Abstract
Objective
To explain the association of out‐of‐pocket (OOP) cost, community‐level factors, and individual characteristics on statin therapy nonadherence.
Data Sources
BlueCross BlueShield of Texas claims data for the period of 2008–2011.
Study Design
A retrospective cohort of 49,176 insured patients, aged 18–64 years, with at least one statin refill during 2008–2011 was analyzed. Using a weighted proportion of days covered ratio, differences between adherent and nonadherent groups are assessed using chi‐squared tests, t‐tests, and a clustered generalized linear model with logit link function.
Principal Findings
Statin therapy adherence, measured at 48 percent, is associated with neighborhood‐level socioeconomic factors, including race/ethnicity, educational attainment, and poverty level. Individual characteristics influencing adherence include OOP medication cost, gender, age, comorbid conditions, and total health care utilization.
Conclusions
This study signifies the importance of OOP costs as a determinant of adherence to medications, but more interestingly, the results suggest that other socioeconomic factors, as measured by neighborhood‐level variables, have a greater association on the likelihood of adherence. The results may be of interest to policy makers, benefit designers, self‐insured employers, and provider organizations.
Keywords: Medication adherence, cost sharing, out‐of‐pocket expense, health care utilization
Chronic diseases affect the majority of Americans, costing an estimated $1.3 trillion annually in direct healthcare cost and productivity loss (DeVol et al. 2007). Fifty percent of Americans do not adhere to their prescribed medication regimens (Sabaté 2003), causing adverse health outcomes, emergency care utilization, high cost of care and reduced workforce productivity (Sabaté 2003; Heisler et al. 2004; Sokol et al. 2005; Briesacher, Gurwitz, and Soumerai 2007; Blanchard et al. 2013). Policy initiatives have been undertaken to improve medication adherence, including Medicare Advantage's physician quality reporting and pay‐for‐performance incentive payments (CMS 2014) and the Accountable Care Organization payment model, which targets safe and effective medication use (RTI 2012). These policy reforms financially incent health care providers to implement organizational best practices for prescribing medications, collaborating with pharmacists, and educating patients on medication self‐management (Rosenbaum and Shrank 2013).
Cardiovascular disease is the leading cause of death in the United States (NCHS 2016), and it can be prevented and reduced using statin therapy (Stone et al. 2014; Armstrong et al. 2014). Based on 2013 risk assessment (Goff, Lloyd‐Jones, and Bennett 2014) and cholesterol treatment guidelines (Stone et al. 2014), it is expected that the number of eligible adults for statin therapy will increase by 12.8 million (Pencina et al. 2014). Unfortunately, nonadherence rates for statin therapy vary between 25 and 70 percent, with 12–45 percent of patients abandoning statin use at the end of 1 year (Insull 1997; Perreault et al. 2009), posing a significant clinical and policy concern.
The geography of health and health care, also known as healthography (Greenberg 2014), is often considered in policy making research. Medical sociologists posit that geography may impact an individual's health and well‐being (Macintyre, Ellaway, and Cummins 2002; Poland et al. 2005; Cummins et al. 2007), leading to health disparity (Phelan, Link, and Tehranifar 2010). Health disparities are known to exist across different demographic groups (Craig et al. 2014), suggesting the importance of evaluating community‐level factors on medication adherence.
This study examines the association of nonadherence to statin medication with neighborhood‐level factors that define socioeconomic position of communities, in addition to individual‐level factors. Past research leveraged different theoretical frameworks as predictive or explanatory models to understand the impact of adherence‐improving interventions (Munro, Lewin, and Volmink 2007), including the health belief model (Harrison, Mullen, and Green 1992) and social cognitive theory (Bandura 2004). The emphasis of these frameworks is on individual focused intervention strategies (Magzamen, Brandt, and Tager 2014), often ignoring social and community‐level factors that may provide more sustainable population health (Link and Phelan 1995). An emerging body of research contextualizes medication adherence using the Health Behavior Model (HBM; Andersen 1995) to explain behaviors (Unni and Farris 2011; Vaidya et al. 2014; Thorpe et al. 2015). However, a research gap exists in understanding how medication adherence differs across neighborhood and individual factors.
This study first contributes to the emerging stream of health behavior research by operationalizing the overarching constructs of predisposing factors and enabling/impeding factors. Second, the use of a commercially insured dataset comprising 49,000+ working‐age adults, makes the research unique because commercially insured adults should have higher rates of literacy and better access, as well as less price sensitivity to marginal increases in copayments when compared to publicly insured or uninsured. Furthermore, the study population has a substantial proportion of Hispanics, a population known to have better health and mortality outcomes (Franzini, Ribble, and Keddie 2001; Medina‐Inojosa et al. 2014), though higher risk for cardiovascular issues (Daviglus, Pirzada, and Talavera 2014).
Conceptual Framework
To understand the relationship between neighborhood and individual characteristics with statin nonadherence, the HBM (Aday and Andersen 1974; Andersen 1995) is contextualized. This model conceptualizes factors that may influence utilization, including enabling factors, predisposing factors, and need factors. These factors are contextualized at the individual, environmental, and provider/system level to assess the impact on statin nonadherence (Figure 1).
Figure 1.
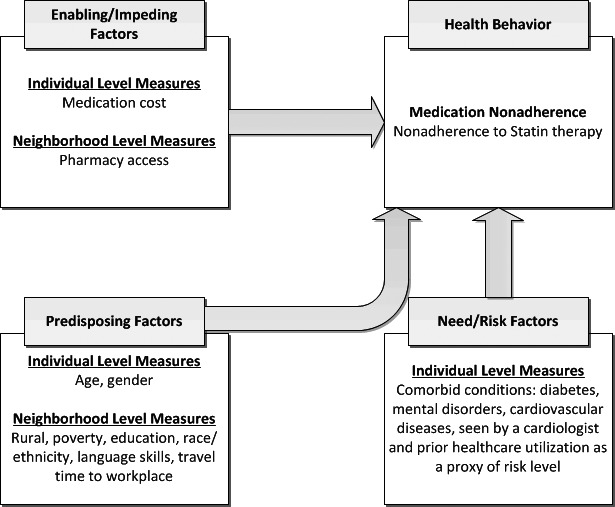
Factors Influencing Medication Nonadherence Behavior
Source: Adapted from behavior model for health care utilization (Aday and Andersen 1974; Andersen 1995).
Enabling/Impeding Factors
The out‐of‐pocket (OOP) cost to receive a health care service can be an enabling (if low) or impeding (if high) factor. Several studies show lowering of copayments is associated with increases in adherence to medications for a broad range of diseases, including diabetes, hyperlipidemia, and coronary heart diseases (Gibson et al. 2006, 2010; Ye et al. 2007). Furthermore, a meta‐synthesis by Eaddy et al. (2012) concludes that increased cost sharing is associated with decreased medication adherence, resulting in worse health outcomes. Moreover, Thiebaud, Patel, and Nichol (2008) show individuals with lower adherence respond more strongly to a reduction in copay than already adherent individuals.
Access to community‐based pharmacies may also influence prescription refill behavior. Xu, Smith, and Borders (2003) report elderly patients living in neighborhoods with higher pharmacy density usually have better access to pharmaceutical systems. Thus, as the number of pharmacies increases in the local neighborhood, individuals may refill prescriptions more regularly.
Predisposing Factors
The Health Behavior Model posits that certain individuals may be prone to use health care services more often than others depending on demographic and socioeconomic characteristics present at the individual or social structure level (Andersen and Newman 1973). A meta‐analysis found the relationship between nonadherence to statin therapy and age is U‐shaped, with nonadherence being minimum around age 60–65 (Mann et al. 2010). A study of the baby boomer generation (aged 44–64 years) reports a monotonically decreasing trend of age effect on medication nonadherence (Wallach‐Kildemoes et al. 2013). Gender is also a predictor of adherence, with the majority of research showing females to be less likely to be adherent to medication (Benner et al. 2004; Ellis et al. 2004; Rasmussen, Chong, and Alter 2007; Chodick et al. 2008; Yu et al. 2008). However, some studies show inconclusive evidence for differences in adherence across gender (Benner et al. 2002; Donnelly et al. 2008).
The social structure of neighborhoods may define position of individuals in the society, and thereby lifestyle patterns, and access to community‐level resources (Aday and Andersen 1974; Andersen 1995). Furthermore, cultural traits could predispose individuals differently in their attitudes toward medical care, physicians, and disease (Andersen and Newman 1973). These neighborhood‐level characteristics can be contextualized based on individual factors, including educational attainment, income (or poverty) level, national origin, language skill, and travel distance between homes and workplace, among others.
Educational attainment has become a prominent predictor, though with mixed evidence, of lifespan, health behavior and health care utilization in the developed nations (Montez and Friedman 2015; Smith et al. 2015). A meta‐analysis by Smith et al. (2015) suggests a negative relationship between educational attainment and onset of chronic disease; however, the authors caution that some studies do not show association. Other studies show a positive association between medication adherence and income (Mann et al. 2010), when viewed at the individual and/or community level (Benner et al. 2004; Rasmussen, Chong, and Alter 2007; Schneeweiss et al. 2007; Chodick et al. 2008; Chan, Shrank, and Cutler 2010). To further emphasize, some posit that lower income groups inadvertently bear the largest proportionate income burden of prescription drugs, including statin therapy (Wallach‐Kildemoes et al. 2013). In the context of racial disparity, several studies suggest minority patients, including Hispanics and African Americans, are less likely to be adherent than white patients (Benner et al. 2002; Ellis et al. 2004; Mann et al. 2007).
Need (Risk) Factors
Patient health risk factors and prior utilization of health care services contribute to predicting health service utilization and behavior. Medication compliance may be confounded by chronic comorbid conditions and/or frequency of interaction with health care providers. Past research shows patients with a history of myocardial infarction or other cardiovascular disease, diabetes, and/or hypertension are more likely to be adherent to statin therapy (Benner et al. 2002, 2004; Schneeweiss et al. 2007; Donnelly et al. 2008; Thiebaud, Patel, and Nichol 2008; Yu et al. 2008). Conversely, some research shows patients with diabetes may be less adherent to statin therapy (Rasmussen, Chong, and Alter 2007). Finally, patients with comorbid mental health disorders are often less adherent to prescription medications for primary conditions like cardiovascular disease and diabetes (Nelson et al. 2011; Hansen et al. 2012; Desai et al. 2014).
Methods
Study Design and Data Sources
This is a retrospective cohort study of individuals on statin therapy during 2008–2012 (study period) in the state of Texas who are privately insured by BlueCross BlueShield of Texas. Data on 49,216 individuals in the age group 18–64 years who have pharmacy benefits through their preferred provider organization are evaluated. The BCBSTX members are not able to select among pharmacy plans, thereby minimizing bias induced by adverse selection or selection induced by adverse selection of lower OOP pharmecutical copyament costs. Socioeconomic status and pharmacy count data are from the RTI Spatial Impact Factor database and the Texas State Board of Pharmacy. Selection of individuals in the age group 18–64 years is limited to only those who with at least two filled statin prescriptions between June 2008 and December 2011 (identification period). All individuals have continuous enrollment 6 months before and 1 year after the first statin prescription fill date (observation period).
Dependent Variable: Nonadherence to Statin Therapy
Medication nonadherence is routinely studied using administrative/pharmacy claims data and defined as a patient who does not refill and/or stops taking a medication. To measure nonadherence, the Pharmacy Quality Alliance endorses the “proportion of days covered” (PDC) (Benner et al. 2002) as a preferred measure for medication adherence (Nau 2014). In 2009, the National Quality Forum endorsed PDC‐based measures (Yeaw 2009), and subsequently the Center for Medicare and Medicaid adopted it in 2010 for measurement of adherence to five therapeutic categories, including statins (CMS 2010).
Medication adherence is measured for each individual as the PDC during the year, immediately following the index date of statin prescription fill (Benner et al. 2002; Leslie 2007). Individuals with less than 80 percent PDC are classified as nonadherent to statin therapy following the established norm in the literature (Benner et al. 2002; Rasmussen, Chong, and Alter 2007; Schneeweiss et al. 2007; Leslie et al. 2008; Choudhry et al. 2010; Wallach‐Kildemoes et al. 2013). The PDC is calculated, using a modified algorithm of Lesley's algorithm, as proposed by Chu, Kawatkar, and Gu (2011), as the number of days within the observation period with medication supply divided by the number of days the patient is eligible for benefits. If fills overlap, the start date of the next fill is adjusted to be the day after the previous fill ended. Also, if patients have supply for more than one statin on a given day, the day is only counted once as being “covered.” If the adherence period for individuals is less than a full year from the index date of the statin prescription fill, the PDC is measured as the number of days the patient is on a statin over the difference in days between the first fill and last refill dates.
Measurement of Explanatory Variables
Out‐of‐pocket medication cost, inclusive of copayments, deductibles, and/or patient cost sharing, for statin medications is the primary explanatory variable. OOP statin medication costs are person‐level costs defined as the average monthly cost for statin prescriptions during the observation period. The OOP cost is determined by computing the difference between the total paid dollar amount and allowed dollar amount listed in the BCBSTX Pharmacy member claim file. The OOP cost is the “patient responsibility”; it is assumed individuals paid this amount to receive their medications. It is likely that some individuals selected lower cost generics, which may influence the results of this research upward. For analysis and ease of interpretation, the monthly average OOP cost is rescaled to a per $10 unit cost.
Demographic variables include gender (female as reference group) and age in years at the time of index claim. The rural location is dichotiomized based on the RUCA 2.0 cross‐walk file available from University of Washington. Comorbid conditions are operationalized as dichotomous variables based on relevant International Classification of Diseases‐Revision 9 codes for presence of diabetes mellitus (ICD9: 250, 375), cardiovascular disease (ICD9: 390–495), and mental disorders (ICD9: 290–319). A visit to a cardiologist within 30 days before the index prescription date is a study variable, in addition to overall prior health care utilization. Patients are categorized into three “prior utilization” groups based on the quartiles of total medical expenditures.
Neighborhood‐level factors are operationalized using zip code‐level aggregate data for Texans age 18–64 years, based on the RTI Spatial Impact Factor database (https://rtispatialdata.rti.org). These variables include educational attainment (proportion of population with college or higher degree; proportion of population with high school or lesser education), poverty level (proportion of household with public assistance), language skills (proportion of English‐only speaking population), racial/ethnic density (proportion of population that are non‐Hispanic White, Hispanic, and African American; proportion of population that are foreign‐born), and the average travel distance to workplace. For the analysis, all zip code‐level variables are categorized corresponding to their quartiles within the observed distribution. In the context of racial/ethnic density, each zip code is dichotomized as dominated by non‐Hispanic whites, Hispanics, or African Americans based on the proportion of the population that belongs to highest quartile of respective race/ethnicity. Finally, the number of community pharmacies in each zip code is derived based on the registry of active pharmacies maintained by the Texas State Board of Pharmacy.
Statistical Analysis
Differences in demographics and clinical characteristics between the adherent and nonadherent groups are assessed using chi‐squared tests for categorical variables and t‐tests for continuous variables. The association between OOP cost and medication nonadherence (i.e., PDC <0.80) is evaluated using clustered generalized linear model‐based regression with logit link function, adjusting for other enabling factors (pharmacy access), predisposing factors (age, gender, rural area, poverty, education, race, language skill, travel time to work), and need factors (comorbid conditions, including diabetes, mental disorder, cardiovascual disease, visit to cardiologist, and prior health service utilization) representing patient‐ or community‐level characteristics. To account for potential bias from varying length of observation periods on which PDC is computed, the PDC is weighted using the exposure period for patients on statins.
Results
Study Population
There are 49,176 patients who met the inclusion criteria. Table 1 provides the summary of sampled individuals with respect to their adherence to statin therapy and various factors that are associated with medication adherence behavior, including enabling/impeding factors, predisposing factors, and need (or risk) factors considered in the research model (see Figure 1).
Table 1.
Description of Medication Nonadherence, Patient‐ and Neighborhood‐Level Enabling/Impeding Factors, Predisposing Factors, and Need (Risk) Factors for 49,176 Commercially Insured Individuals on Statin Therapy during 2009–2011 in Texas State
| Variable | Adherent | Nonadherent | p‐value |
|---|---|---|---|
| Enabling/impeding factors | |||
| OOP cost for statin medications | $21.8 (18.3) | $23.7 (18.8) | <.001 |
| Count of pharmacies per square mile in ZCTA | 0.42 (2.1) | 0.38 (1.28) | .01 |
| Predisposing factors | |||
| Gender, male=yes | 27.1% | 29.3% | .002 |
| Age, in years | 52.4 (7.9) | 49.9 (8.7) | <.001 |
| Rural: Proportion of individuals living in rural ZCTAs | 15.9% | 15.8% | .796 |
| Proportion living in ZCTAs, where percentage of households getting public assistance | 2.42% (2.28) | 2.87% (2.72) | <.001 |
| Proportion living in ZCTAs, where percentage of population with less than high school education | 18.0% (12.3) | 20.5% (13.8) | <.001 |
| Proportion living in ZCTAs, where percentage of population with college or higher education | 27.4% (16.9) | 24.7% (16.2) | <.001 |
| Proportion living in ZCTAs, where percentage of English‐only speaking population | 19.5% (11.7) | 20.9% (12.1) | <.001 |
| Racial/ethnic density | |||
| Proportion living in Non‐Hispanic white dominated ZCTAs | 83.7% | 75.6% | <.001 |
| Proportion living in Hispanic dominated ZCTAs | 14.2% | 21.2% | <.001 |
| Proportion living in African American dominated ZCTAs | 2.03% | 3.20% | <.001 |
| Proportion of foreign‐born population living in ZCTAs | 11.1% (8.8) | 12.5% (9.8) | <.001 |
| Average travel‐to‐work time in ZCTAs, in minutes | 25.3 (5.8) | 25.4 (5.7) | .162 |
| Need/risk factors | |||
| Comorbid conditions | |||
| Proportion of individuals with diabetes | 11.8% | 14.4% | <.001 |
| Proportion of individuals with mental disorders | 31.6% | 32.8% | <.001 |
| Proportion of individuals with cardiovascular disorders | 10.3% | 12.1% | <.001 |
| Proportion of individuals who visited cardiologist 30 days before starting statin therapy | 15.8% | 12.1% | <.001 |
| Prior health care utilization: total medical spending 6 months before starting statin therapy | <.001 | ||
| Low utilizers | 28.9% | 36.2% | – |
| Intermediate utilizers | 34.8% | 37.2% | – |
| High utilizers | 36.2% | 37.3% | – |
Among the 49,176 individuals, about 48 percent are nonadherent to statin therapy. The average OOP cost for statin therapy is about $22.8 (standard deviation [SD]: $18.6). Across the state of Texas, the average number of (noninstitutional) pharmacies is about 60 (SD: ±67) per primary care service area, indicating significant variation in the availability of pharmacies. The average age for sampled individuals is 51.1 years (SD: ±8.5), 56 percent are male, and approximately 16 percent of individuals live in rural zip code tabulation areas (ZCTAs).
Evaluation of the predisposing factors across the four quartile‐based ZCTAs (Table 1) shows 66 percent of the study population live in economically better neighborhoods and almost 70 percent live in areas of higher educational attainment. Nearly 80 percent of sampled individuals live in non‐Hispanic white dominated ZCTAs and less than 3 percent live in African American dominated ZCTAs. Furthermore, about 64 percent of sampled individuals live in ZCTAs that have a higher proportion of foreign‐born residents and 54 percent live in areas, where the average travel‐to‐work time is substantially higher.
Among the sampled individuals, about 27 percent have diabetes, 23 percent have been treated for some type of mental disorder, and about 65 percent have been treated for some type of cardiovascular disorder. Furthermore, about 14 percent of individuals visited a cardiologist within 30 days prior to the first fill of statin medications. As another indicator of individual health risk, the average total medical spending 6 months prior to starting a statin medication averages $1,585 (SD: ±$11,030).
Association of Enabling/Impeding Factors with Medication Nonadherence
The association of OOP cost burden for statin medications is positive and statistically significant with nonadherence to statin therapy. A $10 increase in monthly spending on statin is associated with about 7 percent higher likelihood of an individual becoming nonadherent with the therapy (95 percent CI: 1.06–1.08). The count of active pharmacies per square mile is not associated with nonadherence to statin therapy (95 percent CI: 1.00–1.00) (Table 2).
Table 2.
Association of Medication Adherence with Enabling/Impeding Factors, Predisposing Factors and Need(Risk) Factors, Shown as Adjusted Odds Ratio and 95% Confidence Intervals
| Explanatory Factors | Adjusted Odds Ratio (95% Confidence Interval) |
|---|---|
| Enabling/impeding factors | |
| OOP cost for statin medications | 1.07 (1.057; 1.082) |
| Count of pharmacies per square mile in ZCTA | 1.00 (0.999; 1.000) |
| Predisposing factors | |
| Gender, male=yes | 0.89 (0.854; 0.923) |
| Age, in years | 0.97 (0.963; 0.968) |
| Rural ZCTAs | 1.00 (0.921; 1.088) |
| ZCTAs where percentage households getting public assistance is at | |
| 1st quartile | Reference |
| 2nd quartile | 1.08 (1.020; 1.152) |
| 3rd quartile | 1.09 (0.999; 1.199) |
| 4th quartile | 1.10 (0.980; 1.233) |
| ZCTAs where percentage population with less than high school education is at | |
| 1st quartile | Reference |
| 2nd quartile | 1.00 (0.914; 1.083) |
| 3rd quartile | 0.94 (0.845; 1.038) |
| 4th quartile | 1.01 (0.877; 1.171) |
| ZCTAs where percentage population with college or higher education is at | |
| 1st quartile | Reference |
| 2nd quartile | 0.97 (0.893; 1.058) |
| 3rd quartile | 0.88 (0.790; 0.973) |
| 4th quartile | 0.79 (0.690; 0.901) |
| ZCTAs where percentage of English‐only speaking population is at | |
| 1st quartile | Reference |
| 2nd quartile | 0.96 (0.832; 1.118) |
| 3rd quartile | 1.00 (0.847; 1.174) |
| 4th quartile | 0.93 (0.773; 1.118) |
| Race/ethnicity | |
| Non‐Hispanic white dominated ZCTAs | Reference |
| Hispanic dominated ZCTAs | 1.33 (1.140; 1.545) |
| African American dominated ZCTAs | 1.56 (1.364; 1.787) |
| ZCTAs where foreign‐born population density is at | |
| 1st quartile | Reference |
| 2nd quartile | 1.06 (0.961; 1.170) |
| 3rd quartile | 1.09 (0.974; 1.226) |
| 4th quartile | 1.20 (1.032; 1.399) |
| ZCTAs where average travel‐to‐work time is at | |
| 1st quartile | Reference |
| 2nd quartile | 1.01 (0.939; 1.094) |
| 3rd quartile | 1.06 (0.980; 1.145) |
| 4th quartile | 1.15 (1.075; 1.233) |
| Need/risk factors | |
| Comorbid conditions | |
| Proportion of patients with diabetes | 1.13 (1.077; 1.182) |
| Proportion of patients with mental disorders | 1.11 (1.059; 1.163) |
| Proportion of patients with cardiovascular disorders | 0.88 (0.842; 0.926) |
| Individuals who visited cardiologist 30 days before starting statin medications | 0.79 (0.743; 0.845) |
| Prior health care utilization: total medical costs 6 months prior to starting statins | |
| Low utilizers | 1.11 (1.043; 1.175) |
| Intermediate utilizers | Reference |
| High utilizers | 1.04 (0.993; 1.087) |
| Constant | 5.71 (4.217; 7.719) |
Values set in bold font are statistically significant (p‐value < .05).
Association of Predisposing Factors with Medication Nonadherence
Among the individual‐level characteristics, both gender and age are negatively associated with adherence and statistically significant. In particular, when compared to females, male individuals are 11 percent less likely to be nonadherent with the statin therapy (95 percent CI: 0.85–0.92). Furthermore, an increase of 1 year in age is associated with a 3 percent reduction in the likelihood of an individual becoming nonadherent with statin therapy (95 percent CI: 0.96–0.97) (Table 2).
Among the neighborhood‐related factors considered, partial support is found for the hypothesized relationships. In particular, individuals living in neighborhoods that have a moderately higher proportion of households receiving (economic) public assistance are 8 percent more likely to be nonadherent to statin therapy when compared to neighborhoods with fewer households receiving public assistance (i.e., 1st to 2nd quartile: 95 percent CI: 1.02–1.15). The effect size marginally increases, though statistically not significant, for neighborhoods with a much higher proportion of households receiving public assistance (1st to 3rd quartile, 95 percent CI: 1.00–1.20; 1st to 4th quartile, 95 percent CI: 0.98–1.23) (Table 2).
Individuals living in neighborhoods with a high density of college or higher degreed members are between 12 and 21 percent less likely to be nonadherent with statin therapy, when compared to individuals living in neighborhoods with fewer college or higher graduates (1st to 3rd quartile, 95 percent CI: 0.79–0.97; 1st to 4th quartile, 95 percent CI: 069–0.90). The results also indicate that individuals living in a Hispanic dominated ZCTA or African American dominated neighborhood are 33 percent (95 percent CI: 1.140–1.545) and 56 percent, respectively (95 percent CI: 1.364–1.787), more likely to be nonadherent with statin therapy when compared to individuals living in non‐Hispanic white dominated neighborhoods. Finally, individuals living in neighborhoods with a higher foreign‐born population density are 20 percent more likely to be nonadherent with statin therapy (95 percent CI: 1.032–1.399) when compared to neighborhoods with a lower foreign‐born population density (Table 2).
The remaining predisposing factors which characterize neighborhoods are not associated with medication nonadherence. These include rural areas, educational attainment up to high school, English‐only speaking population density, and average travel‐to‐workplace duration (Table 2).
Association of Need (Risk) Factors with Medication Nonadherence
Individuals with select comorbid conditions, other than cardiovascular disorders, are likely to exhibit a substantial degree of nonadherence to statin therapy when compared to those who do not have the conditions. In particular, individuals diagnosed with diabetes mellitus are 13 percent more likely to be nonadherent (95 percent CI: 1.08–1.18) when compared to their nondiabetic counterparts. Likewise, individuals with mental health disorders are 11 percent more likely to be nonadherent when compared to those who have not been diagnosed for mental health disorders (95 percent CI: 1.06–1.16). Individuals with cardiovascular disorders are 12 percent less likely to be nonadherent (95 percent CI: 0.84–0.93).
Visiting a cardiologist prior to start statin therapy was associated with a 21 percent less likelihood of nonadherence (95 percent CI: 0.74–0.85). Individuals with lower baseline health services utilization are 11 percent more likely to be nonadherent compared to intermediate utilizers (95 percent CI: 1.04–1.18). There was no statistical difference in the likelihood of nonadherence between patients with high baseline health services utilization and the intermediate group (p > .05).
Discussion
Medication nonadherence is a public health challenge due to the rising growth in the chronically ill population. Past research has examined the challenges of medication adherence across numerous contexts, including specific chronic diseases (Cramer 2004), multiple chronic conditions (Williams, Manias, and Walker 2008), and cost‐ and medication‐related factors (Claxton, Cramer, and Pierce 2001; Gellad, Grenard, and Marcum 2011). Researchers have documented the influence of several factors on medication adherence, such as higher cost sharing and OOP expenses (Leibowitz, Manning, and Newhouse 1985; Joyce et al. 2002; Goldman, Joyce, and Karaca‐Mandic 2006; Goldman, Joyce, and Zheng 2007; Gibson et al. 2010; Karaca‐Mandic et al. 2012; Rezayatmand, Pavlova, and Groot 2013), individual‐level characteristics including demographic, socioeconomic, and psychological factors (Cummings et al. 1982; Lewey et al. 2013; Rolnick et al. 2013), and health care system factors (Schmittdiel et al. 2011). Although scholars have synthesized findings using meta‐analyses, there remains a lack of consensus across the factors heavily influencing medication adherence (Eaddy et al. 2012; Conn et al. 2014; Murphy et al. 2014). As policy makers experiment with insurance coverage, benefit designs, and provider incentive programs, it is imperative to better understand the patient‐level drivers of medication adherence. In addition, most empirical studies and theories on medication adherence emphasize individual characteristics with less attention on societal factors. The growing disparities research on health care access, outcomes, and costs further elucidates the need to understand societal influences. This study expands the body of knowledge on medication adherence by examining the impact of cost sharing and individual‐level factors, as well as the impact of contextual factors (neighborhood level variables), on nonadherence to statin medications.
In support of prior research, these results suggest small increases in medication cost sharing are associated with nonadherence, even in a population of working individuals with presumed financial means. A minimal incremental monthly OOP cost burden of $10 is associated with a 7 percent higher nonadherence rate to statin therapy. This is important because many benefit managers and/or insurance plan designs have raised medication copayments by $10, or even $20 during the last few years in an effort to shift cost to consumers and save money for companies. Organizations that are self‐insured may find this cost‐saving strategy to be cost increasing if employees forgo medications and later suffer from more serious conditions. Governmental strategies to increase insurance coverage to the uninsured, through both insurance exchanges and Medicaid, may consider the presumed implications of high medication copayments. Presuming an increase in insurance coverage equates to an increase in medication adherence, in the absence of cost‐sharing considerations, appears incorrect. Furthermore, in the context of cholesterol management, nonadherence to statins may not lead to an immediate need for health care services; however, these individuals will likely have higher health care costs later in life when they are on Medicare. Astute health care leaders and policy makers may well weigh the presumed cost savings from increased copayments against the potential cost increases for unmanaged chronic diseases, both now and in the future.
Mail order pharmacies are another cost‐saving strategy that has become popular because of both the presumed ease of access and typical 3‐month medication supply allowance. Unfortunately, benefit managers and policy makers looking to mail order pharmacies as a method to increase access and reduce costs may be disappointed. This research suggests access to pharmacies, as measured by the number of pharmacies within the service area, does not impact adherence. Furthermore, mail order pharmacies allowing patients to order a 3‐month supply often charge higher copayment rates than if patients refill their medications monthly. Given the price sensitivity shown in this study, patients may be less apt to pay a higher copayment, even if it means more volume (pills). Perhaps insurance plan designers, including Medicaid and Medicare leaders, could consider spending more time negotiating reduced monthly copayments or biweekly refill alternatives, particularly for medications shown to effectively manage chronic diseases.
Among the predisposing factors considered in this study, there is strong evidence of higher medication adherence among males when compared to females and of older patients when compared to younger ones. The latter finding suggests that, among commercially insured Texas residents, individuals on statin therapy are more likely to continue with prescribed medications as they get older, which may reduce the future cost burden on Medicare. This finding suggests educational and marketing strategies tailored for women and/or younger patients are warranted. Legislators involved in expanding insurance coverage, funding wellness/preventative programs, and evaluating the effectiveness of cost‐reducing initiatives might consider the impact of education on disease management. Employers may also use the findings of this study to inform their strategies for targeting health education and disease management to younger employees.
Nonadherence to statin therapy is also associated with select neighborhood‐level socioeconomic factors, including race/ethnicity, educational attainment, and poverty level. In particular, the study results suggest that individuals residing in Hispanic or African American dominated neighborhoods are 33–56 percent more likely to be nonadherent with statin therapy when compared to non‐Hispanic white dominated communities. Communities with lower affluence are also shown to be more likely to be nonadherent. These findings imply targeted policies aimed at increasing medication usage in minority‐dominated and/or less affluent communities may be justified. Policies might include differing medication benefit designs (both governmental and private plans) for disadvantaged groups, aligning physician‐level financial incentives, like the physician quality reporting system, based on patient mix and informing/educating primary care providers and pharmacists in disadvantaged neighborhoods. Understanding the impact of the neighborhood‐level factors might also lead decision makers to create policies, processes, and educational materials specifically aimed at high‐risk populations and in Spanish, when necessary.
The study has several limitations. First, this is an observational study, so the reported findings cannot be interpreted as causal evidence. Second, the medication adherence measure derived from claims data may not necessarily reflect actual adherence behavior. Nonetheless, the PDC provides an estimate of long‐term persistence in medication refills, which is preferred over the medication possession ratio (Benner et al. 2002; Nau 2014). Third, enrollees who purchase statins from low‐cost providers with a fixed fee formulary, such as Walmart's $4 formulary, will not be included in the study. Currently, only one statin, Lovastatin, is being offered on Walmart's fixed fee formulary. The generic version of Lovastatin did not become available until November 2011, which is near the end of our study period and should have minimal effect on the results. Fourth, enrollees who purchase a 90‐day supply of statin medication (approximately 6 percent of enrollees) may bias the results in favor of adherence. Last, while this study focuses on identifying neighborhood‐level factors that may impact medication adherence behavior, the analysis does not include individual specific socioeconomic factors that are likely to be more dominant than the corresponding neighborhood‐level factors. Finally, there is substantial variation in the size of zip code areas, and it is plausible that populations residing in larger zip code areas may exhibit significant variation on the dimensions of socioeconomic factors (Herrin et al. 2015).
These results inform benefit managers, insurance designers, health care payers, providers, and policy makers on strategies for improving medication adherence in the chronically ill population. By devising clinical and educational interventions that smartly target patients based on their age, gender, and neighborhoods, the cost‐effectiveness of statin therapy as an intervention can be optimized. And creating financial incentives for both individuals (lower copayments) and providers (patient mix based) may positively influence medication adherence.
Conclusions
Medication use among chronically ill patients may be influenced by a plethora of factors acting at different levels of the ecological model, ranging from patient to societal level. Through operationalizing the overarching constructs of an HBM, including predisposing factors, neighborhood‐level enabling factors, and individual factors, this analysis studied statin medication nonadherence across 49,000+ privately insured working Texans. The results are consistent and within the range of past research, showing 48 percent of individuals are nonadherent. Increases in OOP expense are associated with a higher probability of nonadherence. Men are more adherent than females and age is negatively associated with nonadherence. Non‐white race status, low educational attainment, and high poverty levels are positively associated with nonadherence. These results reinforce and expand the findings of past research by eliciting the variation in medication adherence is partially explained by select neighborhood level socioeconomic factors.
This study signifies the importance of OOP costs as a determinant of adherence to medications, but more interestingly, the results suggest that other socioeconomic factors, as measured by neighborhood‐level variables, have a greater impact on the likelihood of adherence. For example, decreasing patients OOP costs by $10 was associated with 7 percent less likelihood of nonadherence, while living in neighborhoods dominated by Hispanic or African American populations was associated with 33 and 56 percent more probability of being nonadherent to statin medications. These results indeed emphasize the strong need to look beyond patients' clinical characteristics as the primary subject for interventions to improve adherence and rather focus on the contextual factors of patients' residence.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: Computation of PDC Rates.
Acknowledgments
Joint Acknowledgement/Disclosure Statement: The authors acknowledge Blue Cross Blue Shield of Texas for the use of their claims data for this research and the University of Texas School of Public Health Center for Healthcare Data Research for providing access to the data. The authors also acknowledge their respective institutions for financial salary support.
Disclosures: None.
Disclaimers: None.
References
- Aday, L. A. , and Andersen R. M.. 1974. “A Framework for the Study of Access to Medical Care.” Health Services Research 9: 208–20. [PMC free article] [PubMed] [Google Scholar]
- Andersen, R. M. 1995. “Revisiting the Health Behavioral Model and Access to Medical Care: Does it Matter?” Journal of Health and Social Behavior 36 (1): 1–10. [PubMed] [Google Scholar]
- Andersen, R. M. , and Newman J. F.. 1973. “Societal and Individual Determinants of Medical Care Utilization in the United States.” Milbank Quarterly 51 (1): 95–124. [PubMed] [Google Scholar]
- Bandura, A. 2004. “Health Promotion by Social Cognitive Means.” Health Education and Behavior 31: 143–64. [DOI] [PubMed] [Google Scholar]
- Benner, J. S. , Glynn R. J., Mogun H., Neumann P. J., Weinstein M. C., and Avorn J.. 2002. “Long‐Term Persistence in Use of Statin Therapy in Elderly Patients.” Journal of the American Medical Association 288: 455–61. [DOI] [PubMed] [Google Scholar]
- Benner, J. S. , Tierce J. C., Ballantyne C. M., Prasad C., Bullano M. F., Willey V. J., Erbey J., and Sugano D. S.. 2004. “Follow‐up Lipid Tests and Physician Visits Are Associated with Improved Adherence to Statin Therapy.” Pharmacoeconomics 22 (suppl 3): 13–23. [DOI] [PubMed] [Google Scholar]
- Blanchard, J. , Madden J. M., Ross‐Degnan D., Gresenz C. R., and Soumerai S. B.. 2013. “The Relationship between Emergency Department Use and Cost‐Related Medication Nonadherence among Medicare Beneficiaries.” Annals of Emergency Medicine 62 (5): 475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesacher, B. A. , Gurwitz J. H., and Soumerai S. B.. 2007. “Patients At‐Risk for Cost Related Medication Nonadherence: A Review of the Literature.” Journal of General Internal Medicine 22: 864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, D. C. , Shrank W. H., and Cutler D.. 2010. “Patient, Physician, and Payment Predictors of Statin Adherence.” Medical Care 48 (3): 196–202. [DOI] [PubMed] [Google Scholar]
- Chodick, G. , Shalev V., Gerber Y., Heymann A. D., Silber H., Simah V., and Kokia E.. 2008. “Long‐Term Persistence with Statin Treatment in a Not‐For‐Profit Health Maintenance Organization: A Population‐based Retrospective Cohort Study in Israel.” Clinical Therapeutics 30 (11): 2167–79. [DOI] [PubMed] [Google Scholar]
- Choudhry, N. K. , Fischer M. A., Avorn J., Schneeweiss S., Solomon D. H., Berman C., Jan S., Liu J., Lii J., Brookhart M. A., and Mahoney J. J.. 2010. “At Pitney Bowes, Value‐Based Insurance Design Cut Copayments and Increased Drug Adherence.” Health Affairs 29 (11): 1995–2001. [DOI] [PubMed] [Google Scholar]
- Chu, L. H. , Kawatkar A., and Gu A.. 2011. “SAS Macro Program to Calculate Medication Adherence Rate for Single and Multiple Medication Use” In Western Users of SAS Software Conference, pp. 12–14. San Francisco, CA. [Google Scholar]
- Claxton, A. J. , Cramer J., and Pierce C.. 2001. “A Systematic Review of the Associations between Dose Regimens and Medication Compliance.” Clinical Therapeutics 23 (8): 1296–310. [DOI] [PubMed] [Google Scholar]
- CMS (Centers for Medicare and Medicaid Services). 2010. “Enhancements to Medicare Part D Patient Safety Reports” [accessed on April 16, 2014]. Available at http://www.cms.gov/Medicare/Pre-script-ion-DrugCoverage/PrescriptionDrugCovContra/index.html
- CMS (Centers for Medicare and Medicaid Services) . 2014. “Quality Measures” [accessed on August 1, 2016). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html?redirect=/QUALITYMEASURES/
- Conn, V. S. , Enriquez M., Ruppar T. M., and Chan K. C.. 2014. “Cultural Relevance in Medication Adherence Interventions with Underrepresented Adults: Systematic Review and Meta‐Analysis of Outcomes.” Preventive Medicine 69: 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, B. M. , Reeve B. B., Cella D., Hays R. D., Pickard A. S., and Revicki D. A.. 2014. “Demographic Differences in Health Preferences in the United States.” Medical Care 52: 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, J. A. 2004. “A Systematic Review of Adherence with Medications for Diabetes.” Diabetes Care 27 (5): 1218‐24. [DOI] [PubMed] [Google Scholar]
- Cummings, M. K. , Becker M. H., Kirscht J. P., and Levin N. W.. 1982. “Psychosocial Factors Affecting Adherence to Medical Regimens in a Group of Hemodialysis Patients.” Medical Care 20 (6): 567–80. [DOI] [PubMed] [Google Scholar]
- Cummins, S. , Curtis S., Diez‐Roux A. V., and Macintyre S.. 2007. “Understanding and Representing ‘Place’ in Health Research: A Relational Approach.” Social Science & Medicine 65 (9): 1825–38. [DOI] [PubMed] [Google Scholar]
- Daviglus, M. L. , Pirzada A., and Talavera G. A.. 2014. “Cardiovascular Disease Risk Factors in the Hispanic/Latino Population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).” Progress in Cardiovascular Diseases 57 (3): 230–6. [DOI] [PubMed] [Google Scholar]
- Desai, P. R. , Adeyemi A. O., Richards K. M., and Lawson K. A.. 2014. “Adherence to Oral Diabetes Medications among Users and Nonusers of Antipsychotic Medication.” Psychiatric Services 65 (2): 215–20. [DOI] [PubMed] [Google Scholar]
- DeVol, R. , Bedroussian A., Charuworn A., Chatterjee A., Kim I. K., Kim S., and Klowden K.. 2007. “An Unhealthy America: The Economic Burden of Chronic Disease – Charting a New Course to Save Lives and Increase Productivity and Economic Growth” [accessed on April 16, 2014]. Available at http://www.chronicdiseaseimpact.com/
- Donnelly, L. A. , Doney A. S., Morris A. D., Palmer C. N., and Donnan P. T.. 2008. “Long–term Adherence to Statin Treatment in Diabetes.” Diabetic Medicine 25: 850–5. [DOI] [PubMed] [Google Scholar]
- Eaddy, M. T. , Cook C. L., O'Day K., Burch S. P., and Cantrell R.. 2012. “How Patient Cost–sharing Trends Affect Medication Adherence and Outcomes.” Pharmacy & Therapeutics 37 (1): 45–55. [PMC free article] [PubMed] [Google Scholar]
- Ellis, J. J. , Erickson S. R., Stevenson J. G., Bernstein S. J., Stiles R. A., and Fendrick A. M.. 2004. “Suboptimal Statin Adherence and Discontinuation in Primary and Secondary Prevention Populations: Should We Target Patients with the Most to Gain?” Journal of General Internal Medicine 19: 638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini, L. , Ribble J. C., and Keddie A. M.. 2001. “Understanding the Hispanic Paradox.” Ethnicity and Disease 11 (3): 496‐518. [PubMed] [Google Scholar]
- Gellad, W. F. , Grenard J. L., and Marcum Z. A.. 2011. “A Systematic Review of Barriers to Medication Adherence in the Elderly: Looking Beyond Cost and Regimen Complexity.” The American Journal of Geriatric Pharmacotherapy 9 (1): 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, T. B. , Mark T. L., Azelsen K., Baser O., Rublee D. A., and McGuigan K. A.. 2006. “Impact of Statin Copayments on Adherence and Medical Care Utilization and Expenditures.” The American Journal of Managed Care 12 (12): SP11–9. [PubMed] [Google Scholar]
- Gibson, T. B. , Song X., Alemayehu B., Wang S. S., Waddell J. L., Bouchard J. R., and Forma F.. 2010. “Cost Sharing, Adherence, and Health Outcomes in Patients with Diabetes.” The American Journal of Managed Care 16 (8): 589–600. [PubMed] [Google Scholar]
- Goff, D. C. , Lloyd‐Jones D. M., and Bennett G.. 2014. “American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.” Journal of the American College of Cardiology 63: 2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, D. P. , Joyce G. F., and Karaca‐Mandic P.. 2006. “Varying Pharmacy Benefits with Clinical Status: The Case of Cholesterol‐Lowering Therapy.” The American Journal of Managed Care 12 (1): 21–8. [PubMed] [Google Scholar]
- Goldman, D. P. , Joyce G. F., and Zheng Y.. 2007. “Prescription Drug Cost Sharing: Associations with Medication and Medical Utilization and Spending and Health.” Journal of the American Medical Association 298 (1): 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, M. R. 2014. “Healthography.” American Journal of Public Health 104 (11): 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, R. A. , Maciejewski M., Yu‐Isenberg K., and Farley J. F.. 2012. “Adherence to Antipsychotics and Cardiometabolic Medication: Association with Health Care Utilization and Costs.” Psychiatric Services 63 (9): 920–8. [DOI] [PubMed] [Google Scholar]
- Harrison, J. A. , Mullen P. D., and Green L. W.. 1992. “A Meta‐Analysis of Studies of the Health Belief Model with Adults.” Health Education and Research 7: 107–16. [DOI] [PubMed] [Google Scholar]
- Heisler, M. , Langa K., Eby E., Fendrick A. M., Kabeto M., and Piette J. D.. 2004. “The Health Effects of Restricting Prescription Medication Use Because of Cost.” Medical Care 42: 626–34. [DOI] [PubMed] [Google Scholar]
- Herrin, J. , St. Andre J., Kenward K., Joshi M. S., Audet A. M. J., and Hines S. C.. 2015. “Community Factors and Hospital Readmission Rates.” Health Services Research 50: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insull, W. 1997. “The Problem of Compliance to Cholesterol Altering Therapy.” Journal of Internal Medicine 241 (4): 317–25. [DOI] [PubMed] [Google Scholar]
- Joyce, G. F. , Escarce J. J., Solomon M. D., and Goldman D. P.. 2002. “Employer Drug Benefit Plans and Spending on Prescription Drugs.” Journal of the American Medical Association 288 (14): 1733–9. [DOI] [PubMed] [Google Scholar]
- Karaca‐Mandic, P. , Swenson T., Abraham J. M., and Kane R. L.. 2012. “Association of Medicare Part D Medication Out‐of‐Pocket Costs with Utilization of Statin Medications.” Health Services Research 48 (4): 1311–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz, A. , Manning W. G., and Newhouse J. P.. 1985. “The Demand for Prescription Drugs as a Function of cost‐Sharing.” Social Science & Medicine 21 (10): 1063–9. [DOI] [PubMed] [Google Scholar]
- Leslie, R. S. 2007. “Using Arrays to Calculate Medication Utilization.” Paper# 043–2007, SAS Global Forum [accessed April 15, 2014].
- Leslie, S. R. , Gwadry‐Sridhar F., Thiebaud P., and Patel B. V.. 2008. “Calculating Medication Compliance, Adherence and Persistence in Administrative Pharmacy Claims Databases.” Pharmaceutical Programming 1 (1): 13–9. [Google Scholar]
- Lewey, J. , Shrank W. H., Bowry A. D. K., Kilabuk E., Brennan T. A., and Choudhry N. K.. 2013. “Gender and Racial Disparities in Adherence to Statin Therapy: A Meta‐Analysis.” American Heart Journal 165 (5): 665–78. [DOI] [PubMed] [Google Scholar]
- Link, B. G. , and Phelan J.. 1995. “Social Conditions as Fundamental Causes of Disease.” Journal of Health and Social Behavior 35: 80–94. [PubMed] [Google Scholar]
- Macintyre, S. , Ellaway A., and Cummins S.. 2002. “Place Effects on Health: How Can We Conceptualize, Operationalize and Measure Them?” Social Science & Medicine 55 (1): 125–39. [DOI] [PubMed] [Google Scholar]
- Magzamen, S. , Brandt S. J., and Tager I. B.. 2014. “Examining Household Asthma Management Behavior through a Microeconomic Framework.” Health Education & Behavior 41 (6): 651–62. [DOI] [PubMed] [Google Scholar]
- Mann, D. M. , Allegrante J. P., Natarajan S., Halm E. A., and Charlson M.. 2007. “Predictors of Adherence to Statins for Primary Prevention.” Cardiovascular Drugs Therapeutics 21: 311–6. [DOI] [PubMed] [Google Scholar]
- Mann, D. M. , Woodward M., Muntner P., Falzon L., and Kronish I.. 2010. “Predictors of Nonadherence to Statins: A Systematic Review and Meta‐Analysis.” The Annals of Pharmacotherapy 44 (9): 1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina‐Inojosa, J. , Jean N., Cortes‐Bergoderi M., and Lopez‐Jimenez F.. 2014. “The Hispanic Paradox in Cardiovascular Disease and Total Mortality.” Progress in Cardiovascular Diseases 57 (3): 286–92. [DOI] [PubMed] [Google Scholar]
- Montez, J. K. , and Friedman E. M.. 2015. “Educational Attainment and Adult Health: Under What Conditions Is the Association Causal?” Social Science & Medicine 127 (Feb.): 1–7. [DOI] [PubMed] [Google Scholar]
- Munro, S. S. , Lewin T. Swart., and Volmink J.. 2007. “A Review of Health Behavior Theories: How Useful Are These for Developing Interventions to Promote Long‐Term Medication Adherence for TB and HIV/AIDS?” BMC Public Health 7: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, G. K. , McAlister F. A., Weir D. L., Tjosvold L., and Eurich D. T.. 2014. “Cardiovascular Medication Utilization and Adherence among Adults Living in Rural and Urban Areas: A Systematic Review and Meta‐Analysis.” BMC Public Health 14 (1): 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau, D. P. 2014. “Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence.” Pharmacy Quality Alliance [accessed on April 15, 2014]. Available at http://pqaalliance.org/resources/adherence.asp
- NCHS (National Center for Health Statistics). 2016. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Nelson, L. A. , Graham M. R., Lindsey C., and Rasu R. S.. 2011. “Adherence to Antihyperlipidemic Medication and Lipid Control in Diabetic Veterans Affairs Patients with Psychotic Disorders.” Psychosomatics 52 (4): 310–8. [DOI] [PubMed] [Google Scholar]
- Pencina, M. J. , Navar‐Bogga A. M., D'Agostino R. B., Williams K., Neely B., Sniderman A. D., and Peterson E. D.. 2014. “Application of New Cholesterol Guidelines to a Population Based Sample.” The New England Journal of Medicine 370 (15): 1422–31. [DOI] [PubMed] [Google Scholar]
- Perreault, S. , Ellia L., Dragomir A., Cote R., Blais L., and Berard A.. 2009. “Effect of Statin Adherence on Cerebrovascular Disease in Primary Prevention.” The American Journal of Medicine 122 (7): 647–55. [DOI] [PubMed] [Google Scholar]
- Phelan, J. C. , Link B. G., and Tehranifar P.. 2010. “Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications.” Journal of Health and Social Behavior 51 (S): S28–40. [DOI] [PubMed] [Google Scholar]
- Poland, B. , Lehoux P., Holmes D., and Andrews G.. 2005. “How Place Matters: Unpacking Technology and Power in Health and Social Care.” Health & Social Care in the Community 13 (2): 170–80. [DOI] [PubMed] [Google Scholar]
- Rasmussen, J. N. , Chong A., and Alter D. A.. 2007. “Relationship between Adherence to Evidence‐Based Pharmacotherapy and Long‐Term Mortality after Acute Myocardial Infarction.” Journal of the American Medical Association 297: 177–86. [DOI] [PubMed] [Google Scholar]
- Rezayatmand, R. , Pavlova M., and Groot W.. 2013. “The Impact of Out‐of‐Pocket Payments on Prevention and Health‐Related Life Style: A Systematic Literature Review.” European Journal of Public Health 23 (1): 74–9. [DOI] [PubMed] [Google Scholar]
- Rolnick, S. J. , Pawloski P. A., Hedblom B. D., Asche S. E., and Bruzek R. J.. 2013. “Patient Characteristics Associated with Medication Adherence.” Clinical Medicine & Research 11 (2): 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, L. , and Shrank W. H.. 2013. “Taking Our Medicine–Improving Adherence in the Accountability Era.” New England Journal of Medicine 369 (8): 694‐5. [DOI] [PubMed] [Google Scholar]
- RTI (Research Triangle Institute). 2012. Accountable Care Organization 2012 Program Analysis: Quality Performance Standards Narrative Measure Specifications. Waltham, MA: RTI International. [Google Scholar]
- Sabaté E. (ed.). 2003. Adherence to Long‐Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- Schmittdiel, J. A. , Karter A. J., Dyer W., Parker M., Uratsu C., and Chan J.. 2011. “The Comparative Effectiveness of Mail Order Pharmacy Use vs. Local Pharmacy Use on LDL‐C Control in New Statin Users.” Journal of General Internal Medicine 26: 1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweiss, S. , Patrick A. R., Maculre M., Dormuth C. R., and Glynn R. J.. 2007. “Adherence to Statin Therapy under Drug Cost Sharing in Patients with and without Acute Myocardial Infarction: A Population‐Based Natural Experiment.” Circulation 115 (16): 2128–35. [DOI] [PubMed] [Google Scholar]
- Smith, W. C. , Anderson E., Salinas D., Horvatek R., and Baker D. P.. 2015. “A Meta‐Analysis of Education Effects on Chronic Disease: The Causal Dynamics of the Population Education Transition Curve.” Social Science & Medicine 127: 29–40. [DOI] [PubMed] [Google Scholar]
- Sokol, M. C. , McGuigan K. A., Verbrugge R. R., and Epstein R. S.. 2005. “Impact of Medication Adherence on Hospitalization Risk and Healthcare Cost.” Medical Care 43 (6): 521–30. [DOI] [PubMed] [Google Scholar]
- Solomon, M. D. , Goldman D. P., Joyce G. F., and Escarce J. J.. 2009. “Cost Sharing and the Initiation of Drug Therapy for the Chronically Ill.” Archives of Internal Medicine 169 (8): 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, N. J. , Robinson J., Lichtenstein A. H., Merz N. B., Blum C. B., Eckel R. H., Goldberg A. C., Gordon D., Levy D., Lloyd‐Jones D. M., McBride P., Schwartz J. S., Shero S. T., Smith S. C. Jr, Watson K., and Wilson P. W. F.. 2014. “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.” Circulation 63: 2889–934. [DOI] [PubMed] [Google Scholar]
- Thiebaud, P. , Patel B. V., and Nichol M. B.. 2008. “The Demand for Statin: The Effect of Copay on Utilization and Compliance.” Health Economics 17: 83–97. [DOI] [PubMed] [Google Scholar]
- Thorpe, C. T. , Johnson H., Dopp A. L., Thorpe J. M., Ronk K., Everett C. M., Palta M., Mott D. A., Chewning B., Schleiden L., and Smith M. A.. 2015. “Medication Oversupply in Patients with Diabetes.” Research in Social and Administrative Pharmacy 11 (3): 382–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni, E. , and Farris K. B.. 2011. “Determinants of Different Types of Medication Non‐Adherence in Cholesterol Lowering and Asthma Maintenance Medications: A Theoretical Approach.” Patient Education and Counseling 83: 382–90. [DOI] [PubMed] [Google Scholar]
- Vaidya, V. , Hufstader‐Gabriel M., Gangan N., Shah S., and Bechtol R.. 2014. “Utilization of Smoking‐Cessation Pharmacotherapy among Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer Patients.” Current Medical Research & Opinion 30 (6): 1043–50. [DOI] [PubMed] [Google Scholar]
- Wallach‐Kildemoes, H. , Andersen M., Diderichsen F., and Lange T.. 2013. “Adherence to Preventive Statin Therapy According to Socioeconomic Position.” European Journal of Clinical Pharmacology 69 (8): 1553–63. [DOI] [PubMed] [Google Scholar]
- Williams, A. , Manias E., and Walker R.. 2008. “Interventions to Improve Medication Adherence in People with Multiple Chronic Conditions: A Systematic Review.” Journal of Advanced Nursing 63 (2): 132–43. [DOI] [PubMed] [Google Scholar]
- Xu, K. T. , Smith S. R., and Borders T. F.. 2003. “Access to Prescription Drugs among Noninstitutionalized Elderly People in West Texas.” American Journal of Health System Pharmacy 60 (7): 675–82. [DOI] [PubMed] [Google Scholar]
- Ye, X. , Gross C. R., Schommer J., Cline R., and Peter W. L. S.. 2007. “Association between Copayment and Adherence to Statin Treatment Initiated after Coronary Heart Disease Hospitalization: A Longitudinal, Retrospective, Cohort Study.” Clinical Therapeutics 29 (12): 2748–57. [DOI] [PubMed] [Google Scholar]
- Yeaw, J. 2009. “Comparing Adherence and Persistence across Six Chronic Medication Classes.” Journal of Managed Care Pharmacy 15 (9): 728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, A. P. , Yu Y. F., Nichol M. B., and Gwadry‐Sridhar F.. 2008. “Delay in Filling the Initial Prescription for a Statin: A Potential Early Indicator of Medication Nonpersistence.” Clinical Therapeutics 30: 761–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Computation of PDC Rates.


