Abstract
Cells of the granulocyte-macrophage colony stimulating factor (GM-CSF) or multi-lineage colony stimulating factor (Multi-CSF) dependent line FDC-P1 undergo leukemic transformation after injection into irradiated DBA/2 mice. About one third of factor-independent FDC-P1 variants isolated from leukemic animals express GM-CSF or Multi-CSF, assessed either by bioassay or by sensitive RNA detection using the polymerase chain reaction. All of the GM-CSF-secreting lines studied had a rearrangement in one allele of the GM-CSF gene, three of four Multi-CSF-secreting lines had Multi-CSF gene rearrangements, while factor-independent lines lacking evidence of growth factor production had no demonstrable CSF gene alterations. All rearrangements were characterized by insertions of novel DNA in the 5'-flanking regions of the CSF genes. The inserted segments of DNA varied in size between 0.35 and 6.5 kb and displayed restriction enzyme cleavage maps reminiscent of intracisternal A-particle (IAP) genomes. This was confirmed in two cases by molecular cloning and nucleotide sequence analysis. In these instances, the insertion consisted of solitary IAP long terminal repeats. The transformation system described provides a model for the study of IAP transpositions and their effects on gene activation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Boswell H. S., Srivastava A., Burgess J. S., Nahreini P., Heerema N., Inhorn L., Padgett F., Walker E. B., Geib R. W. Cellular control of in vitro progression of murine myeloid leukemia: progression accompanies acquisition of independence from growth factor and stromal cells. Leukemia. 1987 Nov;1(11):765–771. [PubMed] [Google Scholar]
- Burt D. W., Reith A. D., Brammar W. J. A retroviral provirus closely associated with the Ren-2 gene of DBA/2 mice. Nucleic Acids Res. 1984 Nov 26;12(22):8579–8593. doi: 10.1093/nar/12.22.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. D., Ymer S., Fung M. C., Young I. G. Cloning and nucleotide sequence of the murine interleukin-3 gene. Eur J Biochem. 1985 Jul 15;150(2):297–304. doi: 10.1111/j.1432-1033.1985.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Unger T., Rechavi G., Canaani E., Givol D. Rearrangement of the oncogene c-mos in mouse myeloma NSI and hybridomas. Nature. 1983 Dec 22;306(5945):797–799. doi: 10.1038/306797a0. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
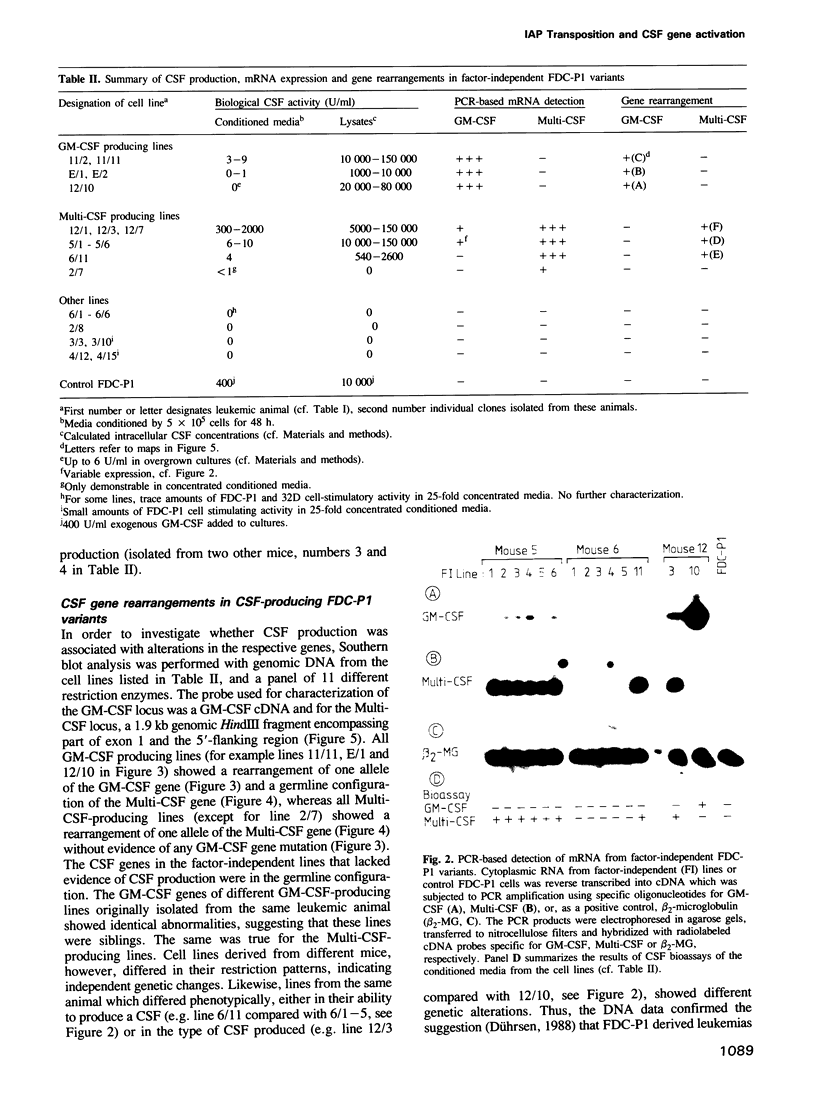
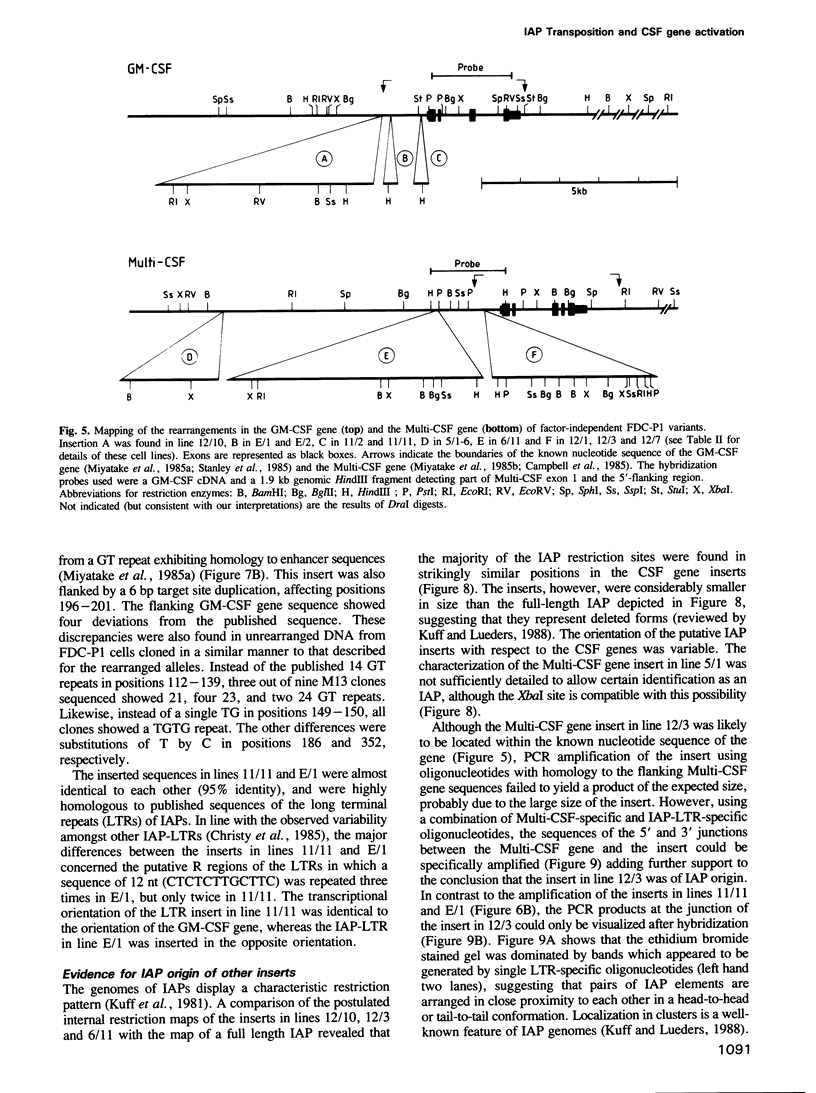
- Dührsen U. In vitro growth patterns and autocrine production of hemopoietic colony stimulating factors: analysis of leukemic populations arising in irradiated mice from cells of an injected factor-dependent continuous cell line. Leukemia. 1988 Jun;2(6):334–342. [PubMed] [Google Scholar]
- Dührsen U., Metcalf D. A model system for leukemic transformation of immortalized hemopoietic cells in irradiated recipient mice. Leukemia. 1988 Jun;2(6):329–333. [PubMed] [Google Scholar]
- Gattoni-Celli S., Hsiao W. L., Weinstein I. B. Rearranged c-mos locus in a MOPC 21 murine myeloma cell line and its persistence in hybridomas. Nature. 1983 Dec 22;306(5945):795–796. doi: 10.1038/306795a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Grail D., Gearing D. P., Metcalf D. Mutagenesis of murine granulocyte/macrophage-colony-stimulating factor reveals critical residues near the N terminus. Eur J Biochem. 1987 Dec 1;169(2):353–358. doi: 10.1111/j.1432-1033.1987.tb13619.x. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Murray K. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J Mol Biol. 1982 Nov 25;162(1):43–67. doi: 10.1016/0022-2836(82)90161-9. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Eckner R. J., Sakakeeny M., Marks P., Reid D., Nabel G., Hapel A., Ihle J. N., Humphries K. C. Interleukin 3-dependent hematopoietic progenitor cell lines. Fed Proc. 1983 Jul;42(10):2762–2771. [PubMed] [Google Scholar]
- Hapel A. J., Vande Woude G., Campbell H. D., Young I. G., Robins T. Generation of an autocrine leukaemia using a retroviral expression vector carrying the interleukin-3 gene. Lymphokine Res. 1986 Fall;5(4):249–254. [PubMed] [Google Scholar]
- Hapel A. J., Warren H. S., Hume D. A. Different colony-stimulating factors are detected by the "interleukin-3"-dependent cell lines FDC-Pl and 32D cl-23. Blood. 1984 Oct;64(4):786–790. [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Young I. G. Fine-structure mapping of the murine IL-3 and GM-CSF genes by pulsed-field gel electrophoresis and molecular cloning. Genomics. 1989 Aug;5(2):359–362. doi: 10.1016/0888-7543(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Leslie K. B., Schrader J. W. Growth factor gene activation and clonal heterogeneity in an autostimulatory myeloid leukemia. Mol Cell Biol. 1989 Jun;9(6):2414–2423. doi: 10.1128/mcb.9.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man Y. M., Delius H., Leader D. P. Molecular analysis of elements inserted into mouse gamma-actin processed pseudogenes. Nucleic Acids Res. 1987 Apr 24;15(8):3291–3304. doi: 10.1093/nar/15.8.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Roberts T. M., Cherington V., Dunn A. R. The in vitro behavior of hemopoietic cells transformed by polyoma middle T antigen parallels that of primary human myeloid leukemic cells. EMBO J. 1987 Dec 1;6(12):3703–3709. doi: 10.1002/j.1460-2075.1987.tb02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A. P., Diamantis I. D., Conscience J. F., Kindler V., Hofer P., Moroni C. A v-H-ras-dependent hemopoietic tumor model involving progression from a clonal stage of transformation competence to autocrine interleukin 3 production. Mol Cell Biol. 1989 Mar;9(3):1183–1190. doi: 10.1128/mcb.9.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naparstek E., Pierce J., Metcalf D., Shadduck R., Ihle J., Leder A., Sakakeeny M. A., Wagner K., Falco J., FitzGerald T. J. Induction of growth alterations in factor-dependent hematopoietic progenitor cell lines by cocultivation with irradiated bone marrow stromal cell lines. Blood. 1986 May;67(5):1395–1403. [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Crapper R. M. Autogenous production of a hemopoietic growth factor, persisting-cell-stimulating factor, as a mechanism for transformation of bone marrow-derived cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6892–6896. doi: 10.1073/pnas.80.22.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Amplification of a specific set of intracisternal A-particle genes in a mouse plasmacytoma. J Virol. 1984 Jan;49(1):171–177. doi: 10.1128/jvi.49.1.171-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Löliger C., Kawai M., Suciu S., Gough N., Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988 Jun 17;53(6):869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wheeler E. F., Askew D., May S., Ihle J. N., Sherr C. J. The v-fms oncogene induces factor-independent growth and transformation of the interleukin-3-dependent myeloid cell line FDC-P1. Mol Cell Biol. 1987 May;7(5):1673–1680. doi: 10.1128/mcb.7.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T., Glöggler K., Baumruker T., Schmidt M., Horak I. Family of middle repetitive DNA sequences in the mouse genome with structural features of solitary retroviral long terminal repeats. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3327–3330. doi: 10.1073/pnas.80.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Kovacic S., Kriz R., Wolf S., Clark S. C., Wellems T. E., Nienhuis A., Epstein N. The human genes for GM-CSF and IL 3 are closely linked in tandem on chromosome 5. Blood. 1988 Apr;71(4):958–961. [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Campbell H. D., Young I. G. Nucleotide sequence of the intracisternal A-particle genome inserted 5' to the interleukin-3 gene of the leukemia cell line WEHI-3B. Nucleic Acids Res. 1986 Jul 25;14(14):5901–5918. doi: 10.1093/nar/14.14.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]








