Introduction
Oral squamous cell carcinoma (OSCC) has historically been treated with surgery or radiation with or without chemotherapy, all of which lead to significant posttreatment morbidity.1 Topical imiquimod cream has been approved by the Food and Drug Administration for treatment of nonhyperkeratotic actinic keratosis, superficial basal cell carcinoma, and external genital warts. Off-label use has demonstrated effectiveness in treatment of mucosal neoplasms.2 Given the considerable risks associated with standard treatment regimens for OSCC, topical imiquimod might offer a reasonable and well-tolerated palliative treatment option for patients with multiple comorbidities. We report the resolution of recurrent OSCC with topical imiquimod cream.
Case report
An 81-year-old white women sought treatment for a superficial fleshy papule on the oral mucosa of the right retromolar trigone (RMT). A computed tomography (CT) scan confirmed a 1.3 cm–soft tissue density of the RMT with minimal adjacent osseous erosion and no pathologically enlarged cervical lymph nodes (Fig 1). Biopsy of the lesion confirmed papillary squamous cell carcinoma with negative margins. The T2N0M0 tumor was resected 1 month later by transoral marginal mandibulectomy with 10-12 mm margins, which were confirmed negative by surgical pathology. Because of the negative margins and favorable histopathology, no postoperative radiotherapy was initiated.
Fig 1.
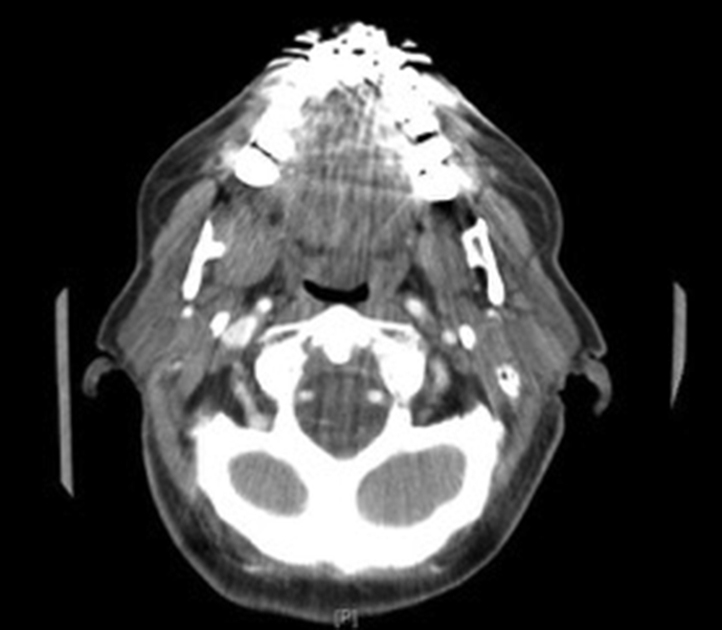
Computed tomography scan of the primary tumor on the neck soft tissue with contrast taken in 2006. A 1-cm soft tissue density is seen in the right retromolar trigone with a suggestion of minimal adjacent osseous erosion. The lesion is partially obscured by a metallic spray artifact secondary to the dental amalgam.
At the 8-month postoperative visit, the patient reported right dental pain and trismus. Physical exam was remarkable for an erythematous, exophytic mass and an adjacent superficial ulceration of the right RMT. Biopsy confirmed recurrent squamous cell carcinoma (Fig 2). A CT scan showed extension of the process along the lateral aspect of the right pterygoid muscles and into the right infratemporal fossa. The tumor was surgically managed with a segmental mandibulectomy and composite resection and reconstructed with a free flap. The patient received postoperative adjuvant radiation therapy to the surgical field and neck. She recovered from surgery and radiation with no complications and continued to follow with otolaryngology and radiation oncology for 3 years.
Fig 2.
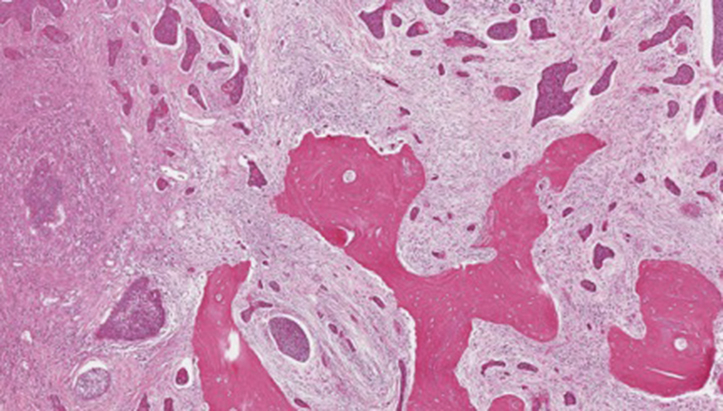
Histologic image of first recurrence demonstrating invasive moderately differentiated squamous cell carcinoma with extensive involvement of the underlying mandibular bone (Hematoxylin-eosin stain; original magnification: ×10.)
Five years following surgery and radiation for recurrent OSCC, the patient presented with a new ulcerating, friable, granular mass of the right lateral floor of the mouth at the base of the free flap. Pathology revealed grade 2 squamous cell carcinoma (Fig 3). A preoperative positron emission tomography-CT scan showed numerous new subcentimeter bilateral pulmonary nodules, highly suspicious for metastatic disease. The decision was made to proceed conservatively without surgery due to the patient's age and comorbidities and the presence of metastatic disease.
Fig 3.
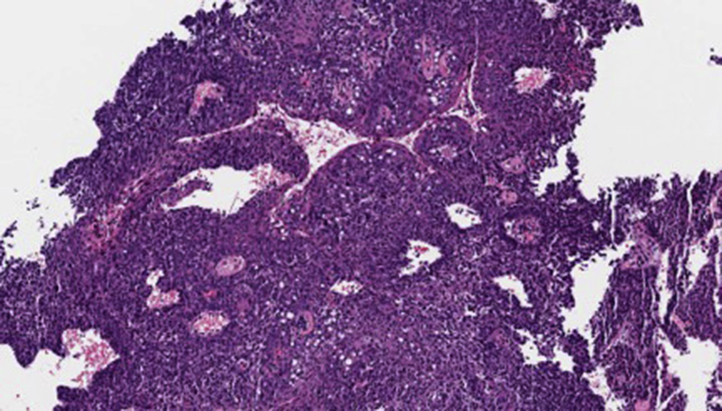
Histologic image of second recurrence demonstrating a fragment of moderately differentiated squamous cell carcinoma (high power). Because of the fragmented and superficial nature of the specimen, the presence of invasion could not be definitively determined. (Hematoxylin-eosin stain; original magnification: ×40.)
This patient presented with recurrent OSCC of the RMT refractory to 2 wide, local excisions and subsequent radiation therapy. Further treatment options included surgery, additional radiotherapy, chemotherapy, and observation. Advanced tumor stage decreased the likelihood of cure from surgical resection, chemotherapy, or radiotherapy. There were considerable risks associated with surgical intervention given the patient's age and extent of the planned procedure. In addition, reconstruction and rehabilitation following salvage surgery was challenging in the setting of previously irradiated tissue. In the presence of lung metastasis, chemotherapy was a noncurative option and of significant morbidity given the age of the patient. Irradiation alone or in combination with chemotherapy was associated with low survival rates and high morbidity.
Tumor directed treatment of the lesion was initiated with topical imiquimod 5% cream daily. A thin layer of 80 mg of imiquimod was applied to the affected area with a cotton swab once per day for 2 weeks. Subsequent examination 2 weeks following the initiation of therapy showed complete regression of the lesion. The imiquimod cream was gradually tapered to once weekly applications without recurrence of the lesion. Two months after starting treatment with topical imiquimod, a shave biopsy was performed of the RMT and found to be negative for disease. No side effects were noted other than slight tenderness from erosions as the lesion was resolving. Now, 4 years following initial treatment with topical imiquimod, the patient continues weekly applications to the RMT. There has not been a recurrence of OSCC (Fig 4).
Fig 4.
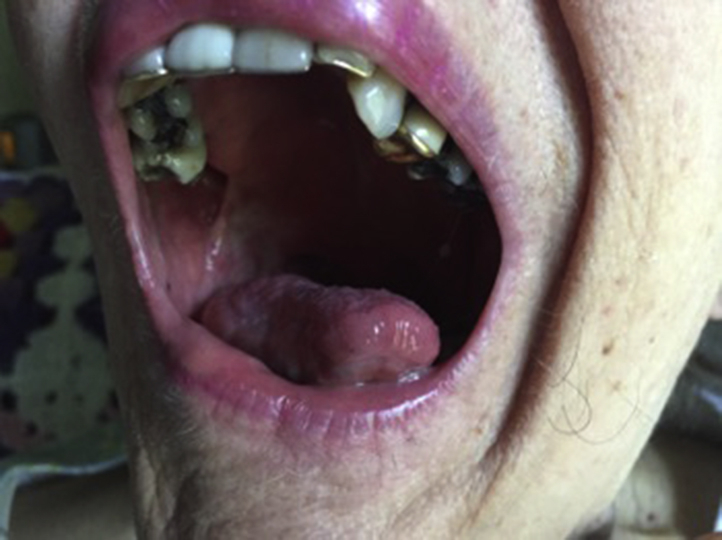
Right retromandibular trigone with no signs of recurrence.
Discussion
OSCC encompasses 90% of all oral malignancies.1, 3 Available treatment options include surgical resection, radiation, and chemotherapy. The utility of these treatment options varies with the stage of disease, tumor site, and surgical accessibility, as well as the functional outcomes and morbidity associated with each modality. OSCC discovered at an advanced stage generally requires a combined modality treatment approach including surgery or radiotherapy with or without chemotherapy and further attention to age, comorbidities, and functional consequences of each treatment.4
Imiquimod is a synthetic imidazoquinoline amide derivative that biases the immune system towards a T helper 1 cell–mediated immune response. This has been exploited clinically in the treatment of viral infections and tumors.5 Imiquimod was approved by the Food and Drug Administration for the treatment of nonhyperkeratotic actinic keratosis, superficial basal cell carcinoma, and external genital warts. It has been used off-label to successfully treat multiple cutaneous diseases including Bowen disease, lentigo maligna melanoma, and extramammary Paget disease.2 Well known side effects of topical imiquimod include erythema, edema, pruritus, and burning, as well as pain, superficial erosions, and ulceration. Topical administration rarely leads to systemic side effects such as fever, headache, and myalgias.6 In general, imiquimod is a well-tolerated, self-applicable treatment with minimal side effects.7 Off-label use of imiquimod has been described for treatment of dysplastic and malignant mucosal surface lesions including focal epithelial hyperplasia, recurrent oral melanoma, and florid oral papillomatosis. Although the treatment of OSCC with topical imiquimod 5% cream was previously described in a mouse model, there are no reported cases of OSCC treated with topical imiquimod in humans.8
Our patient demonstrated a robust clinical response to imiquimod 5% cream in the treatment of recurrent OSCC. Surgery, radiotherapy, and chemotherapy were considered noncurative treatment options with high morbidity due to the advanced stage of OSCC in our patient. Imiquimod 5% cream was a low-morbidity palliative treatment option given the mild adverse effect profile, ease of application, and cost-effective means of maintenance therapy. Topical imiquimod could serve an instrumental role in treatment of lower stage disease in appropriate patients or as a palliative treatment by allowing patients to avoid the considerable adverse effects associated with surgical intervention and radiotherapy.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Ramineni S.K., Dziubla T.D., Cunningham L.L., Jr., Puleo D.A. Local delivery of imiquimod in hamsters using mucoadhesive films and their residence time in human patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:665–673. doi: 10.1016/j.oooo.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Sciubba J. Oral cancer. Am J Clin Dermatol. 2001;2:239–251. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Massano J., Regateiro F.S., Januário G., Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Furness S., Glenny A.M., Worthington H.V. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev. 2011;(4):CD006386. doi: 10.1002/14651858.CD006386.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Stanley M. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 6.Yasar S., Mansur A.T., Serdar Z.A., Goktay F., Aslan C. Treatment of focal epithelial hyperplasia with topical imiquimod: report of three cases. Pediatr Dermatol. 2009;26:465–468. doi: 10.1111/j.1525-1470.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A.K., Cherman A.M., Tyring S.K. Viral and nonviral uses of imiquimod: a review. J Cutan Med Surg. 2004;8:338–352. doi: 10.1007/s10227-005-0023-5. [DOI] [PubMed] [Google Scholar]
- 8.Gkoulioni V., Eleftheriadou A., Yiotakis I. The efficacy of imiquimod on dysplastic lesions of the oral mucosa: an experimental model. Anticancer Res. 2010;30:2891–2896. [PubMed] [Google Scholar]


