Abstract
Objective
There is a need for inexpensive non-invasive tests to identify older healthy persons at risk for Alzheimer’s disease (AD) for enrollment in AD prevention trials. Our objective was to examine whether abnormalities in neuroimaging measures of amyloid and neurodegeneration are correlated with odor identification (OI) in the population-based Mayo Clinic Study of Aging (MCSA).
Methods
Cognitively normal (CN) participants had olfactory function assessed using the Brief Smell Identification Test (B-SIT), underwent magnetic resonance imaging (MRI; n=829) to assess a composite Alzheimer’s disease (AD) signature cortical thickness and hippocampal volume (HVa), and, 11C-Pittsburgh compound B (11C-PiB; n=306) and 18fluorodeoxyglucose (18F-FDG; n=305) positron emission tomography (PET) scanning to assess amyloid accumulation and brain hypometabolism, respectively. The association of neuroimaging biomarkers with OI was examined using multinomial logistic regression and simple linear regression models adjusted for potential confounders.
Results
Among 829 CN participants (mean age 79.2 years; 51.5% men), 248 (29.9%) were normosmic and 78 (9.4%) had anosmia (B-SIT score <6). Abnormal AD signature cortical thickness and reduced HVa were associated with decreased OI as a continuous measure (slope=−.43; (95%CI −.77, −.09); p=.01 and slope=−.72; (95%CI −1.15, −.28); <.01, respectively). Reduced HVa, decreased AD signature cortical thickness and increased amyloid accumulation were significantly associated with increased odds of anosmia.
Interpretation
Our findings suggest that OI may be a non-invasive, inexpensive marker for risk stratification, for identifying participants at the preclinical stage of AD who may be at risk for cognitive impairment, and eligible for inclusion in AD prevention clinical trials. These cross-sectional findings remain to be validated prospectively.
INTRODUCTION
The prevalence and severity of impaired olfaction increases with age.1 It affects more than half of the population aged 65–80 years old, and 62–80% of persons older than 80 years.1 Impaired olfaction negatively affects quality of life, enjoyment of food, physical and mental well-being or safety, and mortality.2, 3 The association with increased mortality in older individuals may be mediated by cognitive impairment.2, 4
Factors that may contribute to olfactory loss in aging include damage to olfactory epithelium, nasal engorgement, sensory loss of receptor cells to odorants, decrease in mucosal metabolizing enzymes, and neurochemical changes in the brain.1, 3 However, olfactory impairment in aging or neurodegenerative disease may result from the expression of aberrant proteins in the olfactory system that cause structural or functional abnormalities in the olfactory system (e.g., olfactory epithelium, olfactory bulb, central olfactory cortex, or olfactory circuitry).1
Olfactory impairment has been associated with cognitive decline,5 mild cognitive impairment (MCI),6 Alzheimer ’s disease (AD) dementia,7–11 vascular dementia,12 Parkinson’s disease,13 dementia with Lewy bodies,14 as well as, the progression from MCI to AD dementia.6, 8 In cognitively normal elderly individuals, worse odor identification (OI) has also been associated with markers of brain pathology, such as increased cortical amyloid and thinner entorhinal cortex,15 and with neurofibrillary pathology in the entorhinal cortex and hippocampus in autopsy studies.16 Thus, changes in olfactory function in cognitively normal persons, could represent “pre-clinical” neurodegenerative disease.3
There is a need for inexpensive non-invasive tests to identify older healthy persons potentially at risk for AD for enrollment in AD prevention trials. One of the earliest brain regions affected by AD is the olfactory system,17, 18 suggesting that olfactory impairment may be an early sign of AD brain pathology, an intermediate marker in the causal pathway from brain biomarker pathology to cognitive impairment. Amyloid-β (Aβ) accumulation has been described in areas of the olfactory network in AD and amnestic MCI participants but also in elderly persons with normal neuropsychological test scores.19 A recent meta-analysis suggested that odor identification and recognition could be the most interesting candidate for inclusion in a group of biomarkers to detect subclinical AD,20 especially when combined with clinical/neuropsychological21 assessment and imaging biomarkers.22
We hypothesized that in vivo neuroimaging biomarkers of AD pathology are associated with olfactory impairment among older cognitively normal (CN) individuals. The objective of the present study, therefore, was to examine the cross-sectional associations between neuroimaging measures of amyloid accumulation and neurodegeneration and a simple measure of OI in a large population-based cohort of cognitively normal older persons.
METHODS
Study population
Details of the MCSA design and methodology have been previously reported.23, 24 Residents in Olmsted County, MN aged 70–89 years on October 1, 2004 (prevalence –index date), were enumerated using the Rochester Epidemiology Project (REP)25 resources and an age and sex-stratified random sample of eligible subjects (without dementia, not terminally ill or in hospice) was invited to participate. Ongoing recruitment was performed beginning from 2008 through 2011, using the same protocols as in 2004. Participants were comprehensively evaluated for a diagnosis of MCI16 or dementia26 and normal cognition as previously described.23, 24 Overall, this analysis includes 829 cognitively normal participants (822 participants were white), who were recruited between 2004 and 2010, completed an in-person evaluation, the Brief Smell Identification Test (B-SIT),27 and underwent magnetic resonance imaging (MRI). Of these individuals, 351 had 11C-PiB PET scans and 350 had 18F-FDG PET scans available during the same MCSA follow-up visit as the B-SIT. Participants with a diagnosis of Parkinson’s Disease (PD) were excluded. During follow-up, two participants developed PD, 138 developed MCI and 13 developed dementia.
Standard Protocol Approvals, Registrations, and Patient Consent
The study was approved by the Institutional Review Boards of the Mayo Clinic and the Olmsted Medical Center. Written informed consent was obtained prior to participation in the study.
Assessment of Olfactory Function
Olfactory impairment was assessed using the B-SIT27 version A that consists of six food-related and six nonfood-related smells (cherry, clove, strawberry, menthol, pineapple, lemon, leather, lilac, smoke, soap, natural gas, and rose). Participants had to scratch, sniff and select one of 4 possible choices. The B-SIT score was calculated as the sum of the correct responses for persons with ≤2 missing responses; for persons with 1 (n=10) or 2 (n=4) missing responses a score of 0.25 was assigned for each missing response.7, 10 Osmia categories were defined by B-SIT score as: anosmia (score <6), hyposmia (or microsmia) (men 6–10, women 6–10.25), and normosmia (men 10.25–12; women 10.5–12)9.
Acquisition of MRI measures
MRI studies were performed at 3 tesla (Signa; GE Healthcare, Waukesha, WI) with an 8-channel phased-array head coil acquiring both a 3-dimensional magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence and a fluid-attenuated inversion recovery (FLAIR) sequence.28 Hippocampal volume (HVa) was measured using the FreeSurfer software (version 5.3), and adjusted for total intracranial volume (n=821).29 Cortical thickness was measured using FreeSurfer (v 5.3) and an AD-signature cortical thickness measure30 was computed by averaging the cortical thickness for entorhinal, inferior temporal, middle temporal, and fusiform cortices (n=826).
18F-FDG PET and 11C-PiB PET Acquisition
PET images were acquired using a PET/CT scanner operating in 3-dimensional mode.31 A detailed 18F-FDG PET and 11C-PiB PET acquisition process is published.31–33 An amyloid PET standardized uptake value ratio (SUVR) was formed from the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest (ROIs) normalized to the whole cerebellum (n=306).31 An AD signature34 18F-FDG PET SUVR (non-sharpened, non-partial volume corrected) was calculated based on glucose metabolic rates from an AD signature meta-ROI and consisted of the average bilateral angular gyri, posterior cingulate, and inferior temporal cortical ROIs from both hemispheres normalized to pons and vermis uptake (n=305).35–37
Neuroimaging biomarkers cut points
The cut-points for biomarker abnormality were defined such that 90% of a group of 75 clinically diagnosed AD dementia subjects from the Mayo Clinic Alzheimer Disease Research Center and MCSA were categorized as abnormal. Abnormal HVa was defined as <−2.40 cm3 (adjusted for total intracranial volume); abnormal FDG PET was defined as SUVR <1.32; abnormal AD signature cortical thickness was defined as <2.74 mm; abnormal amyloid PET was defined as SUVR >1.40 and was validated by autopsy correlation with Thal amyloid phase.30, 34, 38, 39
Covariates
Information on age, sex, years of educational, body mass index (weight in kilograms divided by height in meters squared), smoking habits, self-reported alcohol problems and gait-speed (m/s), was collected at the baseline evaluation; history of type 2 diabetes, hypertension and stroke was obtained from medical record abstraction; apolipoprotein E (APOE) genotyping was performed at baseline.
Statistical Analysis
Cross-sectional associations of abnormal 11C-PiB PET, abnormal AD signature 18F-FDG PET [hypometabolism], abnormal AD signature cortical thickness and abnormal HVa with the osmia categories were examined using multinomial logistic regression models (odds ratios [ORs], 95% confidence intervals [CIs]) having normosmia as the comparison group. Examination of the residuals of regressions of the continuous B-SIT score on abnormal neuroimaging biomarkers, adjusting for age, sex and education, suggested linear associations. We also used multivariable linear regression models to examine the association (slope, 95% CIs) of abnormal biomarkers with continuous B-SIT as the dependent (outcome) variable.
Separate models were fit with each neuroimaging biomarker as the independent (exposure) variable and the B-SIT score (categorical and continuous) as the dependent (outcome) variable. All models were adjusted for age (at B-SIT), sex and education (basic model); TIV was included in models with amygdala volume. We assessed the interaction between abnormal 11C-PiB PET and each of the other three neuroimaging biomarkers (i.e., abnormal AD signature cortical thickness, abnormal HVa and abnormal 18F-FDG PET) and separate analyses were performed in individuals with and without abnormal 11C-PiB PET as there was a statistically significant interaction between abnormal 11C-PiB PET and abnormal 18F-FDG PET.
The association of biomarker groups defined by the combination of abnormality for amyloid (A+/A−) and neurodegeneration (N+/N−) (i.e., A−N−, A+N−, A−N+, and A+N+)34 with the B-SIT score and osmia categories were examined with adjustment for age, sex and education. Presence of neurodegeneration was defined as either HVa ≤−2·40 cm3 or FDG ≤1.32 SUVR.34
Multinomial logistic regression models were also fit to additionally adjust (in separate models) for APOE ε4 carrier status (ε4 carrier vs noncarrier), Unified Parkinson Disease Rating Scale,40 the Boston Naming Test41 and history of head trauma (i.e, the participants were asked “Have you ever experienced any head injuries that led you to see a doctor, stay in the hospital, lose your memory, or become unconscious”). Final model simultaneously adjusted for APOE e4 allele, type 2 diabetes, hypertension, stroke, self-reported alcohol problem and ever smoking; the estimates were essentially unchanged therefore only estimates from the previous more parsimonious models are reported.
In multivariable linear regression models, the associations (slopes [beta estimates], 95% CIs) of MRI and PET ROI measurements in areas of the primary olfactory cortex (e.g. amygdala, entorhinal cortex), the secondary olfactory regions (e.g., e.g., hippocampus, hypothalamus, thalamus, insula, orbitofrontal cortex), and areas where odorant-induced activation has been recorded in previous fMRI and PET studies, including precentral gyrus, superior and inferior temporal gyrus, cingulate gyrus, occipital lobe, parietal lobe,42, 43 and inferior frontal gyrus44 with continuous B-SIT score were examined. No mathematical correction45 was applied for multiple comparisons to avoid increasing type II error, as the investigation broadly examined the associations of smell and neuroimaging measures of amyloid and neurodegeneration so as to generate hypothesis for future prospective studies.
Associations were considered significant at a p value < 0.05, and were performed using SAS statistical software version 9.3 (SAS Institute, Cary, North Carolina) and Stata/SE statistical software version 14.2 (StataCorp LP, College Station, Texas).
RESULTS
Characteristics of participants
Table 1 presents characteristics of 829 participants by osmia categories. Of the 829 participants, (mean age 79.2 years; 51.5% men), 248 (29.9%) were normosmic, 78 (9.4%) had anosmia and 503 (60.7%) had hyposmia; anosmia was significantly more frequent in men. Thirty percent (n=249) of the participants had abnormal cortical thickness and 15.5 % (n=128) had abnormal HVa. Among participants with available PET scans, 38.9 % had abnormal 11C-PiB PET (n=119) and 29.5 % had abnormal 18F-FDG PET (n=90), while 16.9% (n=51) were positive for both β-amyloidosis and neurodegeneration biomarkers (A+N+). The frequencies of abnormal 11C-PiB PET, abnormal AD cortical thickness and abnormal hippocampal volume increased with increasing impairment in OI. Compared to MCSA participants with B-SIT assessment who did not participate in imaging studies (n=595), participants with imaging (n=829) performed better on cognitive tests (i.e., had higher global composite scores) and had a lower frequency of hypertension (data not shown).
Table 1.
Characteristics of Participants - the Mayo Clinic Study of Aging.
| Characteristics | Normosmiaa (N=248) |
Hyposmia (N=503) |
Anosmia (N=78) |
pb |
|---|---|---|---|---|
| Age (years) at time of B-SIT, mean (SD) | 77.7 (4.7) | 79.7 (5.2) | 81.3 (5.4) | <0.001 |
| Female | 145 (58.5) | 227 (45.1) | 30 (38.5) | <0.001 |
| Education (years), mean (SD) | 14.6 (2.7) | 14.2 (2.9) | 14.9 (2.8) | 0.037 |
| Ever Smoker (at time of B-SIT) | 113 (45.6) | 238 (47.3) | 42 (53.8) | 0.441 |
| APOE ε24/ε34/ε44 | 61 (24.6) | 134 (26.6) | 15 (19.2) | 0.357 |
| Depressive symptoms BDI (>=13) (at time of B-SIT) | 8 (3.2) | 32 (6.4) | 4 (5.1) | 0.196 |
| History of diabetes mellitus II (definite/probable) | 33 (13.3) | 104 (20.7) | 9 (11.5) | 0.015 |
| History of stroke | 7 (2.8) | 18 (3.6) | 6 (7.7) | 0.135 |
| History of hypertension | 178 (71.8) | 387 (76.9) | 60 (76.9) | 0.287 |
| History of self-reported alcohol problems | 7 (2.8) | 20 (4.0) | 2 (2.6) | 0.645 |
| Continuous B-SIT score, mean (SD) | 11.4 (0.5) | 8.7 (1.3) | 3.9 (1.2) | <0.001 |
| Abnormal 11C-PiB PETc | 33 (32.4) | 69 (39.2) | 17 (60.7) | 0.024 |
| Abnormal 18F-FDG PETd | 26 (25.5) | 54 (30.9) | 10 (35.7) | 0.481 |
| Abnormal AD Sig. CTe | 54 (21.8) | 159 (31.8) | 36 (46.2) | <0.001 |
| Abnormal HVaf | 21 (8.5) | 85 (17.2) | 22 (28.2) | <0.001 |
| Imaging Biomarker Groupg | 0.016 | |||
| A−/N− | 47 (46.1) | 68 (39.5) | 8 (28.6) | |
| A+/N− | 24 (23.5) | 37 (21.5) | 6 (21.4) | |
| A−/N+ | 22 (21.6) | 36 (20.9) | 3 (10.7) | |
| A+/N+ | 9 (8.8) | 31 (18.0) | 11 (39.3) |
N (%) unless otherwise noted.
Osmia categories were defined as follows based on the B-SIT score: anosmia (score <6), hyposmia (men 6–10, women 6–10.25), normosmia (men 10.25–12; women 10.5–12).
Chi-squared test for categorical variables or Wilcoxon rank sum test for continuous variables.
Abnormal 11C-PiB PET (elevated amyloid) was defined as standardized uptake value ratio (SUVR)>1.40; 306 participants had 11C-PiB PET.
Abnormal 18F-FDG PET (hypometabolism) was defined as SUVR <1.32; 305 participants had 18F-FDG PET.
Abnormal (reduced) AD Sig. CT was defined as <2.74 mm.
Abnormal (reduced) hippocampal volume was defined as <−2.40 cm3 (adjusted for total intracranial volume).
Imaging biomarker groups defined by the combination of abnormality for amyloid (A+/A−) and neurodegeneration (N+/N−).
B-SIT = Brief Smell Identification Test; APOE = apolipoprotein E; BDI = Beck depression inventory; 11C-PiB PET = Pittsburgh compound B positron emission tomography; 18F-FDG = 18fluorodeoxyglucose; AD Sig. CT = Alzheimer’s Disease signature cortical thickness; HVa = hippocampal volume adjusted for total intracranial volume.
Association of olfactory function and imaging biomarkers
Individuals with abnormal 11C-PiB PET, abnormal AD signature cortical thickness and abnormal HVa had significantly increased odds of having anosmia (vs. normosmia) (OR: 2.74, 95% CI: 1.12–6.66; OR=2.20, 95%CI (1.25, 3.86); and OR=2.45, 95%CI (1.21, 4.94), respectively, adjusting for age, sex and education) (Table 2). These estimates remained relatively stable with adjustments for APOE ε4 allele, Unified Parkinson Disease Rating Scale,40 the Boston Naming Test41 and history of head trauma in separate models (Table 2). Biomarkers were not significantly associated with hyposmia.
Table 2.
Association between Neuroimaging Biomarkers and Impaired Odor Identification in Cognitively Normal Individuals - the Mayo Clinic Study of Aging.
| Impaired Olfaction | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Anosmia vs. normosmiaa | Hyposmia vs. normosmiaa | |||||
|
| ||||||
| Imaging Biomarkers | No. Abn /All | OR (95%CI)b | p | No. Abn /All | OR (95%CI) | p |
| Basic modelc | ||||||
| Abnormal 11C-PiB PETd | 28/130 | 2.74 (1.12, 6.66) | .03 | 176/278 | 1.25 (.74, 2.12) | .40 |
| Abnormal 18F-FDG PETe | 28/130 | .99 (.38, 2.55) | .98 | 175/277 | 1.06 (.59, 1.91) | .84 |
| Abnormal AD Sig. CTf | 78/326 | 2.20 (1.25, 3.86) | <.01 | 500/748 | 1.34 (.92, 1.94) | .13 |
| Abnormal HVag | 78/326 | 2.45 (1.21, 4.94) | .01 | 495/743 | 1.61 (.95, 2.72) | .08 |
| Basic model adjusted also for APOE ε4 carrier status | ||||||
| Abnormal 11C-PiB PET | 28/130 | 2.78 (1.11, 7.01) | .03 | 176/278 | 1.23 (.72, 2.12) | .45 |
| Abnormal 18F-FDG PET | 28/130 | .97 (.38, 2.50) | .95 | 175/277 | 1.05 (.58, 1.89) | .86 |
| Abnormal AD Sig. CT | 78/326 | 2.21 (1.25, 3.89) | <.01 | 500/748 | 1.33 (0.91, 1.93) | .14 |
| Abnormal HVa | 78/326 | 2.47 (1.22, 5.00) | .01 | 495/743 | 1.60 (.94, 2.71) | .08 |
| Basic model adjusted also for the Boston Naming Testh | ||||||
| Abnormal 11C-PiB PET | 26/126 | 2.70 (1.08, 6.78) | .03 | 173/273 | 1.24 (.73, 2.11) | .42 |
| Abnormal 18F-FDG PET | 26/126 | .98 (.37, 2.62) | .97 | 172/272 | 1.09 (.60, 1.97) | .78 |
| Abnormal AD Sig. CT | 75/319 | 2.05 (1.15, 3.66) | .01 | 485/729 | 1.29 (.88, 1.88) | .19 |
| Abnormal HVa | 75/319 | 2.30 (1.12, 4.69) | .02 | 480/724 | 1.56 (.92, 2.65) | .10 |
| Basic model adjusted also for UPDRS | ||||||
| Abnormal 11C-PiB PET | 28/130 | 2.80 (1.14, 6.86) | .02 | 176/278 | 1.25 (.74, 2.12) | .40 |
| Abnormal 18F-FDG PET | 28/130 | .96 (.37, 2.49) | .94 | 175/277 | 1.05 (.59, 1.89) | .86 |
| Abnormal AD Sig. CT | 78/326 | 2.15 (1.22, 3.79) | <.01 | 500/748 | 1.32 (.91, 1.92) | .14 |
| Abnormal HVa | 78/326 | 2.43 (1.20, 4.90) | .01 | 495/743 | 1.60 (.94, 2.71) | .08 |
| Basic model adjusted also for history of head traumai | ||||||
| Abnormal 11C-PiB PET | 27/102 | 2.65 (1.04, 6.72) | .04 | 144/219 | 1.12 (.63, 2.02) | .70 |
| Abnormal 18F-FDG PET | 27/102 | .98 (.37, 2.59) | .97 | 143/218 | .97 (.51, 1.85) | .93 |
| Abnormal AD Sig. CT | 75/285 | 2.22 (1.24, 3.98) | <.01 | 454/664 | 1.31 (.88, 1.95) | .18 |
| Abnormal HVa | 75/285 | 2.20 (1.08, 4.49) | .03 | 451/661 | 1.45 (.85, 2.47) | .18 |
Osmia categories were defined based on the Brief Smell Identification Test (B-SIT) score as follows: anosmia (score <6), hyposmia (men 6–10, women 6–10.25), normosmia (men 10.25–12; women 10.5–12).
OR (95% CI) retained from multinomial logistic regression. Separate models were run for each neuroimaging biomarker, represented by one row in the table, having osmia as the outcome variable.
Basic model is adjusted for age, education, sex.
Abnormal 11C-PiB PET (elevated amyloid) was defined as standardized uptake value ratio (SUVR)>1.40.
Abnormal 18F-FDG PET (hypometabolism) was defined as SUVR <1.32.
Abnormal (reduced) AD Sig. CT was defined as <2.74 mm.
Abnormal (reduced) hippocampal volume was defined as <−2.40 cm3 (adjusted for total intracranial volume).
Kaplan EGH, Weintraub S. The Boston Naming Test, 2nd ed. Boston, MA: Lea & Fabiger, 1978.
History of head trauma: “Have you ever experienced any head injuries that led you to see a doctor, stay in the hospital, lose your memory, or become unconscious”.
No. Abn. - the number of persons with abnormal B-SIT; All - participants with and without abnormal biomarker in the specific analysis strata; OR – odds ratio; CI – confidence interval. 11C-PiB PET - Pittsburgh compound B positron emission tomography; 18F-FDG PET - 18fluorodeoxyglucose PET; AD Sig. CT - Alzheimer’s Disease signature cortical thickness; HVa: hippocampal volume adjusted for total intracranial volume; UPDRS - Unified Parkinson’s Disease Rating Scale.
In analyses examining the association of imaging biomarkers with B-SIT as a continuous outcome variable, abnormal AD signature cortical thickness and abnormal HVa were significantly associated with lower B-SIT score (slope (95%CI)=−.43 (−.76, −.09), p=.01; slope (95%CI)=−.72 (−1.15, −.28), p<.01, respectively), adjusting for age, sex and education (Table 3).
Table 3.
Association between Neuroimaging Biomarkers and B-SIT Score in Cognitively Normal Individuals - the Mayo Clinic Study of Aging.
| Continuous B-SIT | ||
|---|---|---|
|
| ||
| Imaging Biomarkers | slope (95%CI)a | p |
| Basic model | ||
| Abnormal 11C-PiB PETb | −.41 (−.91, .08) | .10 |
| Abnormal 18F-FDG PETc | −.12 (−.67, .42) | .66 |
| Abnormal AD Sig. CTd | −.43 (−.76, −.09) | .01 |
| Abnormal HVae | −.72 (−1.15, −.28) | <.01 |
| Basic model adjusted also for APOE ε4 carrier status | ||
| Abnormal 11C-PiB PET | −.36 (−.87, .15) | .16 |
| Abnormal 18F-FDG PET | −.09 (−.64, .46) | .75 |
| Abnormal AD Sig. CT | −.42 (−.76, −.08) | .01 |
| Abnormal HVa | −.71 (−1.15, −.28) | <.01 |
| Basic model adjusted also for the Boston Naming Testf | ||
| Abnormal 11C-PiB PET | −.38 (−.87, .11) | .13 |
| Abnormal 18F-FDG PET | −.15 (−.70, .40) | .60 |
| Abnormal AD Sig. CT | −.37 (−.71, −.02) | .04 |
| Abnormal HVa | −.71 (−1.14, −.27) | <.01 |
| Basic model adjusted also for UPDRS | ||
| Abnormal 11C-PiB PET | −.42 (−.91, .07) | .09 |
| Abnormal 18F-FDG PET | −.09 (−.64, .45) | .74 |
| Abnormal AD Sig. CT | −.40 (−.74, −.06) | .02 |
| Abnormal HVa | −.70 (−1.14, −.26) | <.01 |
| Basic model adjusted also for history of head traumag | ||
| Abnormal 11C-PiB PET | −.37 (−.93, .19) | .20 |
| Abnormal 18F-FDG PET | −.02 (−.64, .60) | .95 |
| Abnormal AD Sig. CT | −.39 (−.75, −.03) | .03 |
| Abnormal HVa | −.67 (−1.13, −.22) | <.01 |
Slopes (95%CI) retained from multivariable linear regression models. Separate models were run for each neuroimaging biomarker, represented by one row in the table, having continuous B-SIT as the dependent (outcome) variable. Basic model is adjusted for age (at B-SIT testing), education, sex.
Abnormal 11C-PiB PET (elevated amyloid) was defined as standardized uptake value ratio (SUVR)>1.40.
Abnormal 18F-FDG PET (hypometabolism) was defined as SUVR<1.32.
Abnormal (reduced) AD Sig. CT was defined as <2.74 mm.
Abnormal (reduced) hippocampal volume was defined as <−2.40 cm3 (adjusted for total intracranial volume).
Kaplan EGH, Weintraub S. The Boston Naming Test, 2nd ed. Boston, MA: Lea & Fabiger, 1978.
History of head trauma: “Have you ever experienced any head injuries that led you to see a doctor, stay in the hospital, lose your memory, or become unconscious”.
B-SIT = Brief Smell Identification Test; CI = confidence interval. 11C-PiB PET = Pittsburgh compound B positron emission tomography; 18F-FDG = 18fluorodeoxyglucose; AD Sig. CT = Alzheimer’s Disease signature cortical thickness; HVa = hippocampal volume adjusted for total intracranial volume; UPDRS = Unified Parkinson’s Disease Rating Scale.
There were statistically significant interactions between abnormal 11C-PiB PET and abnormal 18F-FDG PET (i.e., p=0.017 for interaction for the anosmia (vs. normosmia) comparison and p=0.061 for the hyposmia (vs. normosmia) comparison in the multinomial logistic regression models and p = 0.004 when B-SIT was used as a continuous outcome in the linear regression model), thus we performed separate analyses in those with and without abnormal 11C-PiB PET (Table 4). Of interest the point estimates for associations in the abnormal PiB stratum remained elevated; however, the wide-confidence intervals suggested lower precision and possibly overestimated point estimates due to the small numbers. The association of abnormal HVa with the continuous B-SIT score was significant only in those with abnormal 11C-PiB. There were no statistically significant associations in persons without abnormal PiB. In analyses for imaging biomarker groups defined by the combination of abnormality for amyloid (A+/A−) and neurodegeneration (N+/N−), individuals with A+/N+ imaging biomarker combination (when compared to those with the A−/N− combination) had a 3.84 fold increased odds of having anosmia (OR, 3.84; 95%CI: 1.14, 12.97; p = .03) (Table 5). Having the A+/N+ (vs. A−/N−) imaging biomarker combination was also significantly associated with lower B-SIT score (slope (95%CI) = −.99 (−1.71, −.27).
Table 4.
Association between Imaging Biomarkers and Impaired Odor Identification in Cognitively Normal Individuals by PiB PET status - the Mayo Clinic Study of Aging.
| Impaired Olfaction | Continuous B-SIT | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Anosmia vs. normosmiaa | Hyposmia vs. normosmiaa | |||||||
|
| ||||||||
| Imaging Biomarkers | Abn /All | OR (95%CI)b | p | Abn /All | OR (95%CI) | p | slope (95%CI) | p |
| 11C-PiB SUVR>1.40c,d | ||||||||
| 18F-FDG SUVr<1.32c | 17/50 | 3.10 (0.84, 11.52) | .09 | 68/101 | 2.20 (0.81, 5.99) | .12 | −.96 (−1.84, −.08) | .03 |
| AD Sig. CT <2.74 mm | 17/50 | 2.22 (0.59, 8.39) | .24 | 69/102 | 1.31 (0.49, 3.50) | .59 | −.72 (−1.63, .19) | .13 |
| HVa <−2.40 cm3 | 17/50 | 7.15 (1.36, 37.58) | .02 | 69/102 | 1.76 (0.44, 7.05) | .43 | −1.74 (−2.79, −.69) | <.01 |
| 11C-PiB SUVR≤1.40 | ||||||||
| 18F-FDG SUVr<1.32 | 11/80 | 0.12 (0.01, 1.14) | .07 | 107/176 | 0.64 (0.30, 1.36) | .25 | .65 (−.05, 1.34) | .07 |
| AD Sig. CT <2.74 mm | 11/80 | 2.53 (0.59, 10.82) | .21 | 104/173 | 1.15 (0.50, 2.64) | .74 | −.46 (−1.20, .28) | .23 |
| HVa <−2.40 cm3 | 11/80 | 1.41 (0.22, 9.18) | .72 | 104/173 | 1.53 (0.50, 4.69) | .46 | −.29 (−1.24, .66) | .55 |
Osmia categories were defined as follows: anosmia (score <6), hyposmia (men 6–10, women 6–10.25), normosmia (men 10.25–12; women 10.5–12); B-SIT score was calculated as the sum of the correct responses for persons with ≤2 missing responses.
Odds ratios (95% CI) and slopes (95%CI) were retained from multinomial logistic regression and simple linear regression models, respectively, adjusted for age (at B-SIT testing), education, sex. Separate models were run for each neuroimaging biomarker (i.e., each model is represented by one line in the table, having Osmia or continuous B-SIT as the outcome variable) in participants with 11C-PiB SUVR>1.40 and in participants with 11C-PiB SUVR≤1.40.
Abnormal PiB defined as11C-PiB SUVr>1.40.
p=0.017 for the interaction term between abnormal PiB (SUVR) >1.40) and abnormal FDG (SUVr <1.32) in the anosmia vs. normosmia comparison and p=0.061 for the hyposmia vs. normosmia comparison (in the multinomial logistic regression model) and p = 0.004 when B-SIT was used as a continuous outcome (linear regression model). Interaction terms between abnormal PiB and abnormal AD cortical thickness (<2.74 mm) or abnormal hippocampal volume (<−2.40 cm3) did not reach statistical significance.
B-SIT = Brief Smell Identification Test; OR = odds ratio; CI = confidence interval; 11C-PiB PET = Pittsburgh compound B positron emission tomography; SUVR = standardized uptake value ratio; 18F-FDG = 18fluorodeoxyglucose. AD Sig. CT = Alzheimer’s Disease signature cortical thickness; HVa = hippocampal volume adjusted for total intracranial volume;
Table 5.
Association between Neuroimaging Biomarkers and impaired odor identification (outcome variable) in cognitively normal individuals - the Mayo Clinic Study of Aging.
| Impaired Olfaction | Continuous B-SIT | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Anosmia vs. normosmiaa | Hyposmia vs. normosmiaa | |||||||
|
| ||||||||
| Imaging Biomarkers |
Abn /All | OR (95%CI)b | p | Abn /All | OR (95%CI) | p | slope (95%CI)c | p |
| A+/N− vs. A−/N−d | 14/85 | 1.13 (.33, 3.81) | .85 | 105/176 | .97 (.51,1.85) | .92 | .14 (−.49, .77) | .66 |
| A−/N+ vs. A−/N− | 11/80 | .40 (.09, 1.78) | .23 | 104/173 | .87 (.43, 1.73) | .68 | .26 (−.41, .94) | .44 |
| A+/N+ vs. A−/N− | 19/75 | 3.84 (1.14, 12.97) | .03 | 99/155 | 1.91 (.80, 4.54) | .14 | −.99 (−1.71, −.27) | <.01 |
Osmia categories were defined as follows: anosmia (score <6), hyposmia (men 6–10, women 6–10.25), normosmia (men 10.25–12; women 10.5–12); B-SIT score was calculated as the sum of the correct responses for persons with ≤2 missing responses.
Odds ratio (95% confidence interval) reported for anosmia vs. normosmia and hyposmia vs. normosmia were retained from a single multinomial logistic regression model, having osmia as the dependent variable and adjusted for age (at B-SIT testing), education, sex.
Slopes (95% CI) retained from a single multivariable linear regression model, adjusted for age (at B-SIT testing), education, sex.
Imaging biomarker groups defined by the combination of abnormality for amyloid (A+/A−) and neurodegeneration (N+/N−); A+, is defined as 11C-PIB standardized uptake value ratio (SUVR)>1.40 and A− if otherwise; and N+ is defined as either HVa <−2·40 cm3 or FDG <1.32 SUVR and N− if otherwise.
B-SIT = Brief Smell Identification Test; Abn. - the number of persons with abnormal B-SIT; All - participants with and without abnormal biomarkers in the specific analysis strata; OR – odds ratio; CI – confidence interval.
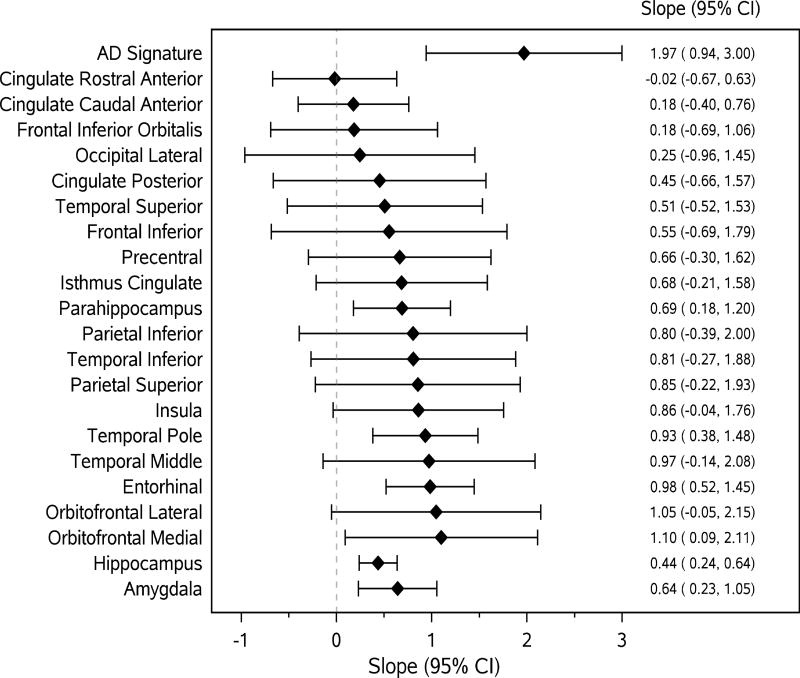
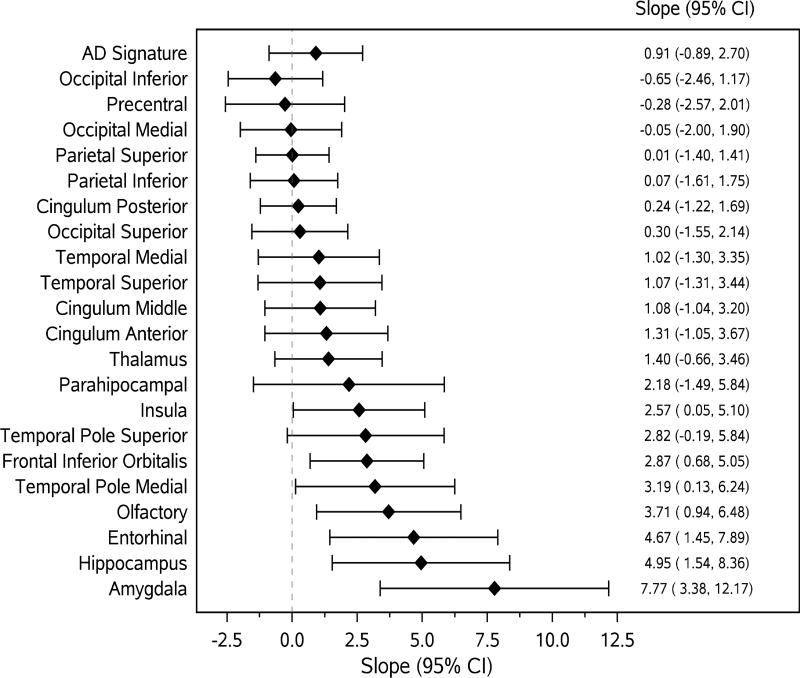
Figures 1 and 2 present the associations (linear regression slopes and 95%CIs) of MRI and 18F-FDG PET ROI measurements with continuous B-SIT score. Associations were stronger (and statistically significant) between MRI (Fig 1) and 18F-FDG PET (Fig 2) ROIs representing areas of the primary olfactory cortex and secondary olfactory regions and B-SIT. None of the associations between 11C-PiB ROIs and B-SIT reached statistical significance.
Figure 1.
Forest plots for associations (slopes, 95%CI) of continuous cortical thickness and volume measures (i.e., amygdala and hippocampus) with continuous B-SIT as the outcome variable. The diamonds represent slopes (estimated change in B-SIT per unit increase in cortical thickness (in mm) and volume measures (in cm3)) from general linear models adjusted for age, sex, education. The solid horizontal lines represent the 95% confidence intervals.
AD signature = an AD-signature cortical thickness measure was computed by averaging the cortical thickness for entorhinal, inferior temporal, middle temporal, and fusiform cortices.
Figure 2.
Forest plots of associations (slopes, 95%CI) of continuous 18-F-FDG PET biomarkers with continuous B-SIT as the outcome variable. The diamonds represent slopes (estimated change in B-SIT per unit increase in FDG PET standardized uptake value ratio) from general linear models, adjusted for age, sex, education. The solid horizontal lines represent the 95% confidence intervals.
AD signature = an AD signature 18F-FDG PET SUVR was calculated based on glucose metabolic rates from an AD signature meta-ROI and consisted of the average bilateral angular gyri, posterior cingulate, and inferior temporal cortical ROIs from both hemispheres normalized to pons and vermis uptake.
DISCUSSION
The present study examined associations of amyloid deposition, markers of neurodegeneration, and neurodegeneration conditioned on amyloid status with OI. In this sample of cognitively normal elders, increased amyloid deposition, abnormal AD signature cortical thickness and abnormal HVa were significantly associated with anosmia Abnormal AD signature cortical thickness and abnormal HVa were significantly associated with decreasing B-SIT score. The association of the B-SIT score with HVa could be driven by abnormal 11C-PiB PET.
The present study examined associations of OI with neurodegeneration conditioned on amyloid status. The association of biomarkers of neurodegeneration (i.e., reduced HVa and hypometabolism) with B-SIT remained statistically significant and was stronger in individuals with abnormal PiB. In addition, the A+N+ biomarker category was strongly associated with severe OI impairment (anosmia). These cross-sectional findings have potential clinical significance if validated prospectively since previous reports suggest that CN individuals with reduced HVa are at risk for MCI46 and those with A+/N+ pathology have higher rates of future medial temporal neurodegeneration.47
These results are partly in agreement with a previous study15 that reported that in PiB positive clinically normal participants, a thinner entorhinal cortex was associated with worse olfactory function. However, previous studies are few and inconclusive. Antemortem OI (using B-SIT) was demonstrated16 to be negatively associated with post-mortem AD brain pathology (amyloid load and tangle density) but authors reported that when both measures of AD pathology were in the same analysis model, the association with tangles persisted but the association with amyloid was 70% attenuated.16 Similar conclusions were reached in studies conducted in mouse models.17 In addition, elevated soluble Aβ has been associated with detrimental effects on neural circuits and altered connectivity of olfactory neurons in mice (overexpressing human APPsw (Swedish mutation)) before the onset of plaques.48 Investigators17 have demonstrated a moderate inverse linear association between OI and PiB binding (global or regional) when pooling together individuals with AD, amnestic MCI or CN individuals, but the association was not verified within each of these groups.
In the present study, abnormal 11C-PiB PET scan was strongly associated with .anosmia.. Although we cannot assess a temporal relationship between amyloid deposition and OI impairment and amyloid deposition, this finding might suggest that a threshold of AD pathology burden in CN individuals might be necessary for severe OI to be present. We did not measure tau, thus we cannot determine whether overall the burden of AD pathology (i.e., both amyloid and tau deposits) was increased but given the study population median age (78.4 years), we could speculate that nearly all participants had some level of neurofibrillary tangle pathology.29, 49
Aging is characterized also by a decrease in cortical thickness,3 and hippocampal volume. We considered atrophy on MRI as a measure of neurodegeneration,29 however hippocampal atrophy is not specific for AD. It could represent other age-related processes leading to neurodegeneration, including tauopathy.29 Investigators18 have found that worse OI was associated with smaller hippocampal volume in a group of MCI and AD patients but not in healthy individuals; however the authors acknowledged the need for larger studies.18 In additional previous reports, OI scores cross-sectionally correlated with hippocampal volume in a study of non-demented elders,50 OI deterioration prospectively correlated with decreased hippocampal volume (and cognitive function deterioration) among MCI patients21 and was associated with greater AD pathology (based on a composite measure including neuritic plaques, diffuse plaques, and neurofibrillary tangles) in an autopsy study of individuals without cognitive impairment.51
The present study examined the association of abnormal (decreased) cortical thickness specifically in AD signature cortical regions OI,30 which we expect would most likely differentiate those individuals at risk for clinical AD. Having abnormal AD signature cortical thickness (vs. not having abnormal AD signature cortical thickness) was most strongly associated with severe OI impairment (anosmia vs. normosmia). We observed significant positive associations between AD signature cortical thickness (as a continuous measure), entorhinal, temporal pole and parahippocampal cortical thicknesses, key regions associated with olfaction and with cortical atrophy in AD dementia, and B-SIT score. In patients with early AD, OI scores were associated with normalized FDG uptake with peaks in the right inferior frontal gyrus, the right fusiform gyrus, precuneus and superior parietal lobe.44 In the present study, FDG ROIs of the olfactory pathway and AD pathology including amygdala, hippocampus, entorhinal, or olfactory ROIs showed significant positive associations with B-SIT score. However, abnormal AD 18F-FDG-PET ratio was associated with B-SIT only in those with abnormal 11C-PiB PET. Potential explanations could include that the abnormal AD 18F-FDG-PET ratio does not include key areas of the olfactory pathway (i.e., it includes average bilateral angular gyri, posterior cingulate, and inferior temporal) or that OI may precede brain hypometabolism in those areas early in the AD process, especially when the Aβ burden is not significant (i.e. in individuals without abnormal PiB PET).
AD pathology develops several decades52 prior to AD symptomatology. Current cross-sectional findings if validated in prospective studies are clinically relevant as the olfactory system demonstrates measurable impairment in the early pre-clinical stages of the disease when the AD pathological markers are also present.17 Because of the nature of the logistic models, when analysis direction is reversed, the results are quite similar, suggesting that in a clinical setting OI could be used to as a marker for neuroimaging abnormality.
The association of imaging biomarker abnormalities with OI suggests that OI may be a non-invasive, inexpensive marker for risk stratification, for identifying participants at the preclinical stage of AD who may be at risk for cognitive impairment, and eligible for inclusion in AD prevention clinical trials. Although, olfactory assessment cannot currently be used as a stand-alone diagnostic test,17 a combination of olfactory assessment with other AD biomarkers (e.g., imaging biomarkers) could strengthen the AD diagnostic sensitivity and specificity.17, 53 However, our findings are preliminary and remain to be validated prospectively.
Limitations of the study should be considered when interpreting the results. The cross-sectional study design precludes our ability to assess causality. Participants in imaging studies performed slightly better cognitively and had a slightly lower frequency of hypertension than individuals without imaging studies, suggesting a potential bias of our estimates toward the null. Analysis stratified by PiB status (normal/abnormal) suggested lower precision and could have overestimated the point estimates. Lastly, the population of Olmsted County, MN, 70 years and older, is predominantly white of European ancestry; thus assessment of these associations in more heterogeneous populations would be of interest.
Despite the potential limitations, the study has several important strengths. The population-based design diminishes potential selection bias. Cognitive status was comprehensively assessed using published criteria, without consideration of any previous diagnoses, thereby reducing the potential bias in ascertainment of CN status. In addition, reliable measures of brain pathology were ascertained by multimodal, state-of-the-art, imaging and the study had a relatively large sample size.
Acknowledgments
The study was supported by National Institutes of Health grants U01 AG006786, K01 AG028573, P50 AG016574, R01 AG011378, R01 AG041851; by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and the Alice Weiner Postdoctoral Research Fellowship in Alzheimer’s Disease Research and was made possible by the Rochester Epidemiology Project (R01 AG034676).
The authors thank Ms. Dana Swenson-Dravis, operations manager of the Mayo Clinic Study of Aging, the staff of the Abigail Van Buren Alzheimer’s Disease Research Center for recruitment and evaluation of study participants, and study participants for their participation in the study.
Footnotes
AUTHOR CONTRIBUTIONS
ROR, MV and RCP contributed to study concept and design; MV, ROR, TJC, WKK, MMMi, MMMa, DSK, YEG, CRJ, VJL and RCP contributed to data acquisition, analysis and interpretation; MV, ROR and TJC contributed to drafting the manuscript and preparing the figures. All authors critically revised the manuscript for important intellectual content.
POTENTIAL CONFLICTS OF INTEREST
Nothing to report.
References
- 1.Attems J, Walker L, Jellinger KA. Olfaction and Aging: A Mini-Review. Gerontology. 2015;61:485–490. doi: 10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- 2.Gopinath B, Sue CM, Kifley A, Mitchell P. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:204–209. doi: 10.1093/gerona/glr165. [DOI] [PubMed] [Google Scholar]
- 3.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devanand DP, Lee S, Manly J, et al. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78:401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84:182–189. doi: 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RO, Christianson TJ, Kremers WK, et al. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2015:1–9. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 8.Tabert MH, Liu X, Doty RL, et al. A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol. 2005;58:155–160. doi: 10.1002/ana.20533. [DOI] [PubMed] [Google Scholar]
- 9.Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88:456–462. doi: 10.1212/WNL.0000000000003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray AJ, Staples V, Murren K, Dhariwal A, Bentham P. Olfactory identification is impaired in clinic-based patients with vascular dementia and senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 2001;16:513–517. doi: 10.1002/gps.383. [DOI] [PubMed] [Google Scholar]
- 13.Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 14.McShane RH, Nagy Z, Esiri MM, et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer's pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84:2153–2160. doi: 10.1212/WNL.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahar-Fuchs A, Chetelat G, Villemagne VL, et al. Olfactory deficits and amyloid-beta burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis. 2010;22:1081–1087. doi: 10.3233/JAD-2010-100696. [DOI] [PubMed] [Google Scholar]
- 18.Kjelvik G, Saltvedt I, White LR, et al. The brain structural and cognitive basis of odor identification deficits in mild cognitive impairment and Alzheimer's disease. BMC Neurol. 2014;14:168. doi: 10.1186/s12883-014-0168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 20.Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer's disease and Parkinson's disease on olfaction: A meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Lojkowska W, Sawicka B, Gugala M, et al. Follow-Up Study of Olfactory Deficits, Cognitive Functions, and Volume Loss of Medial Temporal Lobe Structures in Patients with Mild Cognitive Impairment. Curr Alzheimer Res. 2011;8:689–698. doi: 10.2174/156720511796717212. [DOI] [PubMed] [Google Scholar]
- 22.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 27.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurol. 2015;72:511–519. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CR, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138:3747–3759. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138:1370–1381. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fahn SER Members of the UPDRS Development Committee. In: Unified Parkinson's Disease Rating Scale. Fahn SMC, Calne DB, Goldstein M, editors. Florham Park: Macmillan Health Care Information; 1987. [Google Scholar]
- 41.Kaplan EGH, Weintraub S. The Boston Naming Test. 2. Boston, MA: Lea & Fabiger; 1978. [Google Scholar]
- 42.Sobel N, Johnson BN, Mainland J, Yousem DM. Functional Neuroimaging of Human Olfaction. In: Doty RL, editor. Handbook of olfaction and gustation. 2. New York: M. Dekker; 2003. pp. 251–272. [Google Scholar]
- 43.Gottfried JA. Structural and Functional Imaging of the Human Olfactory System. In: Doty RL, editor. Handbook of olfaction and gustation. 3. Hoboken, New Jersey: John Wiley & Sons Inc.; 2015. pp. 279–303. [Google Scholar]
- 44.Forster S, Vaitl A, Teipel SJ, et al. Functional representation of olfactory impairment in early Alzheimer's disease. J Alzheimers Dis. 2010;22:581–591. doi: 10.3233/JAD-2010-091549. [DOI] [PubMed] [Google Scholar]
- 45.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 46.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis. JAMA Neurol. 2013;70:1030–1038. doi: 10.1001/jamaneurol.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao L, Schrank BR, Rodriguez S, et al. Abeta alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun. 2012;3:1009. doi: 10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 50.Devanand DP, Tabert MH, Cuasay K, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nordberg A. Dementia in 2014. Towards early diagnosis in Alzheimer disease. Nat Rev Neurol. 2015;11:69–70. doi: 10.1038/nrneurol.2014.257. [DOI] [PubMed] [Google Scholar]
- 53.Wesson DW, Wilson DA, Nixon RA. Should olfactory dysfunction be used as a biomarker of Alzheimer's disease? Expert Rev Neurother. 2010;10:633–635. doi: 10.1586/ern.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]