
We acknowledge the work and dedication of Celina Ledesma and Maria Angelica Cardona who contributed to data collection for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Contributions: SAS contributed with data collection and interpretation, and wrote the first draft of the manuscript; SOC designed the research, analyzed and interpreted the data, contributed to manuscript writing and edited the manuscript; DRM performed statistical analysis, interpreted the statistical results, and contributed to manuscript writing; AB, MAM, AKU, RR contributed with patient care, data, manuscript writing, reviewed and approved the manuscript; GR, SB, RP, MS contributed with data collection; PK, SS, SJF, REC contributed with data interpretation, reviewed and approved the manuscript.
Conflict of interest
The authors declare no competing financial interests to disclose for this work.
References
- 1.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 4.Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: a SEER analysis. Sci. Rep. 2014;4:4227. doi: 10.1038/srep04227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 7.Gokbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 8.Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kebriaei P, Poon LM. The role of allogeneic hematopoietic stem cell transplantation in the therapy of patients with acute lymphoblastic leukemia. Curr Hematol Malig. Rep. 2012;7:144–152. doi: 10.1007/s11899-012-0116-3. [DOI] [PubMed] [Google Scholar]
- 10.Gupta V, Richards S, Rowe J Acute Leukemia Stem Cell Transplantation Trialists' Collaborative G. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121:339–350. doi: 10.1182/blood-2012-07-445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messori A, Fadda V, Maratea D, Trippoli S. Acute lymphoblastic leukemia in first complete remission: temporal trend of outcomes in studies comparing allogeneic transplant with autologous transplant or chemotherapy. Ann Hematol. 2013;92:1221–1228. doi: 10.1007/s00277-013-1766-5. [DOI] [PubMed] [Google Scholar]
- 12.Oliansky DM, Larson RA, Weisdorf D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukemia: update of the 2006 evidence-based review. Biol Blood Marrow Transplant. 2012;18:16–17. doi: 10.1016/j.bbmt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J. Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–947. doi: 10.1182/blood-2015-09-671834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 20.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Majhail NS, Farnia SH, Carpenter PA, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863–1869. doi: 10.1016/j.bbmt.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 24.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 25.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2009;113:1375–1382. doi: 10.1182/blood-2008-07-168625. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Cho BS, Kim SY, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1083–1094. doi: 10.1016/j.bbmt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Marks DI, Perez WS, He W, et al. Unrelated donor transplants in adults with Philadelphia-negative acute lymphoblastic leukemia in first complete remission. Blood. 2008;112:426–434. doi: 10.1182/blood-2007-12-128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 30.Yan CH, Jiang Q, Wang J, et al. Superior survival of unmanipulated haploidentical hematopoietic stem cell transplantation compared with chemotherapy alone used as post-remission therapy in adults with standard-risk acute lymphoblastic leukemia in first complete remission. Biol Blood Marrow Transplant. 2014;20:1314–1321. doi: 10.1016/j.bbmt.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Sun YQ, Wang J, Jiang Q, et al. Haploidentical hematopoietic SCT may be superior to conventional consolidation/maintenance chemotherapy as post-remission therapy for high-risk adult ALL. Bone Marrow Transplant. 2015;50:20–25. doi: 10.1038/bmt.2014.195. [DOI] [PubMed] [Google Scholar]
- 32.Marks DI, Wang T, Perez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohty M, Labopin M, Volin L, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 34.Marks DI, Alonso L, Radia R. Allogeneic hematopoietic cell transplantation in adult patients with acute lymphoblastic leukemia. Hematol Oncol Clin North. Am. 2014;28:995–1009. doi: 10.1016/j.hoc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Couban S, Savoie L, Mourad YA, et al. Evidence-based guidelines for the use of tyrosine kinase inhibitors in adults with Philadelphia chromosome-positive or BCR-ABL-positive acute lymphoblastic leukemia: a Canadian consensus. Curr Oncol. 2014;21:e265–309. doi: 10.3747/co.21.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]


