Abstract
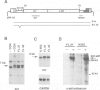
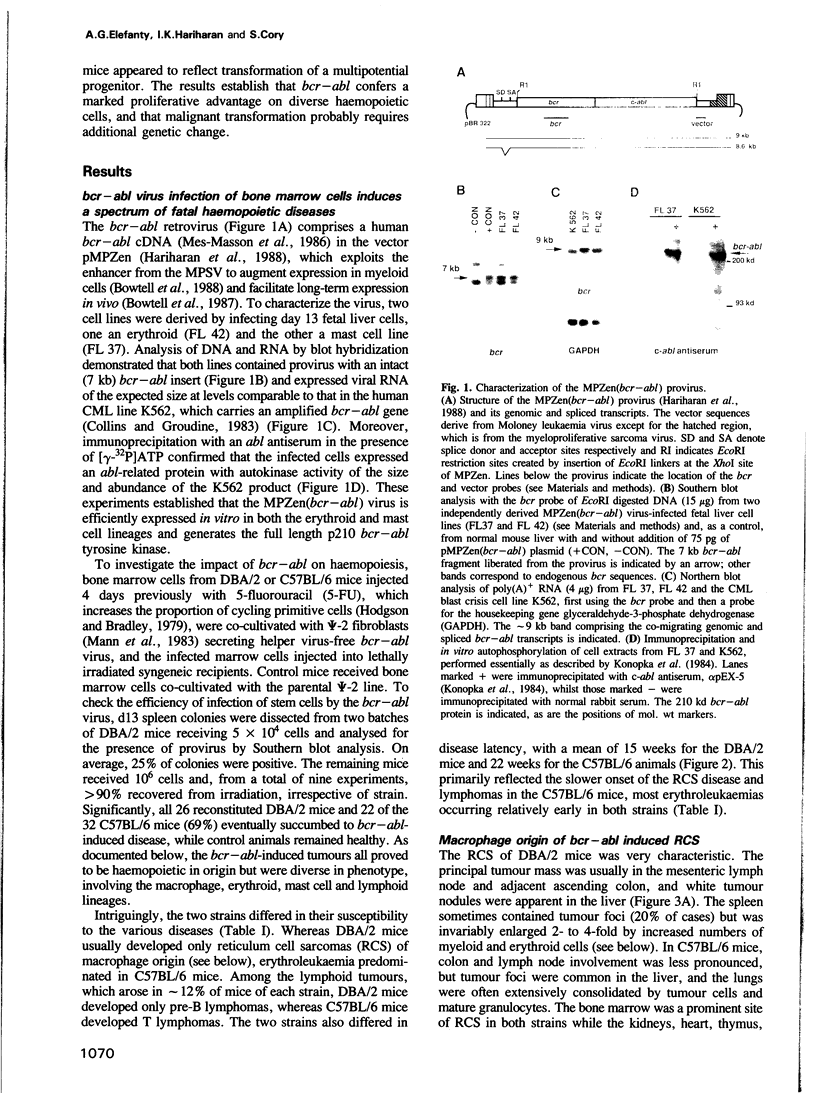
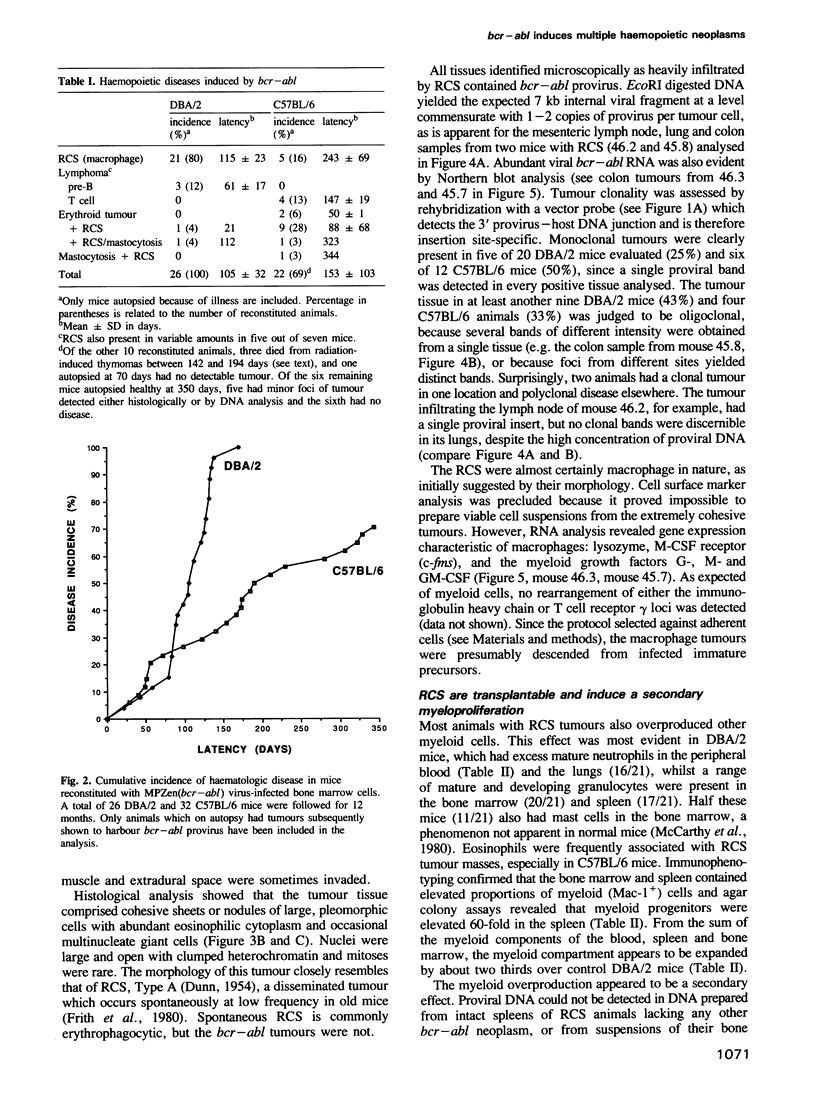
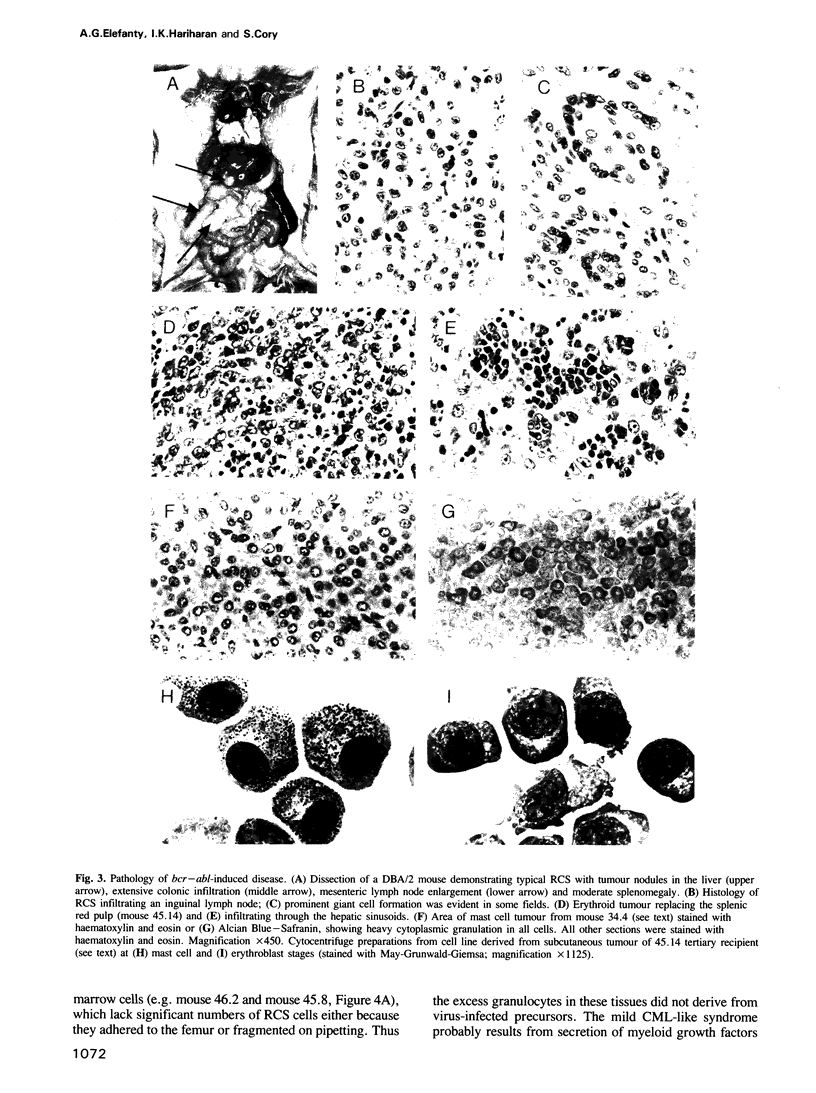
The chromosome translocation forming the hybrid bcr-abl gene is thought to be the initiating event in chronic myeloid leukaemia (CML) and some cases of acute lymphoblastic leukaemia. To assess the impact of bcr-abl upon haemopoiesis, lethally irradiated mice were reconstituted with bone marrow cells enriched for cycling stem cells and infected with a bcr-abl bearing retrovirus. The mice developed several fatal diseases with abnormal accumulations of macrophage, erythroid, mast and lymphoid cells, and marked strain differences in disease distribution and kinetics. Some mice exhibited more than one neoplastic cell type and, in some instances, these were clonally related, indicating that a progenitor or stem cell had been transformed. While classical CML was not observed, the macrophage tumours were accompanied by a mild CML-like syndrome, probably due to myeloid growth factor production by tumour cells. The erythroid and mast cell diseases were rarely transplantable, in contrast to the macrophage tumours and lymphomas, but all disease types displayed limited clonality. These results establish that bcr-abl confers a proliferative advantage on diverse haemopoietic cells but complete transformation probably involves additional genetic changes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Cory S., Johnson G. R., Gonda T. J. Comparison of expression in hemopoietic cells by retroviral vectors carrying two genes. J Virol. 1988 Jul;62(7):2464–2473. doi: 10.1128/jvi.62.7.2464-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Breton-Gorius J., Reyes F., Vernant J. P., Tulliez M., Dreyfus B. The blast crisis of chronic granulocytic leukaemia: megakaryoblastic nature of cells as revealed by the presence of platelet-peroxidase--a cytochemical ultrastructural study. Br J Haematol. 1978 Jul;39(3):295–303. doi: 10.1111/j.1365-2141.1978.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Champlin R. E., Golde D. W. Chronic myelogenous leukemia: recent advances. Blood. 1985 May;65(5):1039–1047. [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Gonda T. J., Johnson G. R. Long-term exposure to retrovirally expressed granulocyte-colony-stimulating factor induces a nonneoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. J Clin Invest. 1989 Nov;84(5):1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Mangelsdorf I., Wedel A., Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN T. B. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954 Jun;14(6):1281–1433. [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLamarter J. F., Hession C., Semon D., Gough N. M., Rothenbuhler R., Mermod J. J. Nucleotide sequence of a cDNA encoding murine CSF-1 (Macrophage-CSF). Nucleic Acids Res. 1987 Mar 11;15(5):2389–2390. doi: 10.1093/nar/15.5.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Frith C. H., Davis T. M., Zolotor L. A., Townsend J. W. Histiocytic lymphoma in the mouse. Leuk Res. 1980;4(6):651–662. doi: 10.1016/0145-2126(80)90076-4. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Kishi K., Ohnishi Y., Shibata A. Bipotential cell differentiation of KU-812: evidence of a hybrid cell line that differentiates into basophils and macrophage-like cells. Blood. 1987 Sep;70(3):612–619. [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N. The BCR/ABL hybrid gene. Baillieres Clin Haematol. 1987 Dec;1(4):983–999. doi: 10.1016/s0950-3536(87)80035-5. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M. cDNA sequence for human bcr, the gene that translocates to the abl oncogene in chronic myeloid leukaemia. EMBO J. 1987 Jan;6(1):115–119. doi: 10.1002/j.1460-2075.1987.tb04727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan I. K., Harris A. W., Crawford M., Abud H., Webb E., Cory S., Adams J. M. A bcr-v-abl oncogene induces lymphomas in transgenic mice. Mol Cell Biol. 1989 Jul;9(7):2798–2805. doi: 10.1128/mcb.9.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Roussel M. F., Rettenmier C. W., Sherr C. J. Multilineage hematopoietic disorders induced by transplantation of bone marrow cells expressing the v-fms oncogene. Cell. 1987 Nov 20;51(4):663–673. doi: 10.1016/0092-8674(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Abraham S., Krystal G., Lansdorp P., Lemoine F., Eaves C. J. Activation of multiple hemopoietic growth factor genes in Abelson virus-transformed myeloid cells. Exp Hematol. 1988 Oct;16(9):774–781. [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S., BROWN M. B. A quantitative dose-response study of lymphoid-tumor development in irradiated C 57 black mice. J Natl Cancer Inst. 1952 Aug;13(1):185–208. [PubMed] [Google Scholar]
- Kantarjian H. M., Keating M. J., Talpaz M., Walters R. S., Smith T. L., Cork A., McCredie K. B., Freireich E. J. Chronic myelogenous leukemia in blast crisis. Analysis of 242 patients. Am J Med. 1987 Sep;83(3):445–454. doi: 10.1016/0002-9343(87)90754-6. [DOI] [PubMed] [Google Scholar]
- Keating A. Ph positive CML cell lines. Baillieres Clin Haematol. 1987 Dec;1(4):1021–1029. doi: 10.1016/s0950-3536(87)80037-9. [DOI] [PubMed] [Google Scholar]
- Keller G., Wagner E. F. Expression of v-src induces a myeloproliferative disease in bone-marrow-reconstituted mice. Genes Dev. 1989 Jun;3(6):827–837. doi: 10.1101/gad.3.6.827. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Activation of the abl oncogene in murine and human leukemias. Biochim Biophys Acta. 1985 Nov 12;823(1):1–17. doi: 10.1016/0304-419x(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Gale R. P., Tomonaga M., Ikeda S., Yao E., Klisak I., Whelan K., Yakir H., Ichimaru M., Sparkes R. S. CML-T1: a cell line derived from T-lymphocyte acute phase of chronic myelogenous leukemia. Blood. 1989 Sep;74(4):1381–1387. [PubMed] [Google Scholar]
- Kurzrock R., Gutterman J. U., Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988 Oct 13;319(15):990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Najfeld V., Fialkow P. J. B-lymphoid cell involvement in chronic myelogenous leukemia: implications for the pathogenesis of the disease. Cancer Genet Cytogenet. 1982 Aug;6(4):359–368. doi: 10.1016/0165-4608(82)90092-9. [DOI] [PubMed] [Google Scholar]
- McCarthy J. H., Mandel T. E., Garson O. M., Metcalf D. The presence of mast cells in agar cultures. Exp Hematol. 1980 May;8(5):562–567. [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., McLaughlin J., Daley G. Q., Paskind M., Witte O. N. Overlapping cDNA clones define the complete coding region for the P210c-abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. [PubMed] [Google Scholar]
- Metcalf D., Moore M. A., Sheridan J. W., Spitzer G. Responsiveness of human granulocytic leukemic cells to colony-stimulating factor. Blood. 1974 Jun;43(6):847–859. [PubMed] [Google Scholar]
- Mori T., Nakazawa S., Nishino K., Sugita K., Takane K., Mori M., Sagawa K., Hayashi Y., Sakurai M. Ph1-positive CML-derived myeloid-monocytoid precursor cell line producing substance(s) that stimulates normal CFU-C. Leuk Res. 1987;11(3):241–249. doi: 10.1016/0145-2126(87)90047-6. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Nakazawa M., Mitjavila M. T., Debili N., Casadevall N., Mayeux P., Rouyer-Fessard P., Dubart A., Roméo P. H., Beuzard Y., Kishi K. KU 812: a pluripotent human cell line with spontaneous erythroid terminal maturation. Blood. 1989 May 15;73(7):2003–2013. [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal S., Cancellos G. P., Gralnick H. P. Erythroblastic transformation of chronic granulocytic leukemia. Am J Med. 1977 Jul;63(1):116–124. doi: 10.1016/0002-9343(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Shepherd P. C., Ganesan T. S., Galton D. A. Haematological classification of the chronic myeloid leukaemias. Baillieres Clin Haematol. 1987 Dec;1(4):887–906. doi: 10.1016/s0950-3536(87)80031-8. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Wendling F., Shreeve M., McLeod D., Axelrad A. A self-renewing, bipotential erythroid/mast cell progenitor in continuous cultures of normal murine bone marrow. J Cell Physiol. 1985 Oct;125(1):10–18. doi: 10.1002/jcp.1041250103. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. The complexity of virus--cell interactions in Abelson virus infection of lymphoid and other hematopoietic cells. Adv Immunol. 1985;37:73–98. doi: 10.1016/s0065-2776(08)60338-7. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. C., Witte O. N. Selective transformation of primitive lymphoid cells by the BCR/ABL oncogene expressed in long-term lymphoid or myeloid cultures. Mol Cell Biol. 1988 Oct;8(10):4079–4087. doi: 10.1128/mcb.8.10.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]